Abstract
Nanotechnology is the development of an engineered device at the atomic, molecular, and macromolecular level in the nanometer range. Advances in nanotechnology have proven beneficial in therapeutic fields such as drug-delivery and gene/protein delivery. Antigen delivery systems are important for inducing and modifying immune responses. In cellular immunity, cytotoxic T lymphocytes (CTLs) are important in the host defense against tumors. Key to the development of CTL-inducible vaccines is the ability to deliver antigens to antigen-presenting cells efficiently and to induce the subsequent activation of T cell-mediated immunity without adjuvants, as they can induce excessive inflammation leading to systemic febrile disease. Since expression and cloning methods for tumor-associated antigens have been reported, cancer vaccines that induce effective cell immunity may be promising therapeutic candidates, but Th2 cells are undesirable for use in cancer immunotherapy. Peptide vaccines have immunological and economic advantages as cancer vaccines because CTL epitope peptides from tumor-associated antigens have high antigen-specificity. However, cancer vaccines have had limited effectiveness in clinical responses due to the ability of cancer cells to “escape” from cancer immunity and a low efficiency of antigen-specific CTL induction due to immunogenic-free synthetic peptides. In contrast, carbohydrate-decorated particles such as carbohydrate-coated liposomes with encapsulated antigens might be more suitable as antigen delivery vehicles to antigen-presenting cells. Oligomannose-coated liposomes (OML) can eliminate established tumors in mouse cancer models. In addition, OMLs with an encased antigen can induce antigen-specific CTLs from peripheral blood mononuclear cells obtained from patients. Feasibility studies of OML-based vaccines have revealed their potential for clinical use as vaccines for diseases where CTLs act as effector cells. Furthermore, use of the hepatitis B core particle, in which tumor-antigen epitopes are set, has consistently been shown to induce strong CTL responses without the use of an adjuvant. Thus, nanoparticles may provide a new prophylactic strategy for infectious disease and therapeutic approaches for cancer via the induction of T-cell immunity.
Introduction
Recent developments in multifunctional nanoparticles offer a great potential for targeted delivery of therapeutic compounds and imaging contrast agents to specific cell types, enhancing therapeutic effects and minimizing side effects. For pharmaceutical purposes, nanoparticles are defined as solid colloidal particles ranging in size from 10 nm to 400 nm. They consist of macromolecular materials in which the active agent (drug or biologically active material) is dissolved, entrapped, and/or encapsulated, or to which the active agent is adsorbed or attached.Citation1 The ability to target liposomal drugs to tumors or target cells represents an attractive strategy for developing more effective cancer therapies.
Antigen-specific CD8+ cytotoxic T lymphocyte (CTL) induction is an attractive immunotherapeutic strategy against hematological malignancies, other cancers, and infectious diseases.Citation2–Citation4 Cluster of differentiation (CD) molecules are expressed on most normal cells. However, CD molecules are often overexpressed on tumor cells. Viral-specific and mutated antigens are clearly foreign, and viral antigens are present only on tumors that are virally induced. Therefore, CTL responses may be initiated against overexpressed cell surface markers or viral antigens expressed by major histocompatibility complex (MHC) molecules to target tumor cells. For instance, impaired host CTL function abrogates protection against the accumulation of human T-cell leukemia virus-1 (HTLV-1)-transformed cells. Thus, preventing the loss of, or inducing host CTL functions, may yield an effective immune strategy against leukemogenesis such as adult T-cell leukemia-lymphoma (ATL).Citation5,Citation6 In particular, CD4+ T lymphocytes differentiate into distinct T helper (Th) cell subsets depending on the type of disease. Among these effector Th subsets, Th1 cells support the expansion and persistence of CTLs, but Th2 cells are undesirable for immunotherapy of cancer or for vaccination against intracellular pathogens. Therefore, a potent vaccine for these diseases should activate both CD8+ and CD4+ T lymphocytes to generate antigen-specific CTLs and Th1 cells, respectively.
The difficulty of inducing antigen-specific CTLs in individual patients vitiates the more widespread use of adoptive T cell therapy. Liposomes can encapsulate large and broad antigenic sequences, immunomodulatory factors, and drugs, and thus can serve as potent delivery vehicles. Whereas free synthetic peptides have proven to be relatively poor immunogens, the use of peptides with oligomannose-coated liposomes (OML) can induce strong cellular immunity.Citation7–Citation10 Virus-like particles (VLPs) have also been shown to induce strong CTLs, even without adjuvant.Citation11,Citation12 Furthermore, liposome-protamine-DNA (LPD) nanoparticles are currently being employed as effective peptide carriers.Citation13 The immunostimulatory and adjuvant properties of LPD nanoparticles have made them suitable for delivering antigens for the purpose of vaccination.Citation14
Thus, the use of liposomes and VLPs to target drugs to tumors represents an attractive therapeutic strategy, especially when used with convenient targeting moieties such as peptides. This review presents an overview of the current attempts to use nanoparticles for vaccines.
Liposomal vaccines
In the study of potent immune responses, adjuvants are crucial. However, adjuvants can induce unfavorable immune responses such as systemic fever, hepatotoxicity, and hypersensitivity “flare reactions” around the injection site. Liposome co-encapsulated CpG oligodeoxynucleotides with recombinant Leishmania major stress-inducible protein 1, enhanced immune responses, and protection against leishmaniasis in immunized BALB/c mice when compared with CpG given in soluble form with antigen or antigen without adjuvant.Citation15 This study indicated the superiority of CpG oligodeoxynucleotides in its liposomal form compared to the soluble form, and that it has an important role in vaccine development strategies against leishmaniasis.Citation16
LPD nanoparticles are spheres (around 150 nm) generated by spontaneous rearrangement of a lipid shell composed of 1,2-dioleoyl-3-trimethylammonium-proprane and cholesterol around a protamine-condensed DNA to develop a virus-like structure.Citation17 The immunostimulatory and adjuvant properties of LPD nanoparticles have made them suitable for delivering antigens for the purpose of vaccination.Citation14 The small diameter and positively charged characteristics of LPD nanoparticles allow lymphatic delivery of peptides and dendritic cell (DC) targeting.Citation18,Citation19
Implications of antigen delivery to antigen-presenting cells (APCs) by carbohydrate-coated liposomes
The critical requirements for an antigen delivery system for vaccines include efficient delivery to the cell of choice, the ability to load the antigen onto MHC molecules, and the ability to activate APCs to express costimulatory molecules to aid the induction of CTL responses. Liposomes have been widely exploited as antigen delivery systems for treating a variety of diseases. These vesicles can be prepared in various ways, which may affect the immunogenicity of the encapsulated antigens.Citation20 Efficient delivery of antigens to immune cells can be facilitated by agents that bind selectively to molecular structures on the surface of the targeted cells. The conjugation of liposomes with antibodies directed to cell surface receptors, recombinant ligand proteins, or chemical ligands, such as carbohydrates, can facilitate uptake by specific cells and therefore have been tested for targeting liposomes to cells and tissues.Citation21–Citation23 The recognition and phagocytosis of pathogens and their subsequent destruction is an important mechanism of immune defense. APCs express many C-type lectin receptors (CLRs) that act as phagocytic receptors.Citation24,Citation25 Some CLRs contain internalization motifs in their cytoplasmic domains, which direct the uptake of ligands and subsequent sorting of CLRs and attached cargo into late endosomes. CLRs also contain an immunoreceptor tyrosine-based activation motifs or immunoreceptor tyrosine-based inhibitory motifs in their cytoplasmic domains that trigger or modulate distinct signaling pathways to induce the expression of specific cytokines, which then determine T cell polarization.Citation25 CLRs recognize distinct carbohydrate structures expressed on pathogens, and therefore the use of carbohydrates that are preferentially recognized by CLRs as targeting signals may be a more sophisticated alternative.Citation26 Thus, carbohydrate-decorated particles such as carbohydrate-coated liposomes with encapsulated antigens may be more suitable antigen delivery vehicles to target CLRs on APCs.
Molecular structures containing terminal mannose are uncommon elements of mammalian tissues, but abundant and highly conserved in microorganisms. Mannose-recognizing CLRs can facilitate the binding and endocytosis of ligands with terminal mannose, fucose, and N-acetylglucosamine.Citation27 Mannose residues can be recognized by DC-specif ic intracellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN, CD209) family molecules expressed by APCs, langerin (CD207) on Langerhans cells and DCs and by dectin-2 on monocytes or macrophages.Citation28,Citation29 Thus, carbohydrate-modified antigens may be preferentially delivered to APCs due to DC-SIGN-mediated uptake of antigens, resulting in effective antigen presentation to T cells.Citation30 Therefore, mannose residues attached to antigens or as a coating on antigen-carrying liposomes have promise for the induction of immune responses using the carbohydrate recognition pathways of APCs.
Oligomannose-coated liposomes as a drug delivery system
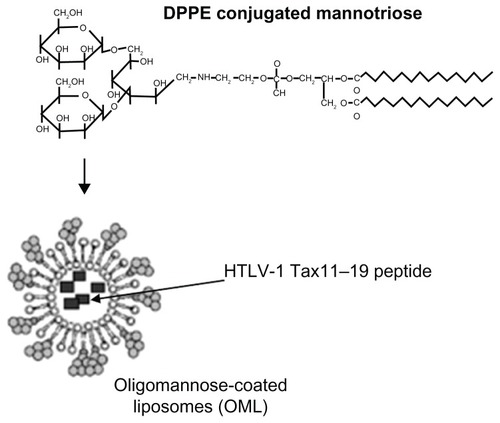
Kojima et al have developed a new active agent (drug, peptide, or biologically active material) delivery system for the control of diseases such as infection, cancer and allergy, using OMLs.Citation31–Citation34 OMLs were prepared from dipalmitoylphosphatidylethanolcholine, cholesterol, and mannotriose-dipalmitoylphosphatidylethanolamine at a molar ratio of 10:10:1 and extruded through a 1-μm pore membrane (). This approach relies on the preferential recognition of oligomannose residues on OMLs by APCs expressing APC-specific mannose-binding lectin receptors, and subsequent uptake of OMLs and antigens encased in the OMLs to be loaded on MHC class I and class II molecules.Citation7 OMLs can also activate and induce the maturation of APCs with enhanced expression of costimulatory molecules and proinflammatory cytokines.Citation9
Figure 1 Structure of synthetic neoglycolipids consisting of dipalmitoylphosphatidylethanolamine.
Abbreviation: DPPE, dipalmitoylphosphatidylethanolcholine; HTLV-1, human T-cell leukemia virus-1.
In the efficient induction of CTLs, Th1 cells support the expansion and persistence of CTLs, whereas Th2 cells are undesirable for immunotherapy of cancer or vaccination against intracellular pathogens. OMLs can enhance interleukin (IL)-12 production and the expression of costimulatory molecules (CD40, CD80, CD86, and MHC class II molecules) on peritoneal macrophages.Citation9 Interestingly, the production of inflammatory cytokines such as IL-1 and IL-6 from either DCs or macrophages was suppressed in response to OML incorporation.Citation9 However, spleen cells from mice immunized with ovalbumin (OVA)-containing OMLs (OML/OVA) produced significant levels of interferon (IFN)-γ that exceeded IL-5 production when stimulated with OVA in vitro. In contrast, spleen cells predominantly produced IL-5 over IFN-γ in response to OVA stimulation, if mice were immunized with OVA adsorbed on aluminum hydroxide, an adjuvant for the induction of Th2 immune responses. Thus, to induce antigen-specific Th1 cells, a suitable adjuvant must be used in conjunction with OVA incorporation.
Pathogens share similar structures termed pathogen-associated molecular patterns (PAMPs). Pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) are a primitive part of the immune system.Citation35 Binding of PAMPs to TLRs triggers signal transduction events that activate mitogen-activated protein kinases and transcription factors such as nuclear factor-κB, leading to the induction of inflammatory cytokines such as IL-1β, IL-6, IL-12, and tumor necrosis factor-α.Citation36 OML-stimulated APCs preferentially produce IL-12, but suppress the production of IL-6 and IL-1β. SIGNR1 colocalizes in lipid rafts with several Src family kinases, such as Lyn, which modulate the maturation of DCs.Citation37 Therefore, OMLs may activate APCs through particular signaling pathways distinct from those triggered by interactions between PAMPs and TLRs.
Oligomannose-coated liposomes as a therapeutic vaccine
Kojima et al demonstrated that OMLs can be used as therapeutic vaccines that induce CTLs specific to the encased antigen without adjuvant ().Citation38 Immunization of BALB/c mice with OMLs encasing soluble leishmanial antigen protected against subsequent Leishmania major infection due to the predominant induction of leishmanial antigen-specific Th1 immune responses over Th2 immune responses. Preliminary studies have also examined the effect of OML-based vaccines using soluble protozoan lysates of Toxoplasma gondii, Trypanosoma brucei gambiense, Babesia rodhaini, or Neospora caninum on the corresponding protozoal infections in mice.Citation33 OML-based vaccinations, incorporating a single injection of OMLs containing 1 μg of antigen, induced antitumor activity in an E.G7-OVA tumor-bearing mouse model that suppressed tumor growth and led to tumor rejection, where approximately 50% of the mice showed elimination of an established tumor.Citation32 Recently, Kojima et al reported the induction of CTLs specific for either HLA-A24-restricted epitopes of survivin2B using mixed lymphocyte-peptide cultures with OML-coated survivin2B peptides or human papillomavirus type16 E6 and E7 with OML-coated papillomavirus DNA.Citation38,Citation39 Therefore, OMLs are suitable for use as safe vaccine carriers of peptides and plasmid DNA and as adjuvants.
Table 1 Therapeutic efficiency of oligomannose-coated liposome-based vaccines
OML with an encased allergen may also have an antiallergic effect since IFN-γ produced by Th1 cells induced by OML-based vaccination inhibited the development and activation of allergen-specific Th2 cells, which promotes IgE synthesis and mediates type I allergic reactions. Immunization of OMLs with entrapped Cry j 1, identified as major allergen in Japanese cedar pollen, prevents elevation of serum IgE levels elicited by Cry j 1 administration in both unsensitized mice and Cry j 1-presensitized mice. This indicates that OML-encased allergens may serve as immunotherapeutic agents to control type I allergic diseases. These inhibitory effects may occur due to a shift from Th2 immune responses to allergen-specific Th1 immune responses since Cry j 1-specific IgG1 production mediated by Th2 cells was significantly reduced, but Cry j 1-specific IgG2a production mediated by Th1 cells increased in the sera of OML-based vaccinated mice.Citation31 Nasal administration of OMLs can induce entrapped HA-specific secretory IgA in local tissues and OVA-specific serum IgG and IgA.Citation40 These feasibility studies of OML-based vaccines have revealed their potential for clinical use as vaccinations for diseases where CTLs and/or Th1 cells are effectors.
Prophylactic nanoparticle vaccines for adult T cell leukemia
ATL is an aggressive malignancy of mature peripheral T-lymphocytes.Citation41–Citation43 Despite recent progress in chemotherapy and supportive care for hematological malignancies, the median survival time is still only approximately 13 months.Citation44–Citation46 Therefore, new strategies for the therapy and prophylaxis of ATL (eg, vaccines and novel molecular target agents) are urgently needed. Although the clonal evolution of ATL cells may involve multiple steps,Citation47 an insufficient T cell response to HTLV-1 is a potential risk factor. Citation48 HTLV-1-specific CTLs are critical in the host immune response against HTLV-1 infection.Citation49,Citation50 We previously reported a decreased frequency and function of HTLV-1 Tax-specific CD8+ T cells in ATL patients and the upregulation of the negative immunoregulatory programmed death 1 marker on HTLV-1 Tax-specific CTLs from asymptomatic HTLV-1 carriers and ATL patients.Citation51–Citation54 Impaired host CTL function reduces protection against the accumulation of HTLV-1-transformed cells; thus, targeting this mechanism may yield an effective immune strategy against leukemogenesis. HTLV-1 Tax-targeted vaccines in a rat model of HTLV-1-induced lymphomas showed promising antitumor effects.Citation55 Therefore, HTLV-1-specific CTLs are important for the immunological suppression of HTLV-1-infected cell proliferation and pathogenesis of ATL.
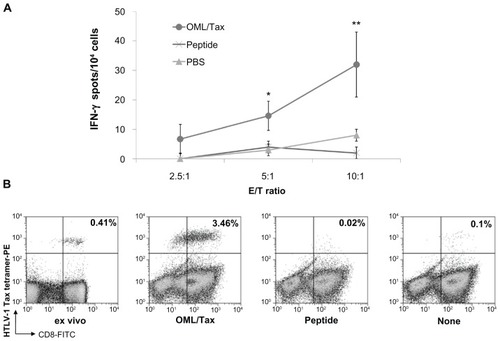
The difficulty in inducing antigen-specific CTLs in individual patients suggests why the use of adoptive T cell therapy is not widespread. OMLs can be an effective antigen delivery system for cancer immunotherapy by activating CTLs and Th subsets.Citation56,Citation57 We examined the efficient induction of HTLV-1-specific CD8+ T cell responses by OMLs encapsulating the HLA-A*0201-restricted HTLV-1 Tax-epitope (OML/Tax; ). Immunization of HLA-A*0201 transgenic mice with OML/Tax induced HTLV-1-specific IFN-γ production from T cells, whereas immunization with the epitope peptide alone did not (). DCs exposed to OML/Tax showed increased expression of CD86 and MHC class I, and class II molecules such as HLA-A02.Citation10 In addition, HTLV-1-specific CD8+ T cells were efficiently induced by OML/Tax derived from HTLV-1 carriers compared with the epitope peptide alone (). OML/Tax increased the number of HTLV-1- specific CD8+ T cells up to 1400-fold, while treatment with peptide alone and without antigen showed increases of 95- and 35-fold, respectively. Furthermore, these HTLV-1- specific CD8+ cells induced apoptosis of HTLV-1 epitope peptide-pulsed T2-A2 cells. CD8+ T cells efficiently lysed Tax peptide-loaded T2-A2 cells, whereas only low-level background lysis was observed in the absence of Tax peptide and for CMV peptide-loaded T2-A2 cells. These results suggest that OML/Tax induces antigen-specific cellular immune responses without the need for adjuvants and may be an effective vaccine carrier for prophylaxis of tumors and infectious diseases.Citation10
Figure 2 Induction of cellular immunity by intradermal immunization of mice with OML/Tax. (A) HLA-A*0201 transgenic mice (n = 5 per group) were intradermally immunized twice with OML/Tax, HTLV-1 peptide (LLFGYPVYV), or PBS on days 0 and 14. Seven days after the last immunization, the spleens and inguinal lymph nodes were collected. Inguinal lymph node cells (2 × 106/well) were stimulated in vitro with HTLV-1 peptide. Six days later, the number of IFN-γ-producing cells per 2.5, 5, or 10 × 104 inguinal lymph node cells upon stimulation with syngeneic bone marrow derived-DCs (1 × 104/well, pulsed with or without each peptide) were determined using an ELISPOT assay. IFN-γ spots were expressed as the number of peptide-loaded to peptide-unloaded target cells. *P < 0.05; **P < 0.01 vs PBS group. All experiments were performed in triplicate. Values represent the mean from five mice. Results represent the mean ± SD. (B) Freshly isolated or cryopreserved PBMCs from HTLV-1 carriers were cultured with OML/Tax, peptide alone, or without antigen. The tetramer assay was performed using fresh (ex vivo) or cultured PBMCs. The numbers in the upper right quadrants represent the percentage of tetramer+CD8+ T cells within the T lymphocyte population.
Abbreviations: DCs, dendritic cells; FITC, fluorescein isothiocyanate; IFN, interferon; HTLV-1, human T-cell leukemia virus-1; OML, oligomannose-coated liposome; PBS, phosphate-buffered saline; PE, phycoerythrin; PBMCs, peripheral blood mononuclear cells.
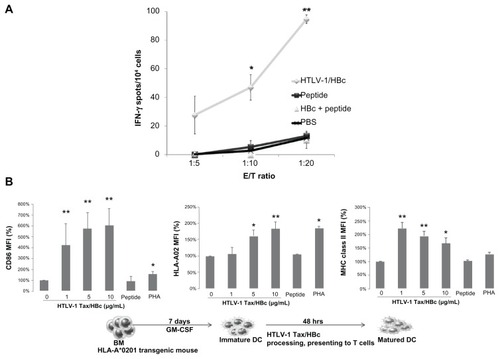
Whereas free synthetic peptides have proven to be relatively poor immunogens, VLPs, such as hepatitis B core (HBc), have consistently been shown to induce strong antibody and CTL responses, even without an adjuvant.Citation11,Citation12 HBcAg, a potent immunogen that elicits strong humoral, T-helper, and CTL responses and is amenable to a variety of heterologous epitopes without an adjuvant,Citation58 may be an effective carrier protein for use in T-cell mediated vaccine development.Citation59 We fused the HTLV-1 Tax11–19 peptide, recognized by HLA-A*0201-restricted HTLV-1-specific CD8+ T-cells with high frequency,Citation52 to HBcAg to yield an HTLV-1/HBc chimeric particle. A synthetic DNA fragment, the HTLV-1 Tax sequence from amino acid positions 8–22, including the Tax11–19 epitope recognized by HLA-A*0201, was inserted into the HBc gene. The chimeric protein was expressed in Pichia pastris KM71. The chimeric particle maintained the capacity to fold correctly and spontaneously assembled into structured capsid particles with a diameter of 36 nm. Immunization of HLA-A*0201 transgenic mice with the chimeric particle induced antigen-specific IFN-γ production as assessed by enzyme-linked immunospot assays, whereas immunization with the epitope peptide alone induced no reaction (). Immunization with the chimeric particle also induced HTLV-1-specific CD8+ T-cells in the spleen and inguinal lymph nodes.Citation60 Furthermore, DCs isolated from HLA-A*0201-transgenic mice exposed to the chimeric particle showed increased expression of CD86, HLA-A02, and MHC class II (). In addition, our results demonstrated that HTLV-1-specific CD8+ T cells could be induced by a peptide with HTLV-1/HBc particles from ATL patients, but not by the peptide or HTLV-1/HBc particles alone, and could lyse immune cells presenting the peptide.Citation60 This suggests that HTLV-1/HBc chimeric particles can induce strong cellular immune responses without the need for an adjuvant via the effective maturation of DCs. Previous studies have demonstrated the efficient processing of lymphocytic choriomeningitis virus-derived p33/HBc chimera via cross-presentation, although only weak CTL responses were induced in C57BL/6 mice.Citation59 Thus, while VLPs alone are inefficient at inducing CTL responses, they become potent vaccines when combined with APC-activating substances such as anti-CD40 mAbs or nonmethylated CG motif-rich DNA (CpGs). These results suggest that the HTLV-1/HBc chimeric particle can induce strong cellular immune responses without adjuvants by inducing the maturation of DCs and is potentially useful as an effective carrier for therapeutic vaccines in tumors or in infectious diseases by substituting the epitope peptide.Citation60
Figure 3 The induction of cellular immunity by intradermal immunization with HTLV-1 Tax/HBc chimeric particles. (A) HLA-A*0201 transgenic mice were intradermally immunized twice with HTLV-1 Tax/HBc chimeric particles (20 μg), HTLV-1 peptide (1 μg: LLFGYPVYV), HTLV-1 peptide (1 μg) plus HBc particles (20 μg) or PBS at day 0 and day 14. Seven days after the last immunization, the spleens and inguinal lymph nodes were collected. The inguinal lymph node cells (2 × 106/well) were stimulated with Tax11–19 peptide in vitro. Six days later, the frequency of cells producing IFN-γ per 5, 10, or 20 × 104 inguinal lymph node cells upon stimulation with syngenic bone marrow-derived DCs (1 × 104/well), pulsed with or without each peptide, was determined by ELISPOT assay. IFN-γ spots are expressed as the number of peptide-loaded to peptide-unloaded target cells. *P < 0.05; **P < 0.01 vs PBS group. The experiments were performed in triplicate. Results represent the mean ± SD. (B) Maturation of DCs induced by HTLV-1 Tax/HBc chimeric particles was assessed by the expression of CD86 and HLA-02 on the surface of DCs after incubation with antigens. Murine immature dendritic cells (iDCs) were obtained from bone marrow (BM) precursors. iDCs were incubated with the indicated concentrations of HTLV-1 Tax/HBc chimeric particles: 10 μg/mL of HTLV-1 Tax peptide or 10 μg/mL of phytohemagglutinin (PHA) at 37°C. Data are expressed as the mean fluorescence intensity (MFI) for each molecule compared to unpulsed (0 μg/mL) iDC controls. Results represent means ± SD of four independent experiments. *P < 0.05; **P < 0.01 vs unpulsed iDC controls.
Abbreviations: DCs, dendritic cells; IFN, interferon; HBC, hepatitis B core; PBS, phosphate-buffered saline.
Conclusion
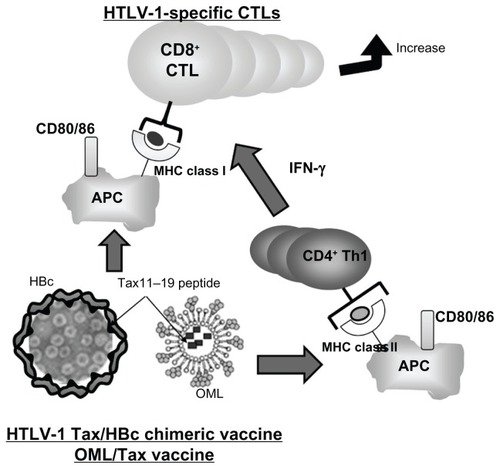
The recent emergence of targeting antigens specific to DCs and their subsequent activation with biomaterials has demonstrated an exciting potential for developing new vaccines. Nanoparticles such as liposomes and VLPs offer the ability to protect antigens from degradation and deliver them specifically to APCs. OML/Tax and HTLV-1 Tax/HBc chimeric particles are capable of inducing strong cellular immune responses without adjuvants and may be useful as effective carriers for prophylactic or therapeutic uses in infectious diseases and tumors such as HTLV-1-infected cells and ATL cells. Thus, novel chemical strategies can be employed to release antigens intracellularly where they can be processed by both MHC class I and class II pathways (). Nanoparticles can also be used as synthetic adjuvants that use different mechanisms to induce DC maturation and initiate adaptive immune responses. Moreover, bioconjugation strategies can be used to attach molecular “danger signals” to particles to amplify the immune response while providing a potential nontoxic alternative adjuvant technology. Finally, the use of these very small nanoparticles offers an antigen-delivery system to unlock the potential of targeting lymph-node-resident DCs to induce adaptive immunity and therapeutic tolerance. The development of biomaterials for immune cell-targeting continues at a rapid pace, and future studies should focus on optimizing antigen delivery and adjuvant activity. These new approaches, inducing antitumor immunity and anti-allergic effects using vaccines, may provide a new prophylactic strategy for infectious disease and therapeutic approaches for cancer.
Figure 4 Strategies targeted at inducing CTLs for the prophylaxis of infectious disease and therapeutic approaches for cancer. Notes: HTLV-1 Tax/HBc chimeric particles and OML/Tax can be phagocytosed by APCs and antigenic fragments presented by either MHC class I or II molecules to activate either CD8+ T cells or CD4+ Th1 cells, respectively. Thus, this strategy could induce strong cellular immune responses without adjuvant as assessed by the increase of CD86 and MHC class I/II expression.
Abbreviations: APC, antigen-presenting cell; CTLs, cytotoxic T lymphocytes; HBC, hepatitis B core; HTLV, human T-cell leukemia virus-1; MHC, major histocompatibility complex; OMLs, oligomannose-coated liposomes; Th, T cell helper.
Acknowledgments
I would like to express my thanks to Dr Sato and Dr Shimizu from BioMedCore Inc (Yokohama, Japan), Dr Kino and Dr Fukada from The Chemo-Sero-Therapeutic Research Institute (Kumamoto, Japan), Dr Suzuki and Mr Toji from the Medical and Biological Laboratories Co, Ltd (Nagoya, Japan), and Dr Nishimura and Dr Hirata from Kumamoto University (Kumamoto, Japan). This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and the Japan Leukemia Research Fund.
Disclosure
The authors declare no potential conflicts of interest relevant to this article.
References
- MuthuMSSinghSTargeted nanomedicines: effective treatment modalities for cancer, AIDS and brain disordersNanomedicine (Lond)20094110511819093899
- AlbertMLSauterBBhardwajNDendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLsNature1998392667186899510252
- KawakamiYFujitaTKudoCDendritic cell based personalized immunotherapy based on cancer antigen researchFront Biosci2008131952195817981682
- KozakoTNew strategy of adult T-cell leukemia treatment targeted for anti-tumor immunity and a longevity gene-encoded proteinYakugaku Zasshi201113171061107221720136
- YasunagaJSakaiTNosakaKImpaired production of naive T lymphocytes in human T-cell leukemia virus type I-infected individuals: its implications in the immunodeficient stateBlood200197103177318311342446
- HarashimaNKuriharaKUtsunomiyaAGraft-versus-Tax response in adult T-cell leukemia patients after hematopoietic stem cell transplantationCancer Res200464139139914729650
- IkeharaYShiuchiNKabata-IkeharaSEffective induction of anti-tumor immune responses with oligomannose-coated liposome targeting to intraperitoneal phagocytic cellsCancer Lett20082601–213714518077084
- MatsuiMShimizuYKoderaYKondoEIkeharaYNakanishiHTargeted delivery of oligomannose-coated liposome to the omental micrometastasis by peritoneal macrophages from patients with gastric cancerCancer Sci201010171670167720507320
- TakagiHFuruyaNKojimaNPreferential production of IL-12 by peritoneal macrophages activated by liposomes prepared from neoglycolipids containing oligomannose residuesCytokine200740324125018060800
- KozakoTHirataSShimizuYOligomannose-coated liposomes efficiently induce human T-cell leukemia virus-1-specific cytotoxic T lymphocytes without adjuvantFEBS J201127881358136621332943
- GrgacicEVAndersonDAVirus-like particles: passport to immune recognitionMethods2006401606516997714
- ZhangSCubasRLiMChenCYaoQVirus-like particle vaccine activates conventional B2 cells and promotes B cell differentiation to IgG2a producing plasma cellsMol Immunol200946101988200119376580
- CuiZHuangLLiposome-polycation-DNA (LPD) particle as a carrier and adjuvant for protein-based vaccines: therapeutic effect against cervical cancerCancer Immunol Immunother200554121180119015846491
- WhitmoreMMLiSFaloLJrHuangLSystemic administration of LPD prepared with CpG oligonucleotides inhibits the growth of established pulmonary metastases by stimulating innate and acquired antitumor immune responsesCancer Immunol Immunother2001501050351411776372
- MalyalaPO’HaganDTSinghMEnhancing the therapeutic efficacy of CpG oligonucleotides using biodegradable microparticlesAdv Drug Deliv Rev200961321822519168103
- BadieeAJaafariMRSamieiASoroushDKhamesipourACoencapsulation of CpG oligodeoxynucleotides with recombinant Leishmania major stress-inducible protein 1 in liposome enhances immune response and protection against leishmaniasis in immunized BALB/c miceClin Vaccine Immunol200815466867418235040
- LiSHuangLIn vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexesGene Ther1997498919009349425
- YanWChenWHuangLMechanism of adjuvant activity of cationic liposome: phosphorylation of a MAP kinase, ERK and induction of chemokinesMol Immunol200744153672368117521728
- ChenWYanWHuangLA simple but effective cancer vaccine consisting of an antigen and a cationic lipidCancer Immunol Immunother200857451753017724588
- BhowmickSMazumdarTSinhaRAliNComparison of liposome based antigen delivery systems for protection against Leishmania donovaniJ Control Release2010141219920719818373
- IkeharaYNiwaTBiaoLA carbohydrate recognition-based drug delivery and controlled release system using intraperitoneal macrophages as a cellular vehicleCancer Res200666178740874816951190
- KawamuraKKadowakiNSuzukiRDendritic cells that endocytosed antigen-containing IgG-liposomes elicit effective antitumor immunityJ Immunother200629216517416531817
- HiraiMMinematsuHKondoNOieKIgarashiKYamazakiNAccumulation of liposome with Sialyl Lewis X to inflammation and tumor region: application to in vivo bio-imagingBiochem Biophys Res Commun2007353355355817189617
- McGrealEPMartinez-PomaresLGordonSDivergent roles for C-type lectins expressed by cells of the innate immune systemMol Immunol200441111109112115476922
- RobinsonMJSanchoDSlackECLeibundGut-LandmannSReis e SousaCMyeloid C-type lectins in innate immunityNat Immunol20067121258126517110942
- AdamsEWRatnerDMSeebergerPHHacohenNCarbohydrate-mediated targeting of antigen to dendritic cells leads to enhanced presentation of antigen to T cellsChembiochem20089229430318186095
- TurnerMWMannose-binding lectin: the pluripotent molecule of the innate immune systemImmunol Today199617115325408961631
- GeijtenbeekTBTorensmaRvan VlietSJIdentification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responsesCell2000100557558510721994
- StambachNSTaylorMECharacterization of carbohydrate recognition by langerin, a C-type lectin of Langerhans cellsGlycobiology200313540141012626394
- SinghSKStephaniJSchaeferMTargeting glycan modified OVA to murine DC-SIGN transgenic dendritic cells enhances MHC class I and II presentationMol Immunol2009472–316417419818504
- IshiiMKoyamaAIsekiHNarumiHYokoyamaNKojimaNAnti-allergic potential of oligomannose-coated liposome-entrapped Cry j 1 as immunotherapy for Japanese cedar pollinosis in miceInt Immunopharmacol20101091041104620584630
- KojimaNBiaoLNakayamaTIshiiMIkeharaYTsujimuraKOligomannose-coated liposomes as a therapeutic antigen-delivery and an adjuvant vehicle for induction of in vivo tumor immunityJ Control Release20081291263218485512
- NishikawaYZhangHIkeharaYKojimaNXuanXYokoyamaNImmunization with oligomannose-coated liposome-entrapped dense granule protein 7 protects dams and offspring from Neospora caninum infection in miceClin Vaccine Immunol200916679279719357313
- ShimizuYTakagiHNakayamaTIntraperitoneal immunization with oligomannose-coated liposome-entrapped soluble leishmanial antigen induces antigen-specific T-helper type immune response in BALB/c mice through uptake by peritoneal macrophagesParasite Immunol200729522923917430546
- JanewayCAJrMedzhitovRInnate immune recognitionAnnu Rev Immunol20022019721611861602
- KawaiTAkiraSPathogen recognition with Toll-like receptorsCurr Opin Immunol200517433834415950447
- KatoCKajiwaraTNumazakiMTakagiHKojimaNOligomannose-coated liposomes activate ERK via Src kinases and PI3K/Akt in J774 A.1 cellsBiochem Biophys Res Commun2008372489890118538131
- KojimaNKawauchiYIshiiMDevelopment of novel carbohydrate-coated liposome-based vaccinesTrends Glycosci Glycotechnol201123134257271
- MizuuchiMHirohashiYTorigoeTNovel oligomannose liposome-DNA complex DNA vaccination efficiently evokes anti-HPV E6 and E7 CTL responsesExp Mol Pathol201292118519022032938
- IshiiMKojimaNMucosal adjuvant activity of oligomannose-coated liposomes for nasal immunizationGlycoconj J201027111512319816665
- HinumaYNagataKHanaokaMAdult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human seraProc Natl Acad Sci U S A19817810647664807031654
- PoieszBJRuscettiFWGazdarAFBunnPAMinnaJDGalloRCDetection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphomaProc Natl Acad Sci U S A19807712741574196261256
- TsukasakiKHermineOBazarbachiADefinition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meetingJ Clin Oncol200927345345919064971
- TaylorGPMatsuokaMNatural history of adult T-cell leukemia/lymphoma and approaches to therapyOncogene200524396047605716155611
- TsukasakiKUtsunomiyaAFukudaHVCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801J Clin Oncol200725345458546417968021
- YamadaYTomonagaMFukudaHA new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan Clinical Oncology Group Study 9303Br J Haematol2001113237538211380402
- YoshidaMMolecular approach to human leukemia: isolation and characterization of the first human retrovirus HTLV-1 and its impact on tumorigenesis in adult T-cell leukemiaProc Jpn Acad Ser B Phys Biol Sci2010862117130
- YasunagaJMatsuokaMLeukaemogenic mechanism of human T-cell leukaemia virus type IRev Med Virol200717530131117621367
- JacobsonSShidaHMcFarlinDEFauciASKoenigSCirculating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological diseaseNature199034862982452482146511
- BanghamCRHTLV-1 infection: role of CTL efficiencyBlood200811262176217718779398
- KozakoTAkimotoMTojiSTarget epitopes of HTLV-1 recognized by class I MHC-restricted cytotoxic T lymphocytes in patients with myelopathy and spastic paraparesis and infected patients with autoimmune disordersJ Med Virol201183350150921264872
- KozakoTArimaNTojiSReduced frequency, diversity, and function of human T cell leukemia virus type 1-specific CD8+ T cell in adult T cell leukemia patientsJ Immunol200617785718572617015761
- KozakoTYoshimitsuMAkimotoMProgrammed death-1 (PD-1)/PD-1 ligand pathway-mediated immune responses against human T-lymphotropic virus type 1 (HTLV-1) in HTLV-1-associated myelopathy/tropical spastic paraparesis and carriers with autoimmune disordersHum Immunol201172111001100621851845
- KozakoTYoshimitsuMFujiwaraHPD-1/PD-L1 expression in human T-cell leukemia virus type 1 carriers and adult T-cell leukemia/lymphoma patientsLeukemia200923237538218830259
- OhashiTHanabuchiSKatoHPrevention of adult T-cell leukemia-like lymphoproliferative disease in rats by adoptively transferred T cells from a donor immunized with human T-cell leukemia virus type 1 Tax-coding DNA vaccineJ Virol200074209610961611000233
- FukasawaMShimizuYShikataKLiposome oligomannose-coated with neoglycolipid, a new candidate for a safe adjuvant for induction of CD8+ cytotoxic T lymphocytesFEBS Lett199844133533569891969
- SugimotoMOhishiKFukasawaMOligomannose-coated liposomes as an adjuvant for the induction of cell-mediated immunityFEBS Lett19953631–253567729553
- MilichDRMcLachlanAThorntonGBHughesJLAntibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell siteNature198732961395475492443856
- StorniTLechnerFErdmannICritical role for activation of antigen-presenting cells in priming of cytotoxic T cell responses after vaccination with virus-like particlesJ Immunol200216862880288611884458
- KozakoTFukadaKHirataSEfficient induction of human T-cell leukemia virus-1-specific CTL by chimeric particle without adjuvant as a prophylactic for adult T-cell leukemiaMol Immunol2009472–360661319889459