Abstract
Background
Vaccine development has been a priority in the fight against leishmaniases, which are vector-borne diseases caused by Leishmania protozoa. Among the different immunization strategies employed to date is inoculation of plasmid DNA coding for parasite antigens, which has a demonstrated ability to induce humoral and cellular immune responses. In this sense, inoculation of plasmid DNA encoding Leishmania kinetoplasmid membrane protein-11 (KMP-11) was able to confer protection against visceral leishmaniasis. However, recently the use of antigen delivery systems such as poly(lactic-co-glycolic acid) (PLGA) nanoparticles has also proven effective for eliciting protective immune responses.
Methods
In the present work, we tested two immunization strategies with the goal of obtaining protection, in terms of lesion development and parasite load, against cutaneous leishmaniasis caused by L. braziliensis. One strategy involved immunization with plasmid DNA encoding L. infantum chagasi KMP-11. Alternatively, mice were primed with PLGA nanoparticles loaded with the recombinant plasmid DNA and boosted using PLGA nanoparticles loaded with recombinant KMP-11.
Results
Both immunization strategies elicited detectable cellular immune responses with the presence of both proinflammatory and anti-inflammatory cytokines; mice receiving the recombinant PLGA nanoparticle formulations also demonstrated anti-KMP-11 IgG1 and IgG2a. Mice were then challenged with L. braziliensis, in the presence of sand fly saliva. Lesion development was not inhibited following either immunization strategy. However, immunization with PLGA nanoparticles resulted in a more prominent reduction in parasite load at the infection site when compared with immunization using plasmid DNA alone. This effect was associated with a local increase in interferon-gamma and in tumor necrosis factor-alpha. Both immunization strategies also resulted in a lower parasite load in the draining lymph nodes, albeit not significantly.
Conclusion
Our results encourage the pursuit of immunization strategies employing nanobased delivery systems for vaccine development against cutaneous leishmaniasis caused by L. braziliensis infection.
Introduction
Leishmaniasis is a group of diseases caused by infection with unicellular protozoan parasites of the genus Leishmania, which are transmitted through the bite of an infected sand fly. Disease burden remains important, involving 88 countries and 350 million people at risk, with 500,000 new cases of visceral leishmaniasis and 1–1.5 million cases of cutaneous leishmaniasis per year.Citation1 Multiple Leishmania species are known to cause disease; L. braziliensis inoculation into the skin leads to the development of an ulcer with elevated borders and a necrotic center. A chronic inflammatory response develops despite the paucity of parasites. In 1%–5% of patients, mucocutaneous leishmaniasis may develop, and in this case, extensive tissue destruction is observed.Citation2 Species such as L. infantum chagasi multiply in the spleen and liver, causing visceral leishmaniasis. The disease may present with acute, subacute, or chronic evolution, but most infected individuals remain completely asymptomatic.Citation3 The visceral form of leishmaniasis is associated with an estimated incidence of 59,000 deaths annually.Citation4 The feasibility of preventing Leishmania infection through vaccination is supported by the fact that individuals who recover from a primary infection are resistant to overt clinical manifestations upon reinfection. In general, the key mediator of resistance to leishmaniasis is cellular immunity, whereby interferon-gamma (IFN-γ) produced by CD4+ Th1 cells promotes the oxidative burst in phagocytes that host the intracellular pathogen, promoting parasite killing.Citation5,Citation6
Vaccination against leishmaniasis has been pursued using different strategies, ranging from inoculation of virulent parasites (leishmanization) to immunization with killed parasite preparations, live attenuated parasites, or with recombinant proteins or plasmid DNA coding for defined Leishmania antigens.Citation7,Citation8 DNA vaccines encode a potent adjuvant, in the form of unmethylated dinucleotides, which are able to activate antigen-presenting cells through Toll-like receptor 9, stimulating the system towards a Th1-type response. DNA vaccination has also been tested in heterologous prime-boost vaccination regimesCitation9 in which the immune system is primed with DNA and boosted with a different formulation of the corresponding antigen. This strategy proved effective in models of visceralCitation10–Citation12 and cutaneous leishmaniasis.Citation13–Citation15 However, the adjuvant effect can also be achieved by encapsulation of antigens into biodegradable and biocompatible particles.Citation16 In this sense, immunization with antigen-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles induced potent immune responses in different experimental models of disease.Citation17–Citation19 Similar and encouraging results have been described regarding encapsulation of Leishmania antigens and the development of leishmaniasis.Citation20,Citation21
The 11 kDa kinetoplastid membrane protein (KMP-11)Citation22 is a promising vaccine candidate against leishmaniasis because it is a strong inducer of IFN-γ production by cells from cured patientsCitation23 and it is highly conserved among the trypanosomatids.Citation24 DNA immunization with KMP-11 was able to confer protection against visceral leishmaniasis caused by L. donovaniCitation25 and against cutaneous leishmaniasis caused by L. major, when used in combination with interleukin (IL)-12.Citation26 Given that there are few published studies regarding vaccine development against L. braziliensis,Citation27 a species prevalent in Brazil and South America, we evaluated the ability of KMP-11 to confer protection against cutaneous leishmaniasis caused by L. braziliensis, employing two strategies. One strategy involved immunization with a naked plasmid DNA coding for KMP-11, whereas a parallel strategy comprised priming with PLGA nanoparticles loaded with a plasmid DNA encoding KMP-11 followed by PLGA nanoparticles loaded with the recombinant KMP-11 protein.
Materials and methods
Mice
Female BALB/c mice (6–8 weeks of age) were obtained from the animal facility at Centro de Pesquisas Gonçalo Moniz, FIOCRUZ. All mice were maintained under pathogen-free conditions. The local Ethics Committee on Animal Care and Utilization approved all procedures involving animals.
Plasmid and recombinant protein purification
The DNA insert containing the coding region of Leishmania KMP-11 was obtained after BamHI/SmaI digestion of the pQE-KMP-11 plasmidCitation28 and was subcloned in the BamHI/EcoRV sites of the pcDNA3 eukaryotic expression plasmid. Plasmid DNA (pcDNA3 and pcDNA3-KMP-11) was purified using an Endofree Plasmid Giga Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The recombinant plasmid, pQE30-KMP-11,Citation28 was transformed into Escherichia coli. Recombinant protein expression was performed as described previously.Citation29 Nonrecombinant pcDNA3, pcDNA3- KMP-11, and recombinant KMP-11 protein were encapsulated into PLGA nanoparticles, as described below.
Preparation and characterization of KMP-11 nanoparticles
Nanoparticles were prepared by employing a solvent evaporation process using a Total Recirculation One-Machine System, which has been used previously for the encapsulation of DNA into PLGA particles.Citation30 Briefly, 100 mg of PLGA Resomer 503 copolymer (4% w/v, polylactic:glycolic acid ratio 50:50, molecular weight 34 kDa, carrying uncapped hydroxyl and carboxyl, Boehringer Ingelheim, Ingelheim, Germany) and 10 mg of cationic lipid 1,2-dioleoyl-sn-glycero- 3-ethylphosphocholine (DOTAP, chloride salt, Avanti Polar Lipids Inc, Alabaster, AL) were dissolved in 2.5 mL dichloromethane and injected through a needle (inner diameter 0.17 mm) under a turbulent regime (50 mL/minute) onto a Pluronic F68® solution (500 μL 6% w/v, Sigma-Aldrich, St Louis, MO) containing 2 mg of either recombinant KMP-11 protein, non-recombinant pcDNA3, or recombinant pcDNA3-KMP-11. This W1/O emulsion was forced to circulate through the system for 4 minutes in order to homogenize the emulsion droplet size. The preformed emulsion was injected into the outer water (W2) phase, ie, 15 mL of polyvinyl alcohol 0.5% w/v (87% hydrolyzed, molecular weight 115,000; BDH Prolabo; VWR International, Radnor, PA) under a constant pump flow. The turbulent injection resulted in formation of a double emulsion (W1/O/W2) that was homogenized by circulation through the system for 8 minutes. The final emulsion was magnetically stirred to allow solvent evaporation and particle formation. The resulting particles (recombinant KMP-11-loaded, nonrecombinant pcDNA3-loaded, pcDNA3-KMP-11-loaded nanoparticles) or unloaded nanoparticles were centrifuged (9300 × g), washed, freeze-dried, lyophilized and stored at −20°C. Nanoparticle size and zeta potential were determined, respectively, by photon correlation spectroscopy and laser Doppler velocimetry using a Zetasizer Nano Series (Malvern Instruments, Worcestershire, UK) after dilution of the samples in distilled water or KCl (1 mM). All measurements were performed in triplicate. The recombinant KMP-11 protein content of the recombinant KMP-11-loaded nanoparticles was determined using the Micro BCA protein assay (Pierce, Rockford, IL) following the manufacturer’s instructions. The colorimetric reaction was measured in a spectrophotometer at 562 nm and compared with the absorbance obtained with nonencapsulated recombinant KMP-11. For this purpose, control calibration curves (1.5–50 μg/mL) were prepared using recombinant KMP-11 dissolved in NaOH 0.1 N. The amount of plasmidial pcDNA3-KMP-11 DNA or nonrecombinant pcDNA3 loaded into the nanoparticles was estimated using a fluorimetric assay (PicoGreen® dsDNA quantitation kit; Molecular Probes, Eugene, OR), following the manufacturer’s instructions. The amount of encapsulated recombinant KMP-11 in the nanoparticles was 3.5 ± 0.5 per mg of recombinant KMP-11-loaded particles; nonrecombinant pcDNA3-loaded nanoparticles contained 7.2 ± 0.7 μg of pcDNA3 per mg of particles, and pcDNA3-KMP-11-loaded nanoparticles contained 6.4 ± 1.2 μg of pcDNA3-KMP-11 DNA per mg of particles.
Immunization with KMP-11 plasmid DNA or recombinant KMP-11-loaded PLGA nanoparticles
BALB/c mice (in groups of six) received 100 μg of nonrecombinant pcDNA3 or pcDNA3-KMP-11 in saline into the right quadriceps on days 0, 14, and 28. Alternatively, mice were primed with pcDNA3-KMP-11-loaded nanoparticles (containing 30 μg of pcDNA3-KMP-11), injected into the left ear dermis, and were boosted 21 days later with recombinant KMP-11-loaded nanoparticles (containing 10 μg of recombinant KMP-11) in the presence of 25 μg of each CpG oligodeoxynucleotide (5′-TCAGCGTTGA-3′ and 5′-GCTAGCGTTAGCGT-3′) (E-OLIGOS).Citation31 Control mice were primed with nonrecombinant pcDNA3-loaded nanoparticles (containing 30 μg of pcDNA3), also injected in the left ear dermis, and were boosted with unloaded (empty) nanoparticles + CpG. Samples of immune sera were collected 2 weeks after the last immunization.
Cytokine detection in mice immunized with KMP-11 plasmid DNA or with recombinant KMP-11-loaded nanoparticles
BALB/c mice were immunized as described above. Two weeks after the last immunization, the mice were euthanized, and single-cell suspensions of lymph nodes draining the immunization site (popliteal for DNA-injected mice and retroaxillary for PLGA nanoparticle-injected mice) were prepared aseptically. Briefly, the draining lymph nodes were homogenized in RPMI 1640 medium and the cells were resuspended in RPMI medium supplemented with 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 10% fetal calf serum (all from Invitrogen, Carlsbad, CA) and 0.05 M β-mercaptoethanol. Cell suspensions were stimulated with recombinant KMP-11 (10 μg/mL) for 48 hours. Culture supernatants were harvested and the presence of cytokines was assayed using a Th1/Th2 cytokine cytometric bead array (BD Biosciences, Franklin Lakes, NJ), which detects murine IL-2, IL-4, IL-5, IFN-γ, and tumor necrosis factor-alpha (TNF-α), following the manufacturer’s instructions. Data were acquired and analyzed using a FACSort flow cytometer (BD Immunocytometry, San Jose, CA) and CBA analysis software (Becton-Dickinson, Franklin Lakes, NJ).
Humoral immune response in mice immunized with KMP-11 plasmid DNA or with recombinant KMP-11-loaded nanoparticles
Enzyme-linked immunosorbent assay microplates were coated overnight at 4°C with recombinant KMP-11 (1 μg/mL) in coating buffer (NaHCO3 0.45 M, Na2CO3 0.02 M, pH 9.6). After washing with phosphate-buffered saline-Tween, the wells were blocked with phosphate-buffered saline- Tween plus 5% dried skim milk for one hour at 37°C. The wells were incubated overnight with sera (diluted 1:100) from mice immunized with pcDNA3-KMP-11 only or with pcDNA3-KMP-11-loaded nanoparticles followed by recombinant KMP-11-loaded nanoparticles, in the presence of CpG. After further washings, wells were incubated with alkaline phosphatase-conjugated antimouse IgG antibody (Promega, Madison, WI) diluted (1:2500) in phosphate-buffered saline- Tween, for one hour at 37°C. Following another washing cycle, wells were developed with p-nitrophenylphosphate in sodium carbonate buffer at pH 9.6 with 1 mg/mL of MgCl2. The absorbance was recorded at 405 nm. Serum IgG subclasses were determined using antimouse IgG1 or IgG2a alkaline phosphatase conjugates (Sigma-Aldrich).
Challenge with L. braziliensis and sand fly saliva
L. braziliensis promastigotes (strain MHOM/BR/01/BA788Citation32) were grown in Schneider medium (Sigma-Aldrich) supplemented with 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 10% heat-inactivated fetal calf serum (all from Invitrogen). Stationary-phase promastigotes were used in all experiments. Adult Lutzomyia intermedia sand flies were captured in Corte de Pedra, Bahia, and used for dissection of salivary glands. Preparation of salivary gland sonicate was conducted as described elsewhere.Citation33 The level of lipopolysaccharide contamination of salivary gland sonicate preparations was determined using a commercially available Limulus amebocyte lysate chromogenic kit (QCL-1000, Lonza Biologics, Newington, NH); the lipopolysaccharide concentration was <0.1 ng/mL. Two weeks after the last immunization, all groups of mice were challenged in the dermis of the right ear with L. braziliensis promastigotes + salivary gland sonicate, as described earlier.Citation34 The progress of infection was monitored weekly, for 10 weeks, by measuring of ear swelling with a digital caliper (Thomas Scientific, Swedesboro, NJ). Parasite load in the infected ear and in its draining lymph nodes was determined as described below.
Parasite load estimate
Parasite load was determined using a quantitative limiting-dilution assay as described elsewhere.Citation32 Briefly, infected ears and lymph nodes draining the infection site were aseptically excised at five weeks following infection with L. braziliensis + salivary gland sonicate and homogenized in Schneider medium (Sigma-Aldrich). The homogenates were serially diluted in Schneider medium supplemented as before and seeded into 96-well plates containing biphasic blood agar (Novy-Nicolle-McNeal) medium. The number of viable parasites was determined from the highest dilution at which the promastigote could be grown out after up to 2 weeks of incubation at 25°C.
Evaluation of cellular immune response after challenge by flow cytometry
Five weeks following infection with L. braziliensis + salivary gland sonicate, the mice were euthanized, and single-cell suspensions of lymph nodes draining the infection site were prepared as described above. Cells were activated in the presence of anti-CD3 10 μg/mL and anti-CD28 10 μg/mL or with Con A 5 μg/mL (Amersham Biosciences, Piscataway, NJ), and were later incubated with Brefeldin A 10 μg/mL (Sigma-Aldrich). Cells were blocked with anti-Fc receptor antibody (2.4G2) and were double-stained simultaneously with antimouse surface CD4 (H129.19) conjugated to FITC. For intracellular staining of cytokines, cells were permeabilized using Cytofix/Cytoperm (BD Biosciences) and incubated with the anticytokine antibodies conjugated to PE:IFN-γ (XMG1.2), IL-4 (BVD4-1D11), and IL-10 (JES5-16E3). The isotype controls used were rat IgG2b (A95-1) and rat IgG2a (R35–95). Data were collected and analyzed using CELLQuest software and a FACSort flow cytometer (Becton-Dickinson). The steady-state frequencies of cytokine positive cells were determined using lymph node cells from control mice.
Cytokine expression at challenge site
Five weeks following infection with L. braziliensis + salivary gland sonicate, the mice were euthanized, infected ears were excised and placed into RLT buffer, and total RNA was extracted using the RNeasy Protect Mini Kit (Qiagen) according to the manufacturer’s instructions. Ear tissue was mechanically lysed with ceramic beads in a MagNALyzer® instrument (Roche Molecular Systems, Pleasanton, CA), according to the manufacturer’s instructions. The resulting tissue lysates were then employed in downstream total RNA extraction. The resulting RNA was resuspended in 20 μL of water and stored at −80°C until use. cDNA synthesis was performed after reverse transcription (Im Prom-II™ reverse transcription system; Promega) of RNA. Real-time polymerase chain reaction was performed in triplicate on the ABI Prism 7500 (Applied Biosystems, Foster City, CA); thermal cycle conditions consisted of a two-minute initial incubation at 50°C followed by ten-minute denaturation at 95°C, and 50 cycles at 95°C for 15 seconds and 60°C for one minute each. Each sample and the negative control were analyzed in triplicate for each run. The comparative method was used to analyze gene expression. Cytokine cycle threshold (Ct) values were normalized to GAPDH expression, as determined by ΔCt = Ct (cytokine) − Ct (GAPDH). Fold change was determined by 2−ΔΔCt, where ΔΔCt = ΔCt (experimental) − ΔCt (control). Citation35 The primers employed herein are described elsewhere.Citation33
Statistical analysis
Data are presented as the mean ± the standard error. The significance of the results was calculated using nonparametric statistical tests, ie, the two-sided Mann-Whitney test for comparisons between two groups. Analyses were conducted using Prism software (version 5.0; GraphPad Software, Inc, San Diego, CA). Differences were considered statistically significant at P ≤ 0.05.
Results
Cellular immune response after immunization with plasmid DNA ± recombinant PLGA nanoparticles
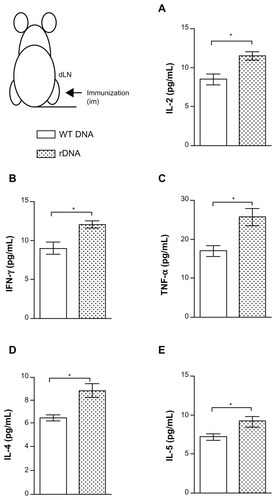
Initially we investigated the anti-KMP-11 cellular immune response induced by immunization using the different strategies. As shown in , in vitro stimulation of draining lymph node cells from mice immunized with pcDNA3-KMP-11 induced a significantly higher production of IL-2 (), IFN-γ (), TNF-α (), IL-4 (), and IL-5 (), when compared with control mice.
Figure 1 Cytokine production in mice immunized with a plasmid DNA encoding KMP-11.
Notes: BALB/c mice were immunized with nonrecombinant pcDNA3 (open bars) or with pcDNA3-KMP-11 (closed bars), as described. Two weeks after the last immunization, draining lymph nodes were collected and the cells were restimulated with recombinant KMP-11. The presence of cytokines in culture supernatants was determined by flow cytometry, using a Th1–Th2 cytometric bead array. Data are presented as the mean ± standard error and are from two independent experiments, each performed with six mice per group. *P < 0.05.
Abbreviations: IFN, interferon; IL, interleukin; KMP-11, kinetoplastid membrane protein-11; TNF, tumor necrosis factor.
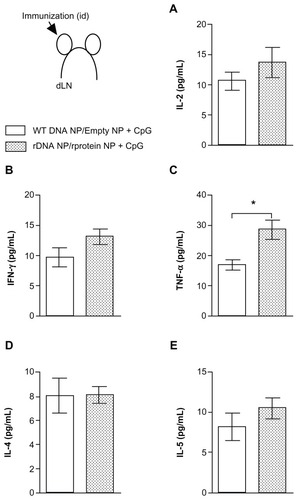
The recombinant nanoparticles used herein had a mean size of 300–450 nm, irrespective of the type of encapsulated antigen (DNA or protein). The mean zeta potential values were between 20 mV and 30 mV, indicating a positive charge at pH 7.4, and independently of the nanoparticle load (recombinant KMP-11 or pcDNA3-KMP-11). Recombinant KMP-11 content per mg of recombinant particle was 3.5 ± 0.5 μg. Regarding plasmid DNA content, one mg of recombinant particles contained 7.2 ± 0.7 μg and 6.4 ± 1.2ug of wild-type and pcDNA3-KMP-11, respectively. When mice were immunized with the recombinant nanoparticle formulations, IL-2 () and IFN-γ () production was also increased. However, in this case, TNF-α levels were significantly higher (), whereas IL-4 () and IL-5 () production was similar to that detected in control mice.
Figure 2 Cytokine production in mice immunized with KMP-11-loaded nanoparticles.
Notes: Control BALB/c mice were immunized with nonrecombinant pcDNA3-loaded nanoparticles followed by unloaded (empty) nanoparticles + CpG (open bars). Experimental BALB/c mice were immunized with pcDNA3-KMP-11-loaded nanoparticles followed by recombinant KMP-11-loaded nanoparticles + CpG (closed bars). Two weeks after the last immunization, draining lymph nodes were collected and cells were restimulated with recombinant KMP-11. The presence of cytokines in culture supernatants was determined by flow cytometry, using a Th1–Th2 cytometric bead array. Data are presented as the mean ± standard error and are from two independent experiments, each performed with six mice per group. *P < 0.05.
Abbreviations: IFN, interferon; IL, interleukin; KMP-11, kinetoplastid membrane protein-11; TNF, tumor necrosis factor.
Humoral immune response on immunization with plasmid DNA or PLGA nanoparticles
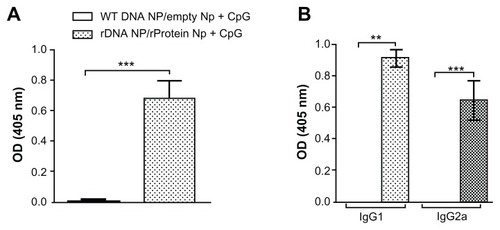
We also probed for the anti-KMP-11 humoral immune response induced by immunization with the different strategies. We did not detect anti-KMP-11 antibodies in mice immunized with either pcDNA3-KMP-11 or with nonrecombinant pcDNA3 (data not shown). However, mice inoculated with pcDNA3-KMP-11-/recombinant KMP-11-loaded nanoparticles + CpG developed a strong and antigen-specific humoral immune response (). IgG1 and IgG2a subclasses () were detected in immune sera which could be associated with the presence of both IL-4/IL-5 and IFN-γ/TNF-α, as seen upon restimulation of draining lymph node cells ().
Figure 3 Humoral immune response in mice immunized with KMP-11-loaded nanoparticles.
Notes: Control BALB/c mice were immunized with nonrecombinant pcDNA3-loaded nanoparticles followed by unloaded nanoparticles + CpG (open bars). Experimental BALB/c mice were immunized with pcDNA3-KMP-11-loaded nanoparticles followed by recombinant KMP-11-loaded nanoparticles + CpG (closed bars). (A) Presence of anti-KMP-11 IgG antibodies was determined by enzyme-linked immunosorbent assay. IgG subclasses present were determined by enzyme-linked immunosorbent assay using IgG1 and IgG2a conjugates (B). Data are presented as the mean ± standard error and are from two independent experiments. *P < 0.01.
Abbreviation: KMP-11, kinetoplastid membrane protein-11.
Outcome of L. braziliensis infection in mice immunized with plasmid DNA or PLGA formulations
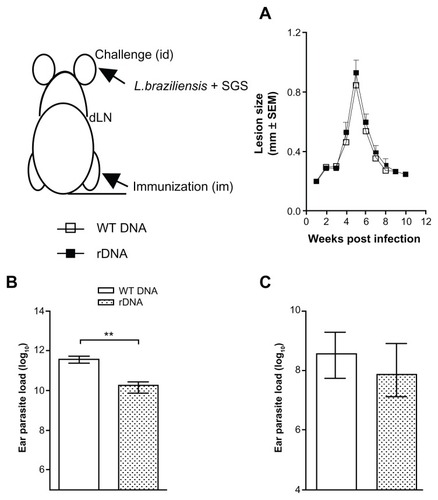
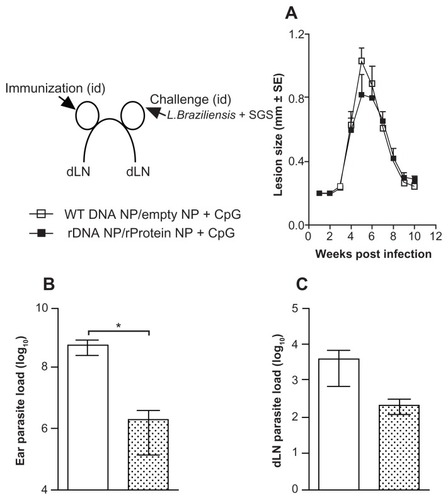
Next, we investigated the outcome of infection with L. braziliensis, in the presence of sand fly saliva. Immunization with pcDNA3-KMP-11 did not alter the course of clinical disease upon a live challenge (), with both immunized and control mice displaying the same outcome followed by spontaneous healing. However, mice inoculated with pcDNA3-KMP-11 had a significantly lower (P < 0.05) parasite load at the ear dermis 5 weeks after infection when compared with control mice (). A similar finding was observed in draining lymph nodes (), although the difference was not significant.
Figure 4 Lesion development and parasite load in mice immunized with a plasmid DNA encoding KMP-11, following a live challenge with parasites.
Notes: BALB/c mice were immunized with nonrecombinant pcDNA3 (open bars) or with pcDNA3-KMP-11 (closed bars), as described. Two weeks after the last immunization, mice were infected in the ear dermis with Leishmania braziliensis + salivary gland sonicate. The course of lesion development was monitored weekly (A), parasite load in the ear (B), and in draining lymph nodes (C) was determined 5 weeks following infection. Data are presented as the mean ± standard error and are from two independent experiments. **P < 0.01.
Abbreviations: KMP-11, kinetoplastid membrane protein-11; SGS, salivary gland sonicate.
Interestingly, immunization with pcDNA3-KMP-11-/recombinant KMP-11-loaded nanoparticles + CpG also did not prevent development of disease () when compared with control animals. Of note, the ear thickness of mice immunized with recombinant nanoparticles was slightly smaller when compared with controls () at 5 weeks after infection. Similar to results obtained upon DNA immunization (), immunization with the recombinant formulations also significantly (P < 0.05) decreased parasite load in the ear dermis (). Interestingly, parasite load in draining lymph nodes was also lower when compared with control mice ().
Figure 5 Lesion development and parasite load in mice immunized with KMP-11-loaded nanoparticles, following a live challenge with parasites.
Notes: Control BALB/c mice were immunized with nonrecombinant pcDNA3-loaded nanoparticles followed by unloaded nanoparticles + CpG (open bars). Experimental BALB/c mice were immunized with pcDNA3-KMP-11-loaded nanoparticles followed by recombinant KMP-11-loaded nanoparticles + CpG (closed bars). Two weeks after the last immunization, mice were infected in the ear dermis with Leishmania braziliensis + salivary gland sonicate. The course of lesion development was monitored weekly (A). Parasite load in the ear (B) and in draining lymph nodes (C) was determined 5 weeks following infection. Data are presented as the mean ± standard error and are from two experiments. *P < 0.05.
Abbreviations: KMP-11, kinetoplastid membrane protein-11; SGS, salivary gland sonicate.
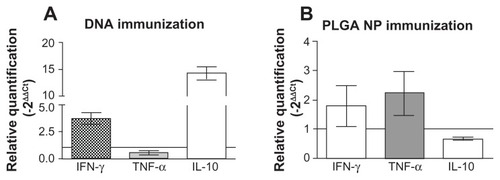
Cytokine production in situ after L. braziliensis + salivary gland sonicate challenge
Because both immunization strategies significantly reduced parasite load at the infection site, we probed for cytokine expression therein 5 weeks after infection. Remarkably, mice immunized with pcDNA3-KMP-11 showed upregulation in both IFN-γ and IL-10 expression () at the infection site; IFN-γ expression was upregulated by approximately 5-fold in comparison with control animals, whereas this increase was about 15-fold for IL-10. TNF-α expression was not detected in mice immunized with pcDNA3-KMP-11. On the other hand, mice immunized with pcDNA3-KMP-11-/recombinant KMP-11-loaded nanoparticles + CpG showed a more moderate upregulation in IFN-γ and TNF-α expression () at the infection site. In contrast with mice immunized using pcDNA3-KMP-11, challenge infection with parasites induced downregulation in IL-10 expression in the mice receiving recombinant PLGA nanoparticles ().
Figure 6 Cytokine expression at the ear dermis following a live challenge with parasites. BALB/c mice were immunized with pcDNA3-KMP-11 (A) or with pcDNA3-KMP-11- loaded nanoparticles followed by recombinant KMP-11-loaded nanoparticles + CpG (B).
Notes: Two weeks after the last immunization, the mice were infected with Leishmania braziliensis + salivary gland sonicate. Relative quantification of IFN-γ, TNF-α, and IL-10 at the infection site was determined 5 weeks after infection, in relation to a housekeeping gene, by real-time polymerase chain reaction (see materials and methods section). Data (mean ± standard error) are presented as the fold increase in gene expression of immunized mice over control mice and are from two independent experiments.
Abbreviations: KMP-11, kinetoplastid membrane protein-11; PLGA, poly(lactic-co-glycolic acid); NP, nanoparticles: IFN-γ, interferon gamma; TNF-α, tumor necrosis factor alpha; IL-10, interleukin-10.
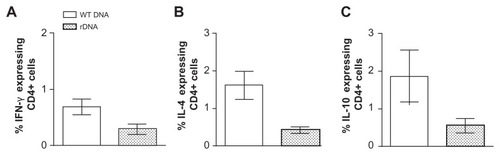
Upon challenge with L. braziliensis + salivary gland sonicate, mice immunized with pcDNA3-KMP-11 or with the recombinant nanoparticle formulations also displayed a lower parasite load within the draining lymph nodes ( and , respectively). Therefore, we also evaluated the frequency of cytokine-secreting cells therein. After infection, mice immunized with pcDNA3-KMP-11 displayed a lower percentage of CD4+ IFN-γ+ (), CD4+ IL-4+ (), and CD4+ IL-10+ () cells when compared with controls.
Figure 7 Intracellular cytokine production by CD4+ and CD8+ T cells in mice immunized with a plasmid DNA encoding KMP-11, following a live challenge with parasites.
Notes: BALB/c mice were immunized with nonrecombinant pcDNA3 (open bars) or with pcDNA3-KMP-11 (closed bars), as described. Two weeks after the last immunization, mice were infected with Leishmania braziliensis + salivary gland sonicate. Five weeks after infection, draining lymph node cells were restimulated in vitro. Data (mean ± standard error) represent the percentages of CD4+ cells secreting IFN-γ, (A), IL-4 (B), or IL-10 (C) and are from two independent experiments.
Abbreviations: KMP-11, kinetoplastid membrane protein-11; IFN-γ, interferon gamma; tumor IL-10, interleukin-10; IL-4, interleukin-4; WT DNA, nonrecombinant pcDNA3; rDNA, pcDNA3-KMP-11.
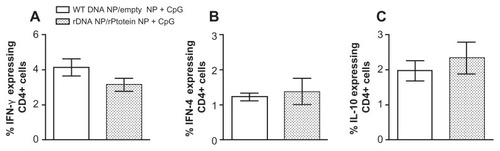
Cells from mice immunized with pcDNA3-KMP-11-/recombinant KMP-11-loaded nanoparticles + CpG also displayed a decreased frequency of CD4+ IFN-γ+ T cells (), after infection. In these animals, the percentage of CD4+ IL-4+ () and CD4+ IL-10+ cells () was similar () or slightly higher () when compared with controls. Similar results were found for cytokine-secreting CD8+ T cells (data not shown).
Figure 8 Intracellular cytokine production by CD4+ and CD8+ T cells in mice immunized with the recombinant nanoparticle formulations, following a live challenge with parasites.
Notes: Control BALB/c mice were immunized with non-recombinant pcDNA3-loaded nanoparticles followed by unloaded nanoparticles + CpG (open bars). Experimental BALB/c mice were immunized with pcDNA3-KMP-11-loaded nanoparticles followed by recombinant KMP-11-loaded nanoparticles + CpG (closed bars). Two weeks after the last immunization, mice were infected with Leishmania braziliensis + salivary gland sonicate. Five weeks after infection, draining lymph node cells were restimulated in vitro. Data (mean ± standard error) represent the percentages of CD4+ cells secreting IFN-γ, (A), IL-4 (B), or IL-10 (C) and are from two independent experiments.
Abbreviations: KMP-11, kinetoplastid membrane protein-11; IFN-γ, interferon gamma; tumor IL-10, interleukin-10; IL-4, interleukin-4; WT DNA, nonrecombinant pcDNA3; rDNA, pcDNA3-KMP-11.
Discussion
In the present work, we evaluated two immunization strategies for their potential to elicit protective immune responses in an experimental model of infection. One strategy consisted of immunization with plasmid DNA and the other involved use of PLGA nanoparticles loaded with plasmid DNA or with the respective recombinant protein. We hypothesized that encapsulation would protect the antigen from degradation and, in parallel, that a heterologous prime-boost strategy would enhance the immune response.
Herein, immunization of BALB/c mice with a plasmid DNA coding for L. infantum chagasi KMP-11 elicited a mixed Th1/Th2-type immune response. However, immunization with the recombinant nanoparticle formulations, in the presence of CpG, induced a significant increase in TNF-α upon restimulation in vitro. Indeed, nanoparticles formulated with DOTAP, the cationic lipid used here, promote a proinflammatory response, with presence of IL-2, IFN-γ, TNF-α,Citation36 and oligodeoxynucleotides, such as CpG motifs, are able to trigger plasmacytoid dendritic cells, resulting in TNF-α production.Citation37 Use of DOTAP in our formulations and of CpG in our immunization scheme may therefore explain the elevated TNF-α levels in immunized mice. We also detected the presence of IgG1 and IgG2a antibodies (), suggesting participation of both IL-4 and IFN-γ in antibody isotype switching, even though levels of these cytokines were not significantly increased in mice immunized with PLGA nanoparticles ().
Following immunization, the mice were challenged with live parasites in the presence of sand fly saliva, mimicking the context of natural infection with Leishmania spp. Upon challenge, neither immunization strategy prevented lesion development. Air pouch stimulation with L. braziliensis + L. intermedia saliva enhances CXCL10, CCL2, TNF-α, and IL-10 expression,Citation33 confirming the immunomodulatory role of saliva from L. intermedia. Although we did not probe for the protective capacity of our immunization strategies in the absence of sand fly saliva, we may speculate that salivary molecules at the time of parasite challenge may have modulated the microenvironment, favoring lesion development.
Despite the inability of the present immunization strategies to prevent disease manifestation, a significant reduction in parasite load was detected at the challenge site. Mice immunized with either DNA alone or with recombinant PLGA nanoparticles displayed increased IFN-γ expression at the infection site. Moreover, mice immunized with recombinant PLGA nanoparticles + CpG also showed elevated TNF-α. IFN-γ and TNF-α act in concert to activate inducible nitric oxide synthase for the production of nitric oxide, and TNF-α stimulates macrophages to produce nitric oxide.Citation6 We can suggest that, in the group immunized with recombinant PLGA nanoparticles, upregulation of IFN-γ and TNF-α combined with downregulation of IL-10, may explain the greater parasite killing at the challenge site. In mice immunized with DNA alone, upregulation of IFN-γ expression was also observed but was accompanied by a strong elevation of IL-10 expression. Mononuclear cells from patients with leishmaniasis produced high levels of IL-10 upon stimulation with recombinant KMP-11,Citation38 whereas addition of recombinant KMP-11 to cells previously stimulated with soluble Leishmania antigen decreased IFN-γ secretion.Citation39 We could speculate that the immune response induced in DNA-immunized mice may have been more prone to modulation exerted by parasite-derived KMP-11 compared with the response elicited by immunization with PLGA nanoparticles.
Parasite load in the draining lymph nodes was also lower following immunization with either DNA alone or with recombinant PLGA nanoparticles, although differences between the experimental and control groups were not significant. One could consider that migration of the effector T cell population (CD4+ IFN-γ+) to the infection site, with parasite killing, explains the lower frequency of cytokine-secreting CD4+ cells in mice immunized with plasmid DNA or with PLGA nanoparticles, when compared with the respective controls. Of note, parasites persist in draining lymph nodes of BALB/c mice inoculated with L. braziliensis,Citation32 despite resolution of dermal lesions and parasite clearance from the infection site. Parasite persistence in cutaneous leishmaniasis has been associated with the presence of regulatory T cells.Citation40 Therefore, another possibility concerns the presence of regulatory T cells within draining lymph nodes preventing parasite clearance. In the draining lymph nodes, these regulatory T cells could counteract the presence of effector cells. Indeed, in mice immunized with PLGA nanoparticles, the frequency of CD4+ IL-10+ T cells was elevated in draining lymph nodes.
A stronger immune response is elicited when antigen is associated with particles, compared with soluble antigen alone.Citation41 In the case of leishmaniasis, immunization CpG and PLGA nanospheres loaded with autoclaved L. major was able to decrease L. major infection and this effect was associated with increased IFN-γ and decreased IL-4 production.Citation21 Doroud et al showed that immunization with solid lipid nanoparticles loaded with plasmid DNA coding for Leishmania cysteine proteinase conferred protection against L. major,Citation42 and was associated with increased IFN-γ levels before challenge and an elevated ratio of IFN-γ/IL-5 after challenge. In addition to the choice of antigen and experimental model, several variables such as particle chemistry, size, and surface charge, affect the ensuing immune response,Citation43,Citation44 and may explain the different outcomes observed in terms of immunity against leishmaniasis. Of note, we performed experiments in which mice were immunized with naked DNA coding for KMP-11 and were boosted with recombinant KMP-11 + CpG. Following this strategy, mice did not develop a strong humoral immune response, nor was parasite load decreased following a challenge with live parasites (data not shown). Therefore, we can suggest that antigen encapsulation enhanced efficacy of the immune response, possibly by protecting the antigen from rapid degradation and or by ascertaining uptake by antigen-presenting cells, as seen in the present results.
Antigens that have proven effective against L. major, such as LACK, LbSTI1, LeIF, and TSA, have not induced similar responses when tested against L. braziliensis.Citation45 Vaccination with soluble L. major promastigote exogenous antigens conferred protection against L. donovani but also failed to induce a similar response against L. braziliensis.Citation46 Since the current findings highlight the need to probe actively for antigens and strategies capable of preventing cutaneous leishmaniasis caused by L. braziliensis, we believe recombinant nanoparticles comprise a platform tailored for such discoveries.
Acknowledgments
We are grateful to José Carlos Miranda for generously providing the Lutzomyia intermedia salivary glands. This work was supported by grants from CNPq, AECID (Government of Navarra), CAN Foundation and CYTED. DMS, TRD, and KF were supported by CAPES fellowships. MWC was supported by a CNPq fellowship. CB, AB, MB-N, and CIO are senior investigators from CNPq.
Disclosure
The authors report no conflicts of interest in this work.
References
- DesjeuxPLeishmaniasis: current situation and new perspectivesComp Immunol Microbiol Infect Dis200427530531815225981
- MarsdenPDMucosal leishmaniasis (“espundia” Escomel, 1911)Trans R Soc Trop Med Hyg19868068598763037735
- BittencourtABarral-NettoMLeishmaniasis82nd edDoerrWSeifertGTropical PathologyBerlinSpringer1995
- AlvarJYactayoSBernCLeishmaniasis and povertyTrends Parasitol2006221255255717023215
- ScottPNatovitzPCoffmanRLPearceESherAImmunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigensJ Exp Med19881685167516842903212
- LiewFYMillottSParkinsonCPalmerRMMoncadaSMacrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginineJ Immunol199014412479447972351828
- de OliveiraCINascimentoIPBarralASotoMBarral-NettoMChallenges and perspectives in vaccination against leishmaniasisParasitol Int200958431932419698801
- OkworIUzonnaJVaccines and vaccination strategies against human cutaneous leishmaniasisHum Vaccin20095529130119221514
- RamshawIARamsayAJThe prime-boost strategy: exciting prospects for improved vaccinationImmunol Today200021416316510740236
- RamiroMJZarateJJHankeTProtection in dogs against visceral leishmaniasis caused by Leishmania infantum is achieved by immunization with a heterologous prime-boost regime using DNA and vaccinia recombinant vectors expressing LACKVaccine20032119–202474248412744881
- DondjiBPerez-JimenezEGoldsmith-PestanaKEstebanMMcMahon-PrattDHeterologous prime-boost vaccination with the LACK antigen protects against murine visceral leishmaniasisInfect Immun20057385286528916041057
- RafatiSZahedifardFAzariMKTaslimiYTaheriTLeishmania infantum: prime boost vaccination with C-terminal extension of cysteine proteinase type I displays both type 1 and 2 immune signatures in BALB/c miceExp Parasitol2008118339340118093586
- GonzaloRMdel RealGRodriguezJRA heterologous prime- boost regime using DNA and recombinant vaccinia virus expressing the Leishmania infantum P36/LACK antigen protects BALB/c mice from cutaneous leishmaniasisVaccine2002207–81226123111803085
- TapiaEPerez-JimenezELopez-FuertesLGonzaloRGherardiMMEstebanMThe combination of DNA vectors expressing IL-12 + IL-18 elicits high protective immune response against cutaneous leishmaniasis after priming with DNA-p36/LACK and the cytokines, followed by a booster with a vaccinia virus recombinant expressing p36/LACKMicrobes Infect200352738412650765
- AbdianNGholamiEZahedifardFSafaeeNRafatiSEvaluation of DNA/DNA and prime-boost vaccination using LPG3 against Leishmania major infection in susceptible BALB/c mice and its antigenic properties in human leishmaniasisExp Parasitol2011127362763621187087
- O’HaganDTRahmanDMcGeeJPBiodegradable microparticles as controlled release antigen delivery systemsImmunology19917322392422071168
- CarcabosoAMHernandezRMIgartuaMRosasJEPatarroyoMEPedrazJLPotent, long lasting systemic antibody levels and mixed Th1/Th2 immune response after nasal immunization with malaria antigen loaded PLGA microparticlesVaccine20042211–121423143215063565
- HamdySMolaviOMaZCo-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunityVaccine200826395046505718680779
- ChongCSWCaoMWongWWEnhancement of T helper type 1 immune responses against hepatitis B virus core antigen by PLGA nanoparticle vaccine deliveryJ Control Release20051021859915653136
- DoroudDZahedifardFVatanaraADelivery of a cocktail DNA vaccine encoding cysteine proteinases type I, II and III with solid lipid nanoparticles potentiate protective immunity against Leishmania major infectionJ Control Release2011153215416221530597
- TafaghodiMKhamesipourAJaafariMRImmunization against leishmaniasis by PLGA nanospheres encapsulated with autoclaved Leishmania major (ALM) and CpG-ODNParasitol Res201110851265127321125294
- JardimAFunkVCaprioliRMOlafsonRWIsolation and structural characterization of the Leishmania donovani kinetoplastid membrane protein-11, a major immunoreactive membrane glycoproteinBiochem J.1995305Pt 13073137826346
- JensenATGasimSIsmailAHumoral and cellular immune responses to synthetic peptides of the Leishmania donovani kinetoplastid membrane protein-11Scand J Immunol19984811031099714418
- ThomasMCGarcia-PerezJLAlonsoCLopezMCMolecular characterization of KMP11 from Trypanosoma cruzi: a cytoskeleton-associated protein regulated at the translational levelDNA Cell Biol2000191475710668791
- BasuRBhaumikSBasuJMNaskarKDeTRoySKinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and -resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1- and Th2-like responses in visceral leishmaniasisJ Immunol2005174117160717115905560
- BhaumikSBasuRSenSNaskarKRoySKMP-11 DNA immunization significantly protects against L. donovani infection but requires exogenous IL-12 as an adjuvant for comparable protection against L. majorVaccine20092791306131619162111
- CostaCHPetersNCMaruyamaSRde BritoECJrSantosIKVaccines for the leishmaniases: proposals for a research agendaPLoS Negl Trop Dis201153e94321468307
- FuertesMAPerezJMSotoMLopezMCAlonsoCCalcium-induced conformational changes in Leishmania infantum kinetoplastid membrane protein-11J Biol Inorg Chem20016110711711191218
- FuertesMABerberichCLozanoRMGimenez-GallegoGAlonsoCFolding stability of the kinetoplastid membrane protein-11 (KMP-11) from Leishmania infantumEur J Biochem1999260255956710095795
- del BarrioGGNovoFJIracheJMLoading of plasmid DNA into PLGA microparticles using TROMS (total recirculation one-machine system): evaluation of its integrity and controlled release propertiesJ Control Release200386112313012490378
- IborraSCarrionJAndersonCAlonsoCSacksDSotoMVaccination with the Leishmania infantum acidic ribosomal P0 protein plus CpG oligodeoxynucleotides induces protection against cutaneous leishmaniasis in C57BL/6 mice but does not prevent progressive disease in BALB/c miceInfect Immun20057395842585216113303
- de MouraTRNovaisFOOliveiraFToward a novel experimental model of infection to study American cutaneous leishmaniasis caused by Leishmania braziliensisInfect Immun20057395827583416113301
- de MouraTROliveiraFRodriguesGCImmunity to Lutzomyia intermedia saliva modulates the inflammatory environment induced by Leishmania braziliensisPLoS Negl Trop Dis201046e71220559550
- de MouraTROliveiraFNovaisFOEnhanced Leishmania braziliensis infection following pre-exposure to sandfly salivaPLoS Negl Trop Dis200712e8418060088
- LivakKJSchmittgenTDAnalysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(t)) methodMethods200125440240811846609
- KedmiRBen-ArieNPeerDThe systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activationBiomaterials201031266867687520541799
- KrugARothenfusserSHornungVIdentification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cellsEur J Immunol20013172154216311449369
- de CarvalhoLPSotoMJeronimoSCharacterization of the immune response to Leishmania infantum recombinant antigensMicrobes Infect20035171212593967
- CarvalhoLPPassosSDutraWOEffect of LACK and KMP11 on IFN-gamma production by peripheral blood mononuclear cells from cutaneous and mucosal leishmaniasis patientsScand J Immunol200561433734215853916
- BelkaidYPiccirilloCAMendezSShevachEMSacksDLCD4+ CD25+ regulatory T cells control Leishmania major persistence and immunityNature2002420691550250712466842
- SinghMBrionesMOttGO’HaganDCationic microparticles: a potent delivery system for DNA vaccinesProc Natl Acad Sci U S A200097281181610639162
- DoroudDZahedifardFVatanaraANajafabadiARRafatiSCysteine proteinase type I, encapsulated in solid lipid nanoparticles induces substantial protection against Leishmania major infection in C57BL/6 miceParasite Immunol201133633534821410716
- Rice-FichtACArenas-GamboaAMKahl-McDonaghMMFichtTAPolymeric particles in vaccine deliveryCurr Opin Microbiol201013110611220079678
- StorniTKundigTMSentiGJohansenPImmunity in response to particulate antigen-delivery systemsAdv Drug Deliv Rev200557333335515560945
- SalayGDortaMLSantosNMTesting of four Leishmania vaccine candidates in a mouse model of infection with Leishmania (Viannia) braziliensis, the main causative agent of cutaneous leishmaniasis in the New WorldClin Vaccine Immunol20071491173118117626159
- TonuiWKTitusRGCross-protection against Leishmania donovani but not L. Braziliensis caused by vaccination with L. major soluble promastigote exogenous antigens in BALB/c miceAm J Trop Med Hyg200776357958417360887