Abstract
Unmethylated cytosine-phosphate-guanosine (CpG) oligodeoxynucleotides (ODNs) are recognized by Toll-like receptor 9 (TLR9) found in antigen-presenting cells and B cells and can activate the immune system. Using CpG ODNs as an adjuvant has been found to be effective for treating infectious diseases, cancers, and allergies. Because natural ODNs with only a phosphodiester backbone are easily degraded by nuclease (deoxyribonuclease [DNase]) in serum, CpG ODNs with a phosphorothioate backbone have been studied for clinical application. CpG ODNs with a phosphorothioate backbone have raised concern regarding undesirable side effects; however, several CpG ODNs with only a phosphodiester backbone have been reported to be stable in serum and to show an immunostimulatory effect. In recent years, research has been conducted on delivery systems for CpG ODNs using nanoparticles (NPs). The advantages of NP-based delivery of CpG ODN include (1) it can protect CpG ODN from DNase, (2) it can retain CpG ODN inside the body for a long period of time, (3) it can improve the cellular uptake efficiency of CpG ODN, and (4) it can deliver CpG ODN to the target tissues. Because the target cells of CpG ODN are cells of the immune system and TLR9, the receptor of CpG ODN is localized in endolysosomes, CpG ODN delivery systems are required to have qualities different from other nucleic acid drugs such as antisense DNA and small interfering RNA. Studies until now have reported various NPs as carriers for CpG ODN delivery. This review presents DNase-resistant CpG ODNs with various structures and their immunostimulatory effects and also focuses on delivery systems of CpG ODNs that utilize NPs. Because CpG ODNs interact with TLR9 and activate both the innate and the adaptive immune system, the application of CpG ODNs for the treatment of cancers, infectious diseases, and allergies holds great promise.
Introduction
Unmethylated cytosine-phosphate-guanosine (CpG) dinucleotide is recognized by Toll-like receptor 9 (TLR9) and induces immune response. Immune activity in DNA was first discovered when a DNA fraction of Bacille Calmette-Guérin was found to produce type I interferon (IFN), leading to the activation of natural killer cells; the antitumor effect of this induction was recognized.Citation1 Krieg et alCitation2 elucidated that immune response is caused only when CpG is included in the DNA, and that immune response is inactivated when the cytosine residue is methylated. This CpG sequence is found with high frequency in bacterial DNA, and only occasionally in mammalian DNA. Because CpG in mammalian DNA is methylated, it is believed that the recognition of unmethylated CpG sequence is an action by the immune system to recognize the DNA of bacteria and eliminate it. It was then discovered that TLR9 is the receptor of DNA containing unmethylated CpG.Citation3
In human beings, TLR9 is mainly expressed by B cells and plasmacytoid dendritic cells (pDCs).Citation4 CpG stimulates these cells and induces innate and adaptive immune responses (). B cells whose TLR9 is activated by CpG secrete cytokines important to the innate immune system – including IL-6, IL-10, and IL-12 – using nuclear factor-kappa B and other signal transduction pathways.Citation2,Citation5,Citation6 IL-6 and IL-12 secreted from B cells are also involved in adaptive immune response. IL-6 promotes the multiplication and activation of B cells; as a result, the production of antibodies is enhanced.Citation7,Citation8
Figure 1 Immunostimulatory effect of cytosine-phosphate-guanosine (CpG) oligodeoxynucleotides (ODNs).
Notes: The immunomodulatory cascade triggered by CpG ODNs includes the activation of T helper 1 (Th1) cells and secretion of proinflammatory cytokines such as interleukin (IL)-6, IL-12, and interferon gamma (IFN-γ). The CpG motifs in either bacterial DNA or synthetic CpG ODNs act as “danger signals” to the innate immune system, triggering a protective immune response against the pathogen. In addition, the adaptive immune response mounted by the host afterward will maintain an immunologic memory and provide long-lasting protection.
Abbreviation: TNFα, tumor necrosis factor-alpha.
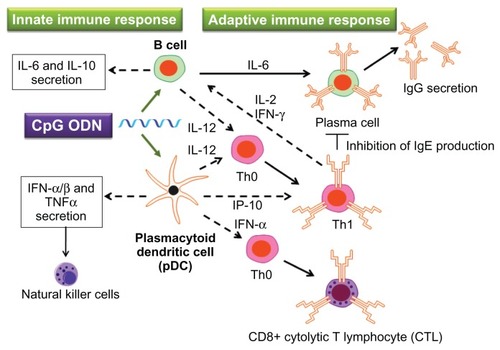
pDCs whose TLR9 is activated by CpG secrete cytokines involved in innate immune response, including type I IFNs and tumor necrosis factor-alpha (TNFα).Citation9 These pDCs also activate natural killer cells.Citation10 Furthermore, pDCs with activated TLR9 secrete IL-12 and promote the differentiation of T helper (Th) 0 into Th1,Citation11–Citation13 as well as inducing Th1 to migrate to B cells through the actions of IFN-γ-inducible protein of 10 kDa (IP10).Citation14,Citation15 B cells that interact with Th1 differentiate into plasma cells, which possess the ability to produce antibodies, playing a central role in adaptive immunity. Also, IFN-α promotes CD8-positive cytotoxic T lymphocyte response.Citation16,Citation17
Because immune response mediated by the activation of TLR9 induces not only the innate immune system but also the adaptive immune system, its application for treating illnesses including infectious diseases, cancers, allergies, and asthma holds great promise.Citation18–Citation21 Until now, various CpG oligodeoxynucleotides (ODNs) have been developed to induce immune response via the activation of TLR9. What is most important in the clinical application of CpG ODN is protecting CpG ODN from DNase and delivering CpG ODN to the TLR9 of pDCs. Chemical modification of CpG ODN is an effective technique to protect against degradation by DNase. However, several severe side effects caused by the modification of DNA backbone have been reported. For example, repeated administration of backbone-modified CpG ODNs has resulted in reduced immune responses, lymphoid follicle destruction, and organ enlargement.Citation22 DNase-resistant natural CpG ODNs consisting entirely of phosphodiester backbone are, therefore, desirable for clinical application, but so far most clinical trials have been conducted using backbone-modified CpG ODNs. Encapsulating CpG ODN and sealing it inside nanoparticles (NPs) is also an effective method to protect ODN against break down by DNase. NPs may make it possible to use naturally occurring CpG ODNs in clinical applications.
Delivery systems for CpG ODNs using NPs as carriers differ greatly from delivery systems for anticancer drugs and nucleic acid drugs such as antisense DNA and small interfering RNA (siRNA). For delivery of anticancer drugs using NPs, the NPs must be delivered from the bloodstream to the cancerous tissues through vascular walls. Therefore, NPs of around 100 nm in size are required. Also, manipulation is required so that immune cells do not capture the NPs before they arrive at cancerous tissues. The target cells for the delivery of CpG ODN, on the other hand, are antigen-presenting cells (APCs) and B cells. These immune cells easily take up relatively large particles, greater than 100 nm in size.Citation23 For delivery of antisense DNA and siRNA, after they have been taken up by cells as a result of endocytosis, their nucleic acids must move from the endosome to the nucleus. However, with the delivery of CpG ODN, because the receptor TLR9 is localized in the endosome, CpG ODNs must be retained in the endosome for a long period of time. Therefore, delivery systems using CpG ODNs require a design strategy different from that of conventional drug delivery systems.
This review summarizes the structural features that depend on base sequences of CpG ODNs consisting of phosphorothioate and phosphodiester backbones and considers their relationship to the capacity of immune mediator cytokine induction. In addition, this review also considers the advantages and disadvantages in a delivery system of these CpG ODNs using various NPs as carriers and describes the possible future direction of studies on CpG ODNs.
Synthetic CpG ODNs with various structures and their immunostimulatory effect
DNase-resisitant CpG ODNs for TLR9 activation
Because ODNs that contain CpG motifs are quickly degraded by DNase, research has been conducted on CpG ODNs resistant to DNase.Citation24–Citation30 DNase-resistant CpG ODNs consisting of a phosphorothioate backbone have been developed by replacing the oxygen in the phosphate group of the nucleic acid targeted by DNase.Citation24,Citation25,Citation31 These chemically modified synthetic CpG ODNs are divided into at least four classes ( and ).
Table 1 Features of each class of cytosine-phosphate-guanosine (CpG) oligodeoxynucleotides (ODNs)
Figure 2 Features of cytosine-phosphate-guanosine oligodeoxynucleotide (ODN) sequences in each class.
Note: Underlining indicates palindromic sequence; black and red hyphens indicate phosphodiester and phosphorothioate bonds, respectively.
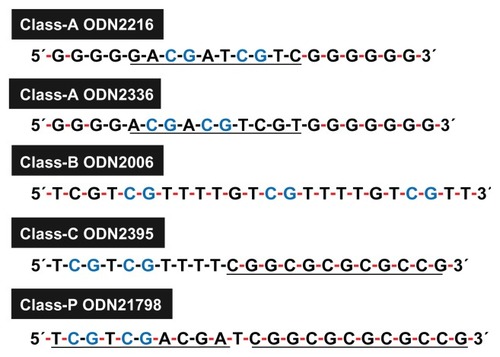
Class A (also known as type D) CpG ODN has a naturally occurring phosphodiester backbone and palindromic CpG motifs at the center of its sequence. Poly(G) sequences on phosphorothioate backbones are attached to the 3′ and 5′ ends.Citation32–Citation34 This class of CpG ODN activates the TLR9 of pDCs and induces IFN-α. However, it almost never induces the multiplication of B cells. The entire sequence of class B (type K) CpG ODN consists of a phosphorothioate backbone.Citation33–Citation36 This class of CpG ODN induces the proliferation and activation of B cells. However, its ability to induce IFN-α with pDCs is low.Citation37 Class C includes one or two CpG motif(s) with a phosphodiester backbone at the 5′ end, and contains a palindromic sequence on a phosphorothioate backbone at the 3′ end. This class of CpG ODN has the capacity to induce the proliferation of B cells and the production of IFN-α via pDCs; it has a quality between classes A and B.Citation38–Citation41 Also, class P CpG ODN has two palindromic motifs on phosphorothioate backbones. It displays a high capacity for producing IFN-α and the ability to activate nuclear factor-kappa B.Citation42
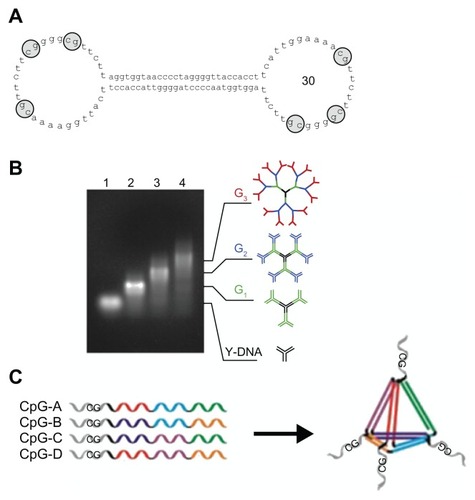
These classes of CpG ODN include a complete or partial phosphorothioate backbone, so that they are resistant to DNase. However, side effects are a cause for concern. Reports of side effects include the prolongation of coagulation time due to the inhibition of the tenase complex,Citation43 distribution of cell signaling due to nonspecific adsorption of proteins including transcription factors,Citation44 and acute toxicity due to complement activation.Citation45,Citation46 These classes of CpG ODN have also been reported to cause renal damage.Citation47,Citation48 Therefore, ideally, naturally occurring CpG ODNs consisting only of phosphodiester backbone is desired. Because DNA is mainly decomposed by exonucleases, it is believed that a ring structure like a plasmid is difficult to break down. When DNA with only a phosphodiester backbone was formed into a dumbbell-like structure, it certainly displayed resistance to DNase.Citation49–Citation51 Schmidt et alCitation52 synthesized an ODN with a phosphodiester backbone in a dumbbell-like structure designed to include CpG motifs in hairpin-loops (). By utilizing the actions of peripheral blood mononuclear cells, immunostimulatory effects similar to linear-structure CpG ODNs with phosphorothioate backbones at both ends were obtained. Immunostimulatory activity that induces TNF-α and IL-6 is found in Y-shaped DNA (Y-DNA) itself, formed from three single-strand DNA having naturally occurring phosphodiester backbones.Citation53 DNA with branch structures, like Y-DNA, has outstanding cellular uptake efficiency. However, it has no resistance to DNase. Rattanakiat et alCitation30 discovered that dendrimer-like DNA (DL-DNA) () with phosphodiester backbones, formed by linking Y-DNA containing CpG motif, has high immunostimulatory activity. One of the causes of this high activity is believed to be DL-DNA’s resistance to DNase. Recently, Nishikawa et alCitation54 observed that TNF-α release from RAW264.7 cells at 8 hours after stimulation by CpG motifs contained X-shaped DNA consisting entirely of phosphodiester backbone. This suggests that X-shaped DNA was stable for 8 hours at least, although Y-DNA has no resistance to DNase. Li et alCitation55 synthesized the CpG-bearing DNA tetrahedral nanostructure with only a phosphodiester backbone (). The core tetrahedral structure comprised four 55-mer ODNs self-assembled with one another by an annealing process. CpG motif sequence was linked to each ODN via a 7-mer oligothiamine spacer. The CpG-bearing DNA tetrahedral nanostructure was efficiently taken up into macrophage-like cells, and induced various pro-inflammatory cytokines such as TNF-α, IL-6, and IL-12 through TLR9 activation. Li et alCitation55 also suggested that the stimulatory effect of the tetrahedral nanostructure is due to resistance to DNase, because the tetrahedral nanostructure DNA was stable in serum for 8 hours.
Figure 3 Structures of cytosine-phosphate-guanosine (CpG) oligodeoxynucleotides (ODNs) consisting of entirely phosphodiester backbone. (A) Sequence and structure of CpG ODN with a dumbbell-like structure: the CpG ODN with a dumbbell-like structure has 30 nucleotides in both loops that contain three CpG dinucleotide motifs.a (B) Structure of dendrimer-like DNA: Y-shaped DNA consists of three single-stranded DNA with 30 nucleotides containing CpG dinucleotide motifs; G1, G2, and G3 dendrimer-like structures were synthesized by ligation of Y-shaped DNA; the sizes of CpG ODNs with G1, G2, and G3 dendrimer-like structures were about 12, 20, and 36 nm, respectively.b (C) Assembly of CpG bearing DNA tetrahedral nanostructure: the core tetrahedral nanostructure consists of assembly with four 55-mer ODNs. The CpG motif is linked to each ODN via a 7-mer oligothyamine spacer.c
aReproduced with permission from Schmidt et al;Citation52 breproduced with permission from Rattanakiat et alCitation30; creproduced with permission from Li et al.Citation55
Furthermore, Meng et alCitation56 discovered that linear-structured CpG ODNs consisting only of a phosphodiester backbone possess high TLR9 activation capacity. TLR9 activation by class B ODNs is most optimal when there are two to four CpG motifs. When four or more CpG motifs were linked, ODN consisting only of a phosphodiester backbone remarkably improved its resistance against DNase. The ODN contained nine or more CpG motifs, remained largely intact in serum for more than 24 hours, and possessed high TLR9 activation capacity even in low concentrations. Because DNA administered inside the body is cleaved from the 3′ end by exonuclease, when the 3′ end of CpG ODN with only a phosphodiester backbone was modified, its resistance to DNase increased.Citation56 When multiple CpG motifs are linked, even when the 3′ end is cleaved by exonuclease, the activation capacity of TLR9 can be maintained when the cell is acted on because many CpG motifs still remain.
Structure-dependent immunostimulatory effect of synthetic CpG ODNs
Class A CpG ODNs induce the production of IFN-α by activating the TLR9 of pDC. However, class B ODNs do not induce the production of IFN-α via pDCs. This shows that the action of CpG ODNs is dependent on base sequence and structure.
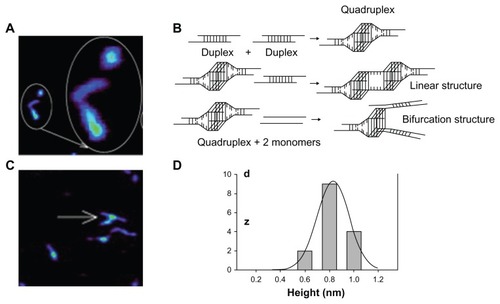
Class A CpG ODNs form nanometer-sized multimers under certain physiological conditions and take on globular and linear structures ().Citation57 This globular structure is split into two forks.Citation57,Citation58 This is because the structure forms a duplex because of the palindromic sequence of base pairs at the center of the ODN. Next, the four poly(G) sequences at the end of these duplexes combine with one another because of Hoogsteen base pairing and become a G-tetrad structure. As a result, they become G-quadruplex structures (). G-quadruplex can form a linear structure when it further forms a G-tetrad with another duplex (). Also, when two strands of monomeric CpG ODN combine with the G-quadruplex, there is a possibility that two-forked NPs can form (). In other words, class A CpG ODNs spontaneously form higher-order structures because of their palindromic sequence and poly(G). For class A CpG ODN2216, in the case of linear structure, its length is more than 100 nm, and in the case of globular structure, the maximum size is 50 nm.Citation57 Also, class A CpG ODN2336 has an average height of 0.8 ± 0.1 nm () and length of 30–70 nm.Citation58 Meanwhile, such higher-order structures are not observed in class B CpG ODNs; they form a linear structure.Citation58
Figure 4 Formation of higher-order structure in class A oligodeoxynucleotides (ODNs). (A) Globular structure of class A cytosine-phosphate-guanosine (CpG) ODN observed by atomic force microscopy (large circled structure is a close-up view of small circle). (B) Possible higher-order structure formation of class A CpG ODNs. Class A CpG ODNs comprise a palindromic sequence in the center and poly(G) sequences at both the 5′ and the 3′ ends. Two monomer molecules form a duplex that is attributed to palindromic sequences. Two duplexes further form a quadruplex through G-tetrad formation of four poly(G) ends. Association of the quadruplex with another duplex causes a linear structure. Two other CpG monomers replace the original duplex by forming two new duplexes, which leads to formation of a bifurcation with three ends. (C) A bifurcation structure of class A CpG ODNs imaged by atomic force microscopy. (D) Height histogram of higher-order structure in class A CpG ODNs. Reproduced with permission from Klein et al.Citation58
Because poly(G) binds with scavenger receptors on the cell surface,Citation59 ODNs that contain poly(G) sequences have been reported to show an increase in cellular uptake efficiency.Citation34,Citation60 This suggests that the high cellular uptake efficiency of class A CpG ODNs with poly(G) also means a high TLR9 activation capacity. On the other hand, Kerkmann et alCitation57 reported that the cellular uptake efficiency of class A and class B CpG ODNs did not change. They suggested that the greater ability of class A CpG ODNs to produce IFN-α than class B CpG ODNs is because of the higher-order structure of class A CpG ODNs. This is because when the palindromic sequence is changed, higher-order structures are not formed, and the ability to produce to IFN-α is decreased. Furthermore, it has been observed that when class B CpG ODNs were loaded onto polystyrene NPs 180 nm in diameter, their production capacity of IFN-α was greater than that of class A CpG ODNs.Citation57 Because the cellular uptake efficiency of free class B CpG ODNs loaded onto polystyrene NPs do not change, it is believed that the high IFN-α production capacity of class A CpG ODNs is due to its spontaneously formed higher-order structure. Other research groups have reported that by artificially causing class B CpG ODNs to form higher-order structures using NPs, an immune profile similar to class A CpG ODNs could be obtained.Citation61–Citation63 Class C CpG ODNs also form a duplex, because of the palindromic sequence at the 3′ end. This structure is believed to affect the activation of TLR9.
Naturally occurring DL-DNA containing CpG motifs synthesized as Y-DNA structural units displayed high immunostimulatory activity.Citation30 The hydrodynamic size of Y-DNA formed from three-strand 30-base ODN was 7.0 ± 0.2 nm; however, second- and third-generation DL-DNA () were 20 ± 1.2 nm and 35.8 ± 3.2 nm, respectively. Receptor-mediated endocytosis is dependent on the size of ligands, and the optimal size for uptake is known to be 25–30 nm.Citation64–Citation67 The size of DL-DNA falls within this range, so its high immunostimulatory activity may have an effect on cellular uptake efficiency in addition to resistance to DNase. What is extremely interesting is that Y-DNA has immunostimulatory activity even if it does not contain CpG motifs.Citation54 This suggests the importance of the higher-order structure of ODNs on immunostimulation.
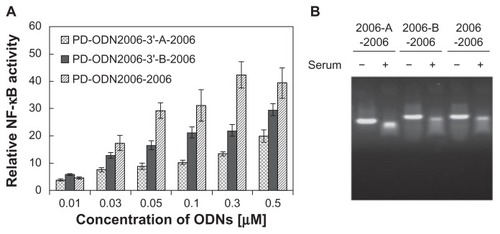
Meng et alCitation56 reported that ODNs consisting only of a phosphodiester backbone and linked with numerous CpG motifs exhibited high TLR9 production capacity. CpG ODN2006, a class B prototype, includes three CpG motifs. PD-ODN2006 is synthesized with a phosphodiester backbone, not the phosphorothioate backbone of the original ODN2006 sequence, and it has no TLR9 activation capacity because DNase degrades it. PD-ODN2006-2006, composed of two PD-ODN2006s linked together, is not degraded much by DNase and possesses high TLR9 activation capacity (). When the size of the DNA is below 250 bases, cellular uptake efficiency increases as the DNA size increases.Citation68 However, a sequence that indirectly connects PD-ODN2006s using a 14-mer ligand sequence without a CpG dinucleotide sequence (PD-2006-linker-2006) showed lowered TLR9 activation capacity (). PD-ODN2006-2006 and PD-ODN2006-linker-2006 both contain six CpG motifs. The difference in TLR9 activation capacity despite this characteristic suggests that aside from the number of CpG motifs and size, TLR9 activation is also dependent on the ODN sequence.
Figure 5 Effect of linker sequences in phosphodiester (PD) cytosine-phosphate-guanosine (CpG) oligodeoxynucleotide 2006 (ODN2006) connection on Toll-like receptor 9 (TLR9) activation.a (Original ODN2006 consists entirely of phosphorothioate backbone, but the backbones of all the CpG ODNs used in this experiment were replaced by naturally occurring phosphodiester bonds.) (A) Activation of TLR9 represented by nuclear factor-kappa B (NF-κB) activity: 293XL-hTLR9 cells were stimulated by series connection of PD-ODN2006 with and without linker sequences. PD-ODN2006-2006, composed of two phosphodiester ODN2006s directly linked together, showed higher capacity for TLR9 activation than PD-ODN2006-A-2006 and PD-ODN2006-B-2006, whose sequences consist of indirectly connected phosphodiester ODN2006s using 14-mer linkers (sequence of linker A, CCTTCAGTGGGACC; sequence of linker B, GGTCCCACTGAAGG). (B) Stability of these CpG ODNs consisting of entirely phosphodiester backbone in solution with and without serum imaged by gel electrophoresis: these CpG ODNs consisting entirely of phosphodiester backbone were resistant to deoxyribonuclease in serum.
Notes: aThe sequence of ODN2006 that contains three CpG motifs is shown in .
Reproduced with permission from Meng et al.Citation56
Delivery of CpG ODNs using NPs
Control of the immune system via TLR9 by CpG ODNs has been shown to be effective for treating infectious diseases, cancers, and allergies. In recent years, various NPs have been developed as carriers of CpG ODNs. The number of papers related to the delivery of CpG ODNs using NPs has increased sharply since 2007.
The advantages of using NPs as CpG ODN carriers include (1) protection from DNase degradation, (2) extension of retention time inside the body, (3) decrease in the amount administered because cellular uptake efficiency is improved, (4) the ability to change the structure of CpG ODNs, (5) the ability to deliver to target tissues, (6) the ability to change localization inside the body, and (7) allow the slow release of CpG ODNs over a long period of time.
Protection of CpG ODNs from degradation by DNase
Concerning the protection of CpG ODNs from DNase by NPs, many research studies use CpG ODNs with a phosphorothioate backbone resistant to DNase, so there is little direct evidence available. Because antisense ODNs encapsulated by cationic lipid NPs or lipid NPs have been reported to be partially or completely protected from DNase degradation in serum,Citation69,Citation70 CpG ODNs with only phosphodiester backbone are believed to have similar effects when encapsulated by these nanopaNPsrticles. Zhu et alCitation71 showed that CpG ODNs with only a phosphodiester backbone joined electrostatically to the surface of NPs acquired resistance to DNase degradation. They observed that all molecules of CpG ODNs with a completely phosphodiester backbone were broken down within an hour in 20% serum. However, CpG ODNs attached electrostatically to mesoporous silica NPs, the surface of which were modified with amino groups, remained after 3 hours without being degraded (). Furthermore, when the conjugate of these NPs and CpG ODNs was coated with poly(allylamine hydrochloride), the efficiency of protection against DNase was discovered to increase. In vivo mice experiments have shown that 272 nm cationic poly(D,L-lactic-co-glycolic acid) (PLGA) NPs were taken up into pDCs in less than an hour,Citation72 so that protection of CpG ODNs against DNase by NPs for several hours is considered to be sufficient. The greatest advantage of using NPs is protection against DNase, as CpG ODNs consisting of chemically unmodified phosphodiester backbone can be used.
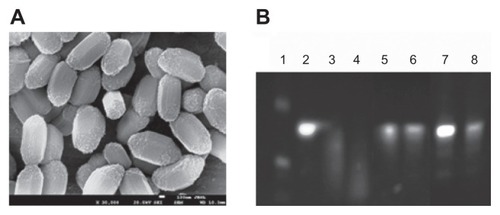
Figure 6 Mesoporous silica nanoparticles (NPs) for delivery of natural cytosine-phosphate-guanosine (CpG) oligodeoxynucleotides (ODNs) consisting entirely of phosphodiester backbone. (A) Scanning electron microscopic image of aminomodified mesoporous silica SBA-15 particles. (B) Stability of CpG ODNs consisting entirely of phosphodiester backbone in 20% serum containing medium: lane 1, DNA marker; lane 2, undigested CpG ODN consisting entirely of phosphorothioate backbone; lanes 3 and 4, free natural CpG ODN after incubation for 1 and 3 hours; lanes 5 and 6, natural CpG ODN loaded on mesoporous silica NPs after incubation for 1 and 4 hours; and lanes 7 and 8, natural CpG ODN loaded on mesoporous silica NPs followed by polycation (poly(allylamine hydrochloride)) treatment after incubation for 1 and 3 hours. This indicates that natural CpG ODN consisting entirely of phosphodiester backbone loaded on mesoporous silica NPs was stable in serum. Reproduced with permission from Zhu et al.Citation71
Prolonged circulation time and increased cellular uptake
In vivo experiments have reported that NPs prolong the circulation lifetime of CpG ODNs in the body. Pan et alCitation73 included a CG sequence in an 18-mer antisense ODN for controlling the Bcl-2 gene and discovered that this could activate the immune system. When free antisense ODN with phosphorothioate backbone was administered intravenously to mice, only 1% remained in plasma after 24 hours. When this antisense ODN was encapsulated in lipid NPs (89 ± 45 nm in diameter) composed of 3β-[N,N-(dimethylaminoethane)carbamoyl] cholesterol (DC-Chol), egg yolk phosphatidylcholine, distearoylphosphatidylethano lamine-N-[methoxy(polyethylene glycol)-2000] (mPEG2000-DSPE), and protamine, 25% of the ODN remained in plasma after 24 hours. This shows that encapsulation in lipid NPs extends blood circulation time. DC-Chol, a cationic lipid, partially deprotonates in near-neutral pH and decreases the surface electrical charge of NPs. Also, mPEG2000-DSPE on the surface of NPs decreases the uptake of NPS by the reticuloendothelial system. These actions are believed to prolong the blood circulation time. When this lipid NP-encapsulated antisense ODN was administered, the cumulative dose to tumorous tissues was about nine times that of free ODN. Traditional cationic lipid NPs are quickly eliminated from the circulatory system,Citation45 but if the electrical charge on the NPs’ surface is reduced, as in the case of DC-Chol, circulation time can be extended. Wilson et alCitation27 discovered neutral cationic lipid NPs ionizable at physiological pH. When ionizable cationic lipid NPs and ODN are mixed under a low pH condition, the surface of the NPs becomes charged, and the ODN is electrostatically attached. Next, when these NPs are transferred to 40% ethanol, the structure of the NPs becomes unstable and ODN is taken up into the interior of the NPs. Afterwards, when the pH is made neutral, the NPs become neutral NPs. In contrast to the free ODN circulation lifetime of a few minutes, the circulation lifetime of ODN encapsulated in ionizable cationic lipid NPs can be extended from several hours to several days.Citation74,Citation75
One of the reasons that CpG ODNs adsorbed into NPs or with encapsulated phosphorothioate backbone show greater TLR9 activity than free CpG ODNs is that the cellular uptake efficiency of CpG ODNs is improved. The cellular uptake efficiency of CpG ODNs is being studied using fluorescently labeled CpG ODNs. When fluorescently labeled free CpG ODN was added to cultured 239XL-TLR9 cells, fluorescence was not observed inside the cells. In contrast, strong fluorescence was observed in CpG ODN adsorbed by mesoporous silica NPs and boron nitride NPs ().Citation71,Citation76 In vivo studies have shown the method of delivering CpG ODN using lipid NPs significantly increased uptake efficiency compared with free CpG ODN when it was administered to mice and the uptake into APCs in the spleen and lymph nodes was observed after 24 hours.Citation27 Also, when CpG ODNs were loaded onto cationized gelatin NPs with an average diameter of 272 ± 33.3 nm, after 2 days DCs in draining lymph nodes were 30% CpG positive and B cells were 20% positive.Citation77 The cause is believed to be the increase in the delivery efficiency of CpG ODNs by NPs, with many CpG ODNs being adsorbed or encapsulated per NP. Klier et alCitation78 also reported that class A CpG ODN delivered by gelatin NPs showed significant immunomodulation effect (Th2/ Th1 shift) on equine bronchoalveolar lavage cells. This effect is believed to be an enhancement of cellular uptake of CpG ODNs by gelatin NPs.
Figure 7 Boron nitride nanospheres (BNNSs) as carrier for cytosine-phosphate-guanosine oligodeoxynucleotide delivery: (A) transmission electron microscopic image of BNNSs; (B) localization of BNNSs taken up into cell (BNNSs [red] are localized in endolysosome [green]); (C) distribution of BNNSs during cell proliferation. Cells and BNNS were stained with DAPI (blue) and rhodamine B (red), respectively. BNNSs (red) were observed even in divided cells that were incubated for 48 and 72 hours (h).
Reproduced with permission from Zhi et al.Citation76
Abbreviation: DIC, differential interference contrast.
![Figure 7 Boron nitride nanospheres (BNNSs) as carrier for cytosine-phosphate-guanosine oligodeoxynucleotide delivery: (A) transmission electron microscopic image of BNNSs; (B) localization of BNNSs taken up into cell (BNNSs [red] are localized in endolysosome [green]); (C) distribution of BNNSs during cell proliferation. Cells and BNNS were stained with DAPI (blue) and rhodamine B (red), respectively. BNNSs (red) were observed even in divided cells that were incubated for 48 and 72 hours (h).Reproduced with permission from Zhi et al.Citation76Abbreviation: DIC, differential interference contrast.](/cms/asset/598ec50d-60c5-4832-be6d-750e8b21c1a2/dijn_a_30197_f0007_c.jpg)
Clathrin-mediated endocytosis, caveolae-mediated uptake, phagocytosis, macropinocytosis, and clathrin- and caveolae-independent endocytosis are all possible methods for the uptake of lipid NPS into cells. The specific method depends on the type of cells.Citation27 When free CpG ODNs were charged negatively, it was difficult for them to attach to a negatively charged cell surface. This electrostatic repulsion is believed to limit the efficiency of free CpG ODN uptake.
The size of NPs affects both cellular uptake and TLR9 activation. Foged et alCitation23 investigated phagocytic activity on DCs by polystyrene NPs of various sizes from 0.04 to 15 μm, and reported that the size of 500 nm was optimal for uptake into DCs. Also, when the NP size was greater than 200 nm, it was reported that the retention time in the endosome was long. However, NPs form aggregates or agglomerates in solution. When NPs form aggregates or agglomerates, cells take in not only NPs by themselves but also NPs in aggregates or agglomerates. Therefore, not the primary size of NPs but their hydrodynamic size should be used as the indicator of the effects of the size of NPs on cellular uptake.
Retention of NPs loaded with CpG ODNs in endolysosome
TLR9 joins with CpG ODNs in the endolysosome; therefore, it is important to retain the CpG ODN in the endolysosome. Vectors and siRNA delivery systems must deliver to the nucleus; therefore, breaking out from the endolysosome is necessary. However, for the delivery systems of CpG ODNs, because TLR9 exists in the endolysosome, breaking out from the endolysosome is not necessary but staying in is. Chen et alCitation79 coated the surface of polystylene NPs with four types of cationic polymers – poly(ethylenimine), chitosan, poly(2-dimethyl-amino)ethyl methacrylate, and poly(L-lysine) – to cause CpG ODN to bind. They reported that with poly(L-lysine), there was least escape of NPs from the endolysosome. This means that the type of polycation has an effect on the retention of NPs in the endosome. When Zhi et alCitation76 added boron nitride NPs to 293XL-TLR9 cells, it was localized in the lysosome after 24 hours. This localization was maintained even after the cell division ().
Sustained release of CpG ODNs
The increase in uptake efficiency of CpG ODNs and in their efficiency of delivery means that the dose of CpG ODN can be decreased. Furthermore, the retention of NPs in the endolysosome and lysosome leads to continuous effects by CpG ODN. Furthermore, the sustained release of CpG ODN by NPs also results in decreased dosage and continuous effects. Sokolova et alCitation80 prepared multishell NPs that causes CpG ODN to be adsorbed to calcium phosphate (). By using a multishell structure, CpG ODN can be protected from DNase. At the same time, calcium phosphate gradually dissolves in the acidic environment of the lysosome’s interior, so the slow release of CpG ODN can be expected. Zhu et alCitation81 have reported on the enzyme-triggered sustainable release of CpG ODNs. They caused naturally occurring CpG ODN to be adsorbed by hollow mesoporous silica NPs with a laminated surface. Furthermore, they coated the surface with poly(L-lysine). By repeating the adsorption of this CpG ODN and poly(L-lysine), a layer-by-layer adsorption can be formed. By dissolving the NP surface of ploy(L-lysine) with α-chymotrypsin, CpG ODN together with low-molecular-weight compounds loaded in the hollow NPs can be slowly released (). Demento et alCitation72 controlled the rate of release of CpG ODN from NPs by attaching biotinylated CpG ODN to the surface of PLGA NPs modified with avidin-palmitate. Usually 100 μg of CpG ODN is administered per mouse, but with NPs, effects were observed with administration of 0.5 μg. By minimizing the release of CpG ODN, side effects including autoimmunity and lymphoid architectural damage that occur when CpG ODN is given in large amounts can be decreased.Citation22
Figure 8 Multishell calcium phosphate (CaP) nanoparticles (NPs) for cytosinephosphate-guanosine (CpG) delivery: (A) preparation of multishell CaP-NPs functionalized with CpG oligodeoxynucleotides and antigen (hemagglutinin, HA): (B) scanning electron micrographs of singe-shell (top panels) and triple-shell NPs (bottom panels).
Reproduced with permission from Sokolova et al.Citation80
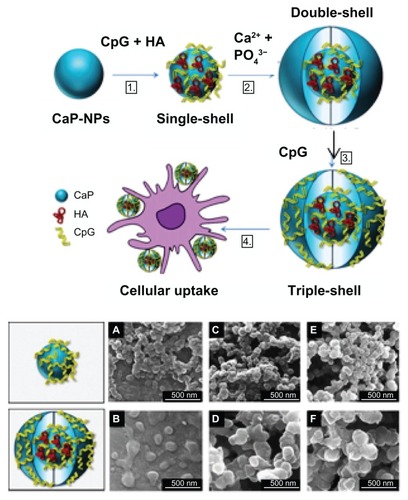
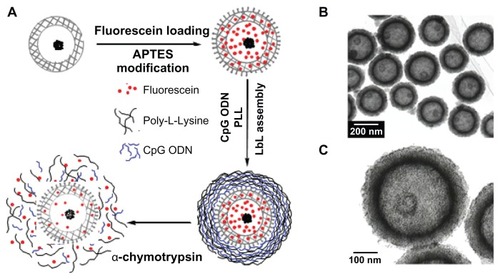
Figure 9 Hollow mesoporous silica/poly(L-lysine) (HMS/PLL) nanoparticles for codelivery of drug and cytosine-phosphate-guanosine (CpG) oligodeoxynucleotides (ODNs): (A) preparation of the fluorescein and CpG ODN-loaded HMS/PLL particles and enzyme triggered release; (B and C) transmission electron microscopic images of HMS nanoparticles.
Reproduced with permission from Zhu et al.Citation81
Abbreviations: APTES, amino-propyltriethoxysilane; LBL, layer-by-layer.
Delivery of CpG ODNs to target tissues
Bourquin et alCitation77 reported that CpG ODN loaded on cationized gelatin NPs had remarkably high antitumor effects compared with free CpG ODN in a mouse melanoma model. They investigated the localization of CpG ODN in vivo, and revealed that while free CpG ODN accumulated in splenocytes, CpG ODN delivered by NPs was selectively stored in APCs inside draining lymph nodes. The activation of adaptive immune response due to the selective transport to APCs inside draining lymph nodes by NPs is believed to be the cause for the high antitumor effect.
The size of NPs also has an effect on transferability into tissues. The molecular weight of drugs and the size of NPs are known to be factors determining the transferability into lymph nodes. Drugs with a molecular weight less than 5000 injected intramuscularly or subcutaneously are absorbed by capillaries and circulated. However, drugs with a molecular weight greater than 20,000 mainly transfer to lymph nodes.Citation82 The commonly used class B CpG ODN with phosphorothioate backbone has a molecular weight of about 8000, and its transferability to lymph nodes is low. For therapeutic NPs administered into the abdominal cavity of mice, it has been reported that NPs with sizes of 100–200 nm are excellent for transferring to lymph nodes and in retention.Citation83 Kuramoto et alCitation84 prepared liposomes 100–200 nm in size composed of N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride, a cationic lipid, and cholesterol, to which CpG ODNs were loaded. When these NPs were administered to the abdominal cavity of mice with peritoneal dissemination, a pronounced antitumor effect was observed compared with CpG ODN alone.
By attaching specific ligands to the surface of NPs, it is possible to deliver them to target cells. Because macrophages and pDCs, which are target cells of CpG ODN, have mannose receptors, it is believed that introducing mannose to the surface of NPs can intensify the effects of CpG ODNs. Kuramoto et alCitation85 loaded CpG ODN on mannose-modified liposome, the surface of which was modified with mannose-modified cholesterol derivative cholesten-5-yloxy-N-(4-((1-amino-2-β-D-thiomannosylbutyl)amino)alkyl)formamide, and administered it to the abdominal cavity of mice with peritoneal dissemination. As a result, by targeting the immune cells in the greater omentum and the mesentery, where numerous lymph nodes exist, increased antitumor effect was reported. Also, Chen et alCitation86 synthesized glycodendrimers with terminals consisting of α-mannose and made this adsorb to the surface of boron nitride nanotubes. Because α-mannnose binds with the mannose receptor of macrophages, CpG ODN delivered by these NPs is selectively taken up into macrophages. Also, because glycodendrimer is recognized by lectins on the cell surface, cellular uptake efficiency can also be expected to increase.
Multicomponent delivery system including CpG ODNs
When antigens and CpG ODNs are delivered at the same time, the advantages of both agents can be maximally drawn out.Citation87,Citation31 Furthermore, when delivering these agents simultaneously with NPs, it has been reported that using separate NPs to deliver each of the agents results in greater immunostimulatory effect.Citation88–Citation91 This suggests that delivering antigens and CpG ODN to APCs at the same time is important.
Standley et alCitation29 encapsulated ovalbumin (OVA), and furthermore prepared acid-degradable NPs (CpG-OVA-NPs) based on a cross-linked polymer network that includeed CpG ODN. The size of these NPs was 200–500 nm, and the bonding amount of the encapsulated OVA and CpG ODN were 40 and 25 μg/mg NPs for CpG ODN. In vitro assays showed CpG-OVA-NPs increased the amount of IL-12 secreted by APCs by 45 times compared with NPs encapsulating only OVA. Also, the induction of CD40, CD80, and CD86, indicators of DC activation also increased. However, co-delivery had no effect on major histocompatibility complex (MHC) I and MHC II. It has been reported that for in vivo assays, co-delivery can induce OVA-specific CD8 T-cell response. Lee et alCitation92 also showed the enhancement of MHC-restricted presentation of antigen by using biodegradable PLGA NPs loaded with CpG ODNs and OVA. Similar profound augmentation of anti-OVA-specific immune response was observed by co-delivery of OVA and CpG ODN using nanoliposomes.Citation93
Nasal and intradermal vaccination is an attractive strategy for CpG ODN delivery. For this strategy in previous studies, mucoadhesive N-trimethyl chitosan NPs loaded with OVA and CpG ODN were prepared.Citation94,Citation95 This delivery system induced a significantly higher level of IgG2a and increased the number of OVA-specific IFN-γ producing T cells in the spleen compared with NPs with only OVA, suggesting that the co-delivery of antigen and CpG ODN improves the immunostimulatory effect.
Many studies have reported on systems that incorporate CpG ODN and antigens in biodegradable NPs.Citation96–Citation101 These systems co-encapsulate CpG with antigens in biodegradable NPs. Demento et alCitation72 minimized the release rate of CpG ODN by using biotin-avidin binding to attach CpG ODN to the surface of biodegradable PLGA NPs. CpG ODN-modified antigen-encapsulating PLGA NPs that encapsulated antigen peptides of the West Nile virus envelope protein were prepared. The average size of these NPs was 272 nm, and the encapsulated antigen peptides were about 4.7 μg/mg NPs. These NPs were administered to mice, and their immunostimulatory effect was compared with Alhydrogel, which includes antigens and aluminum hydroxide (as adjuvant). The results showed that with Alhydrogel, the level of IgG1, associated with Th2-skewed response, was high. With CpG ODN-modified antigen-encapsulating PLGA NPs, the levels of IgG2a and IgG2b, involved in Th1-based response, were high. Concerning infection of the West Nile virus, the test group administered Alhydrogel had a 44% survival rate. The test group administered CpG ODN-modified antigenencapsulating PLGA NPs had a 94% survival rate.
Intratumoral injection of PEGylated unilamellar liposomes bearing surface-conjugated anti-CD40 antibody and CpG ODN has demonstrated synergistic antitumor effects without dose-limiting inflammatory toxicity.Citation102 In addition, co-delivery of polyriboinosinic-polyribocytidylic acid (poly(I:C)), an agonist of CpG and TLR3, with CD40 ligand (CD40L), has been reported to intensify the antitumor effect of CD40L.Citation103 When vector-expressing CD40L (pSP-DCD40L) was directly administered to mouse tumor together with CpG ODN and poly(I:C), antitumor activity was displayed. When pSP-D-CD40L was loaded onto poly(β-amino esters), a cationic polymer, and polyethylenimine NPs, and delivered together with CpG ODN and poly(I:C), even greater antitumor effect was obtained. Wells et alCitation104 observed high antitumor activity as a result of the delivery of anti-CD40 antibody together with CpG ODN, poly(I:C9), and IFN-γ in an emulsion of squalene and Tween 80. These high antitumor activities are believed to be due to the induction of the powerful antitumor response of CD8-positive T cells by the stimulation of CD40 combined with TLR agonists. Also, Sokolova et alCitation80 reported that CpG ODN and poly(I:C) incorporated with hemagglutinin, a model antigen, into multishell calcium phosphate NPs and administered to DCs resulted in a greater amount of IL-12 produced.
Disadvantages of delivery system
Meanwhile, the disadvantages of using NPs include not being able to establish the safety of NPs and not being able to clarify the metabolic process. When mixed with DNA, cationic polymers like poly(L-lysine) and polyethyleneimine form polyplexes.Citation105–Citation107 Also, the method to attach CpG ODN by modifying negatively charged NPs with polycation is the most generally implemented method. However, polycations elicit the nonspecific adsorption of negatively charged molecules, and also promote the formation of NP aggregates.Citation108–Citation110 These results are believed to be causes of side effects. Furthermore, it has been suggested that polycations may cause damage to liver from complement activation.Citation45 Research has been conducted to avoid these disadvantages of polycations by using PEGCitation111 and polyanions.Citation112,Citation113 Also, it has been reported that ionizable cationic liposome eliminates these disadvantages of polycations.Citation27 Kim et alCitation110 prepared amphiphilic NPs composed of hydrophilic poly(γ-glutamic acid) (γ-PGA) and hydrophobic L-phenylalanine. These NPs are negatively charged by ionizing the carboxyl group near the surface of γ-PGA.Citation114 However, it has been reported that free CpG ODN of the same negative charge can be encapsulated. Forming a polyplex of CpG ODN and poly(ɛ-lysine), the CpG ODN load can be increased by encapsulating the polyplex in γ-PGA and hydrophobic L-phenylalanine.
Prospects for the future
Recently, a cytosolic DNA-sensing receptor that functions upstream of the TLR was discovered and named the high-mobility group box (HMGB) protein.Citation115 In addition to being expressed in immune cells, HMGB protein can be found in a variety of other cell types. The protein can recognize DNA and RNA and activate the innate immune system. Furthermore, since HMGB recognizes mammalian DNA, it is thought to be implicated in autoimmune diseases. Class B CpG ODNs with a phosphorothioate backbone demonstrated a high affinity to HMGB,Citation115 whereas interaction was not seen with a natural CpG ODN with a phosphodiester backbone.Citation116 These results suggest that CpG ODNs with a phosphorothioate backbone activate innate and adaptive immune systems in B cells and APCs via TLR9, and inhibit the innate immune system in other types of cells. Conversely, natural CpG ODNs with only a phosphodiester backbone activate the innate and adaptive immune systems in B cells and APCs via TLR9 and do not inhibit the innate immune system in other types of cells. From the standpoint of improvement and maintenance of systemic immune activation, the presence of natural CpG ODNs with a phosphodiester backbone is therefore more advantageous than CpG ODNs with a phosphorothioate backbone.
The number of natural CpG ODNs consisting entirely of phosphodiester backbone developed is significantly smaller than that of CpG ODNs with a phosphorothioate backbone. The development of clinically applicable natural CpG ODNs is therefore expected to grow in the future. On the other hand, since CpG ODNs with a phosphorothioate backbone demonstrate high affinity with HMGB protein, they may be effective in the treatment of HMGB-mediated autoimmune diseasesCitation116 such as rheumatoid arthritis and systemic lupus erythematosus.
There are many benefits of NP-mediated delivery of CpG ODNs. The author found that the binding modes of CpG ODNs to NPs affect the production of immune mediators (cytokines) (unpublished data). That is, the type of the binding mode of class B CpG ODNs to NPs can induce the production of either IL-6 or IFN-α. This ability to control cytokine induction opens the possibility of treatment-specific preparations of CpG ODN carriers. Most CpG ODN NP preparations employ CpG ODNs with a phosphorothioate backbone. As described earlier in this review, natural CpG ODNs do not require encapsulation in NPs such as liposomes to be protected from DNase activity: adsorption to NPs is sufficient. The development of safe delivery systems for natural CpG ODNs in NPs is fast approaching.
Conclusion
The application of CpG ODNs for the treatment of cancers, infectious diseases, and allergies holds great promise, because CpG ODNs interact with TLR9 and activate both the innate and the adaptive immune system. This review paper summarizes the structural features that depend on base sequences of CpG ODNs consisting of phosphorothioate and phosphodiester backbones, and their relationship to the capacity of immune mediator cytokine induction. In addition, advantages and disadvantages in the delivery system of these CpG ODNs using various NPs and future direction of studies on CpG ODNs are described.
The number of natural CpG ODNs consisting entirely of phosphodiester backbone developed is significantly smaller than that of CpG ODNs with a phosphorothioate backbone. The development of clinically applicable natural CpG ODNs is therefore expected to grow in the future. From the standpoint of improvement and maintenance of systemic immune activation, the presence of natural CpG ODNs with a phosphodiester backbone is therefore more advantageous than CpG ODNs with a phosphorothioate backbone. However, since CpG ODNs with a phosphorothioate backbone demonstrate high affinity with HMGB protein, they may be effective in the treatment of HMGB-mediated autoimmune diseasesCitation116 such as rheumatoid arthritis and systemic lupus erythematosus.
There are many benefits of NP-mediated delivery of CpG ODNs, and the development of safe delivery systems for natural CpG ODNs in NPs is fast approaching.
Acknowledgments
This study was funded by a grant-in-aid for scientific research (C-22560777) from the Japan Society for the Promotion of Science and the Ministry of Education, Culture, Sports, Science and Technology.
Disclosures
The author reports no conflicts of interest in this work.
References
- TokunagaTYamamotoHShimadaSAntitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG: I. Isolation, physicochemical characterization, and antitumor activityJ Natl Cancer Inst19847249559626200641
- KriegAMYiAKMatsonSCpG motifs in bacterial DNA trigger direct B-cell activationNature199537465225465497700380
- HemmiHTakeuchiOKawaiTA Toll-like receptor recognizes bacterial DNANature2000408681374074511130078
- HornungVRothenfusserSBritschSQuantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligonucleatidesJ Immunol200216894531453711970999
- KlinmanDMYiAKBeaucageSLConoverJKriegAMCpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gammaProc Natl Acad Sci U S A1996937287928838610135
- AkiraSUematsuSTakeuchiOPathogen recognition and innate immunityCell2006124478380116497588
- JungJYiAKZhangXChoeJLiLChoiYSDistinct response of human B cell subpopulations in recognition of an innate immune signal, CpG DNAJ Immunol200216952368237312193703
- BernasconiNLTraggiaiELanzavecchiaAMaintenance of serological memory by polyclonal activation of human memory B cellsScience200229856012199220212481138
- Asselin-PaturelCBrizardGCheminKType I interferon dependence of plasmacytoid dendritic cell activation and migrationJ Exp Med200520171157116715795237
- BallasZKRasmussenWLKriegAMInduction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNAJ Immunol19961575184018458757300
- NapolitaniGRinaldiABertoniFSallustoFLanzavecchiaASelected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cellsNat Immunol20056876977615995707
- RomanMMartin-OrozcoEGoodmanJSImmunostimulatory DNA sequences function as T helper-1-promoting adjuvantsNat Med1997388498549256274
- HartmannGWeinerGJKriegAMCpG DNA: a potent signal for growth activation, and maturation of human dendritic cellsProc Natl Acad Sci U S A199996169305931910430938
- BlackwellSEKriegAMCpG-A-induced monocyte IFN-gamma-inducible protein-10 production is regulated by plasmacytoid dendritic cell-derived IFN-alphaJ Immunol200317084061406812682235
- VollmerJJurkMSamulowitzUCpG oligodeoxynucleotides stimulate IFN-gamma-inducible protein-10 production in human B cellsJ Endotoxin Res200410643143815588427
- SchwarzKStorniTManolovaVRole of Toll-like receptors in costimulating cytotoxic T cell responsesEur J Immunol20033361465147012778463
- HeitAMaurerTHochreinHCutting edge: Toll-like receptor 9 expression is not required for CpG DNA-aided cross-presentation of DNA-conjugated antigens but essential for cross-priming of CD8 T cellsJ Immunol200317062802280512626528
- KriegAMTherapeutic potential of Toll-like receptor 9 activationNat Rev Drug Discov20065647148416763660
- JahrsdörferBWeinerGJCpG oligodeoxynucleotides for immune stimulation in cancer immunotherapyCurr Opin Investig Drugs200346686690
- FonsecaDEKlineJNUse of CpG oligonucleotides in treatment of asthma and allergic diseaseAdv Drug Deliv Rev200961325626219167442
- KlinmanDMKlaschikSSatoTTrossDCpG oligonucleotides as adjuvants for vaccines targeting infectious diseasesAdv Drug Deliv Rev200961324825519272313
- HeikenwalderMPolymenidouMJuntTLymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxy-nucleotide administrationNat Med200410218719214745443
- FogedCBrodinBFrokjaerSSundbladAParticle size and surface charge affect particle uptake by human dendritic cells in an in vitro modelInt J Pharm2005298231532215961266
- KurreckJAntisense technologies: improvement through novel chemical modificationsEur J Biochem200327081628164412694176
- AgrawalSZhaoQAntisense therapeuticsCurr Opin Chem Biol1998245195289736926
- MalyalaPO’HaganDTSinghMEnhancing the therapeutic efficacy of CpG oligonucleotides using biodegradable microparticlesAdv Drug Deliv Rev200961321822519168103
- WilsonKDde JongSDTamYKLipid-based delivery of CpG oligonucleotides enhances immunotherapeutic efficacyAdv Drug Deliv Rev200961323324219232375
- BiancoAHoebekeJGodefroySCationic carbon nanotubes bind to CpG oligodeoxynucleotides and enhance their immunostimulatory propertiesJ Am Chem Soc20051271585915631447
- StandleySMMendeLGohSLIncorporation of CpG oligonucleotide ligand into protein-loaded particle vaccines promotes antigen-specific CD8 T-cell immunityBioconjug Chem2007181778317226959
- RattanakiatSNishikawaMFunabashiHLuoDTakakuraYThe assembly of a short linear natural cytosine-phosphate-guanine DNA into dendritic structures and its effect on immunostimulatory activityBiomaterials200930295701570619604576
- MutwiriGKNichaniAKBabiukSBabiukLAStrategies for enhancing the immunostimulatory effects of CpG oligodeoxynucleotidesJ Control Release200497111715147800
- KrugARothenfusserSHornungVIdentification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cellsEur J Immunol20013172154216311449369
- KriegAMCpG motifs in bacterial DNA and their immune effectsAnnu Rev Immunol20022070976011861616
- GürselMVerthelyiDGürselIIshiiKJKlinmanDMDifferential and competitive activation of human immune cells by distinct classes of CpG oligonucleotideJ Leukoc Biol200271581382011994506
- HartmannGWeeratnaRDBallasZKDelineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivoJ Immunol200016431617162410640783
- HartmannGKriegAMMechanism and function of a newly identified CpG DNA motif in human primary B cellsJ Immunol2000164294495310623843
- KrugATowarowskiABritschSToll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amount of IL–12Eur J Immunol200131103026303711592079
- HartmannGBattianyJPoeckHRational design of new CpG oligonucleotides that combine B cell activation with high IFN-α induction in plasmacytoiddendritic cellsEur J Immunol20033361633164112778481
- MarshallJDFearonKAbbateCIdentification of a novel CpG DNA class and motif that optimally stimulate B cell and plasmacytoid dendritic cell functionsJ Leukoc Biol200373678179212773511
- PoeckHWagnerMBattianyJPlasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell helpBlood200410383058306415070685
- VollmerJWeeratnaRPayettePCharacterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activitiesEur J Immunol200434125126214971051
- SamulowitzUWeberMWeeratnaRA novel class of immune-stimulatory CpG oligodeoxynucleotides unifies high potency in type I interferon induction with preferred structural propertiesOligonucleotides20102029310120384481
- SheehanJPLanHCPhosphorothioate oligonucleotides inhibit the intrinsic tenase complexBlood1998925161716259716589
- BrownDAKangSHGryaznovSMEffect of phosphorothioate modification of oligodeoxynucleotides on specific protein bindingJ Biol Chem19942694326801268057929417
- LevinAAA review of the issues in the pharmacokinetics and toxicology of phosphorothioate antisense of oligonucleotidesBiochim Biophys Acta199914891698410806998
- HenrySPBeattieGYehGComplement activation is responsible for acute toxicities in rhesus monkeys treated with a phosphorothioate oligonucleotideInt Immunopharmacol20022121657166612469940
- ChavanyCConnellYNeckersLContribution of sequence and phosphorothioate content to inhibition of cell growth and adhesion caused by c-myc antisense oligomersMol Pharmarcol1995484738746
- CrookeRMIn vitro toxicology and pharmacokinetics of antisense oligonucleotidesAnticancer Drug Des1991666096461772572
- ErieDSinhaNOlsonWJonesRBreslauerKADumbbell-shaped, double-hairpin structure of DNA: a thermodynamic investigationBiochemistry19872622715071593427065
- ChuBCOrgelLEThe stability of different forms of double-stranded decoy DNA in serum and nuclear extractsNucleic Acids Res19922021585758581454556
- CluselCUgarteEEnjolrasNVasseurMBlumenfeldMEx vivo regulation of specific gene expression by nanomolar concentration of double-stranded dumbbell oligonucleotidesNucleic Acids Res19932115340534117688452
- SchmidtMAntonKNordhausCJunghansCWittigBWormMCytokine and Ig-production by CG-containing sequences with phosphorodiester backbone and dumbbell-shapeAllergy2006611566316364157
- NishikawaMMatonoMRattanakiatSMatsuokaNTakakuraYEnhanced immunostimulatory activity of oligodeoxynucleotides by Y-shape formationImmunology2008124224725518217956
- NishikawaMMizunoYMohriKBiodegradable CpG DNA hydrogels for sustained delivery of doxorubicin and immunostimulatory signals in tumor-bearing miceBiomaterials201132248849420932569
- LiJPeiHZhuBSelf-assembled multivalent DNA nanostructures for noninvasive intracellular delivery of immunostimulatory CpG oligonucleotidesACS Nano20115118783878921988181
- MengWYamazakiTNishidaYHanagataNNuclease-resistant immunostimulatory phosphodiester CpG oligodeoxynucleotides as human Toll-like receptor 9 agonistsBMC Biotechnol201111889621943407
- KerkmannMCostaLTRichterCSpontaneous formation of nucleic acid-based nanoparticles is responsible for high interferon-alpha induction by CpG-A in plasmacytoid dendritic cellsJ Biol Chem200528098086809315591070
- KleinDCLatzEEspevikTStokkeBTHigher order structure of short immunostimulatory oligonucleotides studied by atomic force microscopyUltramicroscopy2010110668969320202756
- LeeSWSongMKBaekKHEffects of a hexameric deoxyriboguanosine run conjugation into CpG oligodeoxynucleotides on their immunostimulatory potentialsJ Immunol200016573631363911034366
- DalpkeAHZimmermannSAlbrechtIHeegKPhosphodiester CpG oligonucleotides as adjuvants: polyguanosine runs enhance cellular uptake and improve immunostimulative activity of phosphodiester CpG oligonucleotides in vitro and in vivoImmunology2002106110211211972638
- HondaKOhbaYYanaiHSpatiotemporal regulation of MyD88-IRF-7 signaling for robust type-I interferon inductionNature200543470361035104015815647
- MarshallJDHesselEMGregorioJNovel chimeric immunomodulatory compounds containing short CpG oligodeoxynucleotides have differential activities in human cellsNucleic Acids Res200331175122513312930963
- FearonKMarshallJDAbbateCA minimal human immunostimulatory CpG motif that potently induces IFN-gamma and IFN-alpha productionEur J Immunol20033382114212212884285
- AoyamaYKanamoriTNakaiTArtificial viruses and their application to gene delivery: size-controlled gene coating with glycocluster nanoparticlesJ Am Chem Soc2003125123455345712643707
- NakaiTKanamoriTSandoSAoyamaYRemarkably size-regulated cell invasion by artificial viruses: saccharide-dependent self-aggregation of glycoviruses and its consequences in glycoviral gene deliveryJ Am Chem Soc2003125288465846712848552
- OsakiFKanamoriTSandoSSeraTAoyamaYA quantum dot conjugated sugar ball and its cellular uptake: on the size effects of endocytosis in the subviral regionJ Am Chem Soc2004126216520652115161257
- GaoHShiWFreundLBMechanics of receptor-mediated endocytosisProc Natl Acad Sci U S A2005102279469947415972807
- RobertsTLDunnJATerryTDDifferences in macrophage activation by bacterial DNA and CpG-containing oligonucleotidesJ Immunol200517563569357616148100
- TakeshitaFLeiferCAGurselICutting edge: role of Toll-like receptor 9 in CpG DNA-induced activation of human cellsJ Immunol200116773555355811564765
- SempleSCKlimukSKHarasymTOHopeMJLipid-based formulations of antisense oligonucleotides for systemic delivery applicationsMethods Enzymol200031332234110595364
- ZhuYMengWLiXGaoHHanagataNDesign of mesoporous silica/ cytosine-phosphodiester-guanin oligodeoxynucleotide complexes to enhance delivery efficiencyJ Phys Chem C20111152447452
- DementoSLBonaféNCuiWTLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitisJ Immunol201018552989299720660705
- PanXChenLLiuSYangXGaoJXLeeRJAntitumor activity of G3139 lipid nanoparticles (LNPs)Mol Pharm20096121122019072654
- SempleSCKlimukSKHarasymTOEfficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizeable aminolipids: formation of novel small multilamellar vesicle structuresBiochim Biophys Acta200115101–215216611342155
- YuRZGearyRSLeedsJMPharmacokinetics and tissue disposition in monkeys of an antisense oligonucleotide inhibitor of Ha-ras encapsulated in stealth liposomesPharm Res19991681309131510468036
- ZhiCMengWYamazakiTBN nanospheres as CpG ODN carriers for activation of Toll-like receptor 9J Mater Chem2011211452195222
- BourquinCAnzDZwiorekKTargeting CpG oligonucleotides to the lymph node by nanoparticles elicits efficient antitumoral immunityJ Immunol200818152990299818713969
- KlierJMayAFuchsSImmunostimulation of bronchoalveolar lavage cells from recurrent airway obstruction-affected horses by different CpG-classes bound to gelatin nanoparticlesVet Immunol Immunopathol20111441–2798721831455
- ChenHCSunBTranKKShenHEffects of particle size on Toll-like receptor 9-mediated cytokine profilesBiomaterials20113261731173721126760
- SokolovaVKnuschkeTKovtunABuerJEppleMWestendorfAMThe use of calcium phosphate nanoparticles encapsulating Toll-like receptor ligands and the antigen hemagglutinin to induce dendritic cell maturation and T cell activationBiomaterials201031215627563320417963
- ZhuYMengWGaoHHanagataNHollow mesoporous silica/poly(L-lysine) particles for codelivery of drug and gene with enzyme-triggered release propertyJ Phys Chem C2011115281363013636
- BallardBEBiopharmaceutical considerations in subcutaneous and intra-muscular drug administrationJ Pharm Sci19685733573784871917
- HiranoKHuntCALymphatic transport of liposome-encapsulated agents: effects of liposome size following intraperitoneal administrationJ Pharm Sci19857499159214067845
- KuramotoYNishikawaMHyoudouKYamashitaFHashidaMInhibition of peritoneal dissemination of tumor cells by single dosing of phosphodiester CpG oligonucleotide/cationic liposome complexJ Control Release2006115222623316996162
- KuramotoYKawakamiSZhouSFukudaKYamashitaFHashidaMUse of mannosylated cationic liposomes/immunostimulatory CpG DNA complex for effective inhibition of peritoneal dissemination in miceJ Gene Med200810439239918181219
- ChenXWuPRousseasMBoron nitride nanotubes are non-cytotoxic and can be functionalized for interaction with proteins and cellsJ Am Chem Soc2009131389089119119844
- TigheHTakabayashiKSchwartzDConjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunityEur J Immunol20003071939194710940883
- GürselMTuncaSOzkanMOzcengizGAlaeddinogluGImmunoadjuvant action of plasmid DNA in liposomesVaccine19991711–121376138310195773
- GürselIGürselMIshiiKJKlinmanDMSterically stabilized cationic liposomes improve the uptake and immunostimulatory activity of CpG oligonucleotidesJ Immunol200116763324332811544321
- Kaiser-SchulzGHeitAQuintanilla-MartinezLPolyactide-coglycolide microspheres co-encapsulating recombinant tandem prion protein with CpG-oligonucleotide break self-tolerance to prion protein in wild-type mice and induce CD4 and CD8 T cell responsesJ Immunol200717952797280717709493
- NierkensSden BrokMHSutmullerRPIn vivo colocalization of antigen and CpG [corrected] within dendritic cells is associated the efficacy of cancer immunotherapyCancer Res200868135390539618593941
- LeeYRLeeYHImSABiodegradable nanoparticles containing TLR3 or TLR9 agonists together with antigen enhance MHC-restricted presentation of the antigenArch Pharm Res201033111859186621116790
- ErikçiEGürselMGürselIDifferential immune activation following encapsulation of immunostimulatory CpG oligodeoxynucleotide in nanoliposomesBiomaterials20113261715172321112627
- SlütterBJiskootWDual role of CpG as immune modulator and physical crosslinker in ovalbumin loaded N-trimethyl chitosan (TMC) nanoparticles for nasal vaccinationJ Control Release2010148111712120600405
- BalSMSlütterBVerheulRBouwstraJAJiskootWAdjuvanted, antigen loaded N-trimethyl chitosan nanoparticles for nasal and intradermal vaccination: adjuvant- and site-dependent immunogenicity in miceEur J Pharm Sci201245447548122009113
- SinghMOttGKazzazJCationic microparticles are an effective delivery system for immune stimulatory CpG DNAPharm Res200118101476147911697476
- DiwanMTafaghodiMSamuelJEnhancement of immune responses by co-delivery of a CpG oligodeoxynucleotide and tetanus toxoid in biodegradable nanospheresJ Control Release2002851–324726212480329
- ZhangXQDahleCEBamanNKRichNWeinerGJSalemAKPotent antigen-specific immune responses stimulated by codelivery of CpG ODN and antigens in degradable microparticlesJ Immunother200730546947817589287
- BorgesOCordeiro-da-SilvaATavaresJImmune response by nasal delivery of hepatitis B surface antigen and codelivery of a CpG ODN in alginate coated chitosan nanoparticlesEur J Pharm Biopharm200869240541618364251
- XieHGurselIIvinsBECpG oligodeoxynucleotides adsorbed onto polylactide-co-glycolide microparticles improve the immunogenicity and protective activity of the licensed anthrax vaccineInfect Immun200573282883315664922
- HeitASchmitzFHaasTBuschDHWagnerHAntigen co-encapsulated with adjuvants efficiently drive protective T cell immunityEur J Immunol20073782063207417628858
- KwongBLiuHIrvineDJInduction of potent anti-tumor responses while eliminating systemic side effects via liposome-anchored combinatorial immunotherapyBiomaterials201132225134514721514665
- StoneGWBarzeeSSnarskyVNanoparticle-delivered multimeric soluble CD40L DNA combined with Toll-like receptor agonists as a treatment for melanomaPLoS One2009410e733419812695
- WellsJWCowledCJFarzanehFNobleACombined triggering of dendritic cell receptors results in synergistic activation and potent cytotoxic immunityJ Immunol200818153422343118714014
- HaradaAKawamuraMMatsuoTTakahashiTKonoKSynthesis and characterization of a head-tail type polycation block copolymer as a nonviral gene vectorBioconjug Chem20061713516417245
- WagnerEKloecknerJGene delivery using polymer therapeuticsAdv Polym Sci2006192135173
- NguyenDNGreenJJChanJMLangerRAndersonDGPolymeric materials for gene delivery and DNA vaccinationAdv Mater2009218847867
- MeyerOKirpotinDHongKCationic liposomes coated with polyethylene glycol as carriers for oligonucleotidesJ Biol Chem19982732515621156279624154
- MahatoRIAnwerKTagliaferriFBiodistribution and gene expression of lipid/plasmid complexes after systemic administrationHum Gene Ther1998914208320999759935
- KimHAkagiTAkashiMPreparation of CpG ODN-encapsulated anionic poly(amino acid) nanoparticles for gene deliveryChem Lett2010393278279
- PackDWHoffmanASPunSStaytonPSDesign and development of polymers for gene deliveryNat Rev Drug Discov20054758159316052241
- TrubetskoyVSWongSCSubbotinVRecharging cationic DNA complexes with highly charged polyanions for in vitro and in vivo gene deliveryGene Ther200310326127112571634
- KurosakiTKitaharaTFumotoSTernary complexes of pDNA, polyethylenimine, and gamma-polyglutamic acid for gene delivery systemsBiomaterials200930142846285319232715
- KimHAkagiTAkashiMPreparation of size tunable amphiphilic poly(amino acid) nanoparticlesMacromol Biosci20099984284819422015
- YanaiHBanTWangZHMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responsesNature200946272699910319890330
- YanaiHChibaSBanTSuppression of immune responses by nonimmunogenic oligodeoxynucleotides with high affinity for high-mobility group box proteins (HMGBs)Proc Natl Acad Sci U S A201110828115421154721709231