Abstract
Background
Multidrug resistance is the main obstacle to the efficiency of systemic chemotherapy against hematologic malignancy. This study investigated the reversible effect of the copolymer wogonin and daunorubicin coloaded into Fe3O4 magnetic nanoparticles, and the mechanism potentially involved.
Methods
The growth inhibition rate of K562/A02 cells was investigated by MTT assay, and apoptosis of cells and the intracellular daunorubicin concentration were detected by flow cytometry. Distribution of nanoparticles taken up by K562/A02 cells was observed under a transmission electron microscope and demonstrated by Prussian blue staining. The transcription level of MDR1 mRNA and expression of P-glycoprotein were determined by reverse transcriptase polymerase chain reaction and Western blotting assay, respectively.
Results
The reversible effect of daunorubicin-wogonin magnetic nanoparticles was 8.87-fold that of daunorubicin + wogonin and of daunorubicin magnetic nanoparticles. Transmission electron microscopy and Prussian blue staining revealed that the nanoparticles were located in the endosome vesicles of cytoplasm. Also, the apoptosis rate and accumulation of intracellular daunorubicin in the daunorubicin-wogonin magnetic nanoparticle group were significantly higher than that in the daunorubicin, daunorubicin + wogonin, and daunorubicin magnetic nanoparticle groups. Furthermore, transcription of MDR1 mRNA and expression of P-glycoprotein in K562/A02 cells were significantly downregulated in the daunorubicin-wogonin magnetic nanoparticle group compared with the other groups.
Conclusion
These findings suggest that the remarkable effects of the novel daunorubicin-wogonin magnetic nanoparticle formulation on multidrug resistant K562/A02 leukemia cells would be a promising strategy for overcoming multidrug resistance.
Introduction
Multidrug resistance is the major obstacle to the efficiency of chemotherapy in the treatment of leukemia.Citation1 The mechanisms associated with multidrug resistance in cancer have been widely explored, and chemotherapy-induced upregulation of P-glycoprotein is considered the major event in establishing multidrug resistance in cancer cells.Citation2 Much research attention has been focused on the discovery and development of agents that can inhibit P-glycoprotein with high efficiency and low toxicity.Citation3–Citation5 However, these compounds, with their low efficiency and/or high toxicity, are often nonspecific.Citation6,Citation7 The first and second generations of P-glycoprotein inhibitors have now been tested in clinic trials, but their therapeutic effects and safety profiles have not been ideal.Citation6,Citation7 Successful management of cancers with overexpressed P-glycoprotein would be greatly aided by novel agents with high efficiency and/or low toxicity. Wogonin (5,7-dihydroxy-8-methoxy flavone) is a flavone originating from the roots of Scutellaria baicalensis Georgi. One study has shown that wogonin 10–30 μmol/L acted as an inhibitor of P-glycoprotein and consequently increased the cellular content of chemotherapeutic agents in multidrug resistant cancer cells.Citation8 On the other hand, wogonin can inhibit apoptosis induced by chemotherapeutic agents in normal cells, such as thymocytes.Citation9
However, sequential or concurrent administration of a chemosensitizer and a cytotoxic drug or a combination of drugs cannot guarantee the co-action of intended drugs in the same cancer cells because of their different pharmacokinetics and tissue disposition. It is exciting that magnetic nanoparticles, with their biodegradable nature, biocompatibility, and low toxicity, possess the capability to encapsulate a single drug or multiple drugs with a variety of properties, ranging from highly water-soluble to poorly water-soluble.Citation10–Citation13 In addition, its passive targeting properties may reduce side effects during chemotherapy,Citation14 rendering it a promising drug delivery system.
Therefore, in this study, to overcome the dose-limiting side effects of conventional chemotherapeutic agents, as well as to reduce the risk of therapeutic failure as a result of multidrug resistance, we undertook a rational design of biocompatible magnetic nanoparticles for sustained delivery of wogonin and daunorubicin and also investigated the potential mechanisms involved.
Materials and methods
Chemicals
Daunorubicin hydrochloride (Huifengda Chemical Co, Jinan, China), wogonin (Key Laboratory of Carcinogenesis and Intervention, Jiangsu Province, China Pharmaceutical University, China), RPMI 1640 medium (Gibco/BRL, Gaithersburg, MD), newborn bovine serum (Sijiqing, Hangzhou, China), adriamycin (Hisun Phamaceutical Co, Zhejiang, China), TRIzol® (Invitrogen, Carlsbad, CA) were used. A reverse transcriptase polymerase chain reaction (RT-PCR) kit was purchased from Takara Biotechnology (Dalian, China). Monoclonal antibodies of P-glycoprotein and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA). All other chemicals were of analytic grade.
Preparation of drug-loaded Fe3O4 magnetic nanoparticles
Wogonin was dissolved in absolute dimethyl sulfoxide to give a 1 mol/L solution, and the final concentration of dimethyl sulfoxide in the medium of wogonin-treated cells was less than 0.05% (v/v), showing no toxicity in K562/A02 cells.Citation15 Magnetic nanoparticles were produced using the electrochemical deposition methodCitation13 for use in the present experiment in order to obtain a colloidal suspension of magnetic nanoparticles.
In total, 10 μmol of magnetic nanoparticles were well distributed by ultrasound in 10 mL of RPMI 1640 medium containing 10% (v/v) inactivated newborn bovine serum.Citation16 Daunorubicin was dissolved in RPMI 1640 medium containing 10% (v/v) inactivated newborn bovine serum to obtain a 1 mmol/L solution. Various concentrations of daunorubicin and wogonin 20 μmol/L were added into the aqueous dispersion of magnetic nanoparticles at a molar ratio of 1/2/4/8/16/32/64:20:100. The mixture was kept below 4°C for 48 hours to enable the drugs to conjugate with the magnetic nanoparticles by mechanical absorption polymerization.Citation10,Citation13
Cell culture
K562, a human chronic myeloid leukemia blast crisis cell line, and K562/A02, a K562 cell line resistant to adriamycin, were cultured in RPMI 1640 medium supplemented with 10% newborn bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2, and passaged every two days. The resistant cell line was incubated in the presence of adriamycin 1 μg/mL until at least 3 days before starting the experiments.
Cytotoxicity assay
In vitro cytotoxicity was measured using the MTT assay. K562/A02 cells were seeded in 96-well plates at a density of 2 × 103 cells/well per 0.1 mL of medium, and incubated with daunorubicin 1–64 μmol/L, Fe3O4 magnetic nanoparticles 0.1–0.5 (v/v), wogonin 10–50 μmol/L, daunorubicin 1–64 μmol/L + wogonin 20 μmol/L, daunorubicin magnetic nanoparticles at a molar ratio of 1/2/4/8/16/32/64:100, or daunorubicin-wogonin magnetic nanoparticles at a molar ratio of 1/2/4/8/16/32/64:20:100 at 37°C for 24, 48, and 72 hours. Meanwhile, RPMI 1640 medium was used as the blank control. After incubation, 20 μL of MTT 0.5 mg/mL were added to each well and cultured for 4 hours at 37°C. Thereafter, 150 μL of dimethyl sulfoxide were added to each well to dissolve the formazan crystals using an automated shaker to stir the cells slightly. Absorbance of the suspension was measured by optical density (OD) at a wave length of 490 nm. The cell inhibition ratio (%) was calculated as (1 − OD treated group/OD control group) × 100. The IC50 was defined as the concentration required for 50% inhibition of cell growth.
Qualitative and quantitative evaluation of Fe3O4 magnetic nanoparticle uptake in cells
Transmission electron microscopy
K562/A02 cell suspensions were seeded at a density of 5 × 105 cells/well in 6-well plates and incubated with 10% (v/v) magnetic nanoparticles for 48 hours at 37°C. After incubation, the cells were collected and fixed for 4 hours at 4°C in 2.5% glutaraldehyde, and fully washed in 0.1 mol/L phosphate-buffered saline three times. The cells were then post-fixed at 4°C in 2% osmium tetroxide for 2 hours, and dehydrated in a graded series of ethanols and embedded in epoxy resin. Thereafter, the embedded cells were cut into ultrathin sections (75 nm) and stained with uranyl acetate and lead citrate. Finally, the sections were viewed by transmission electron microscopy (JEM-2100, JEOL, Tokyo, Japan).
Optical microscopy
After incubating with 0.1 (v/v) magnetic nanoparticles for 48 hours at 37°C, the cells were harvested and made into cell smears. Before observation by optical microscopy, the cells were stained with Prussian blue. The cells on the slide were continuously incubated for 30 minutes in 2% potassium ferrocyanide and 6% hydrochloric acid, and then counterstained with nuclear fast red for 3 minutes. The smears were viewed under an optical microscope (B × 41M, Olympus, Tokyo, Japan) at 1000 × amplification.
Apoptosis assay by flow cytometry
After incubation with different drugs, the cells were collected at 48 hours, washed twice with phosphate-buffered saline, and suspended in 200 μL of binding buffer and 10 μL of Annexin V-FITC for 20 minutes in the dark. Analyses were done using a FACSCalibur® flow cytometer (Becton Dickinson, San Antonio, TX) with Cell Quest™ software.
Cellular accumulation of daunorubicin
Cellular accumulation of daunorubicin was analyzed by flow cytometry. In brief, after incubation with different drugs for 48 hours, the cells were collected and washed with 0.1 mol/L phosphate-buffered saline three times. Thereafter, 500 μL of phosphate-buffered saline was added to each sample to resuspend the cells. The cellular accumulation of daunorubicin in each sample was determined using the flow cytometer at a wave length of 488 nm.
RT-PCR assay
The RT-PCR method was used to evaluate the qualitative efficacy of MDR1 with drugs as previously described at the transcription level. After incubation, the cells were lysed and total RNA was extracted with TRIzol. 4 μg of total RNA was added to reverse transcriptase buffer, 25 mmol/L of MgCl2, 10 mmol/L deoxyribonucleotide triphosphates, random 9 mers (50 pmol/μL), RNase inhibitor (40 U/μL), and avian myeloblastosis virus reverse transcriptase (5 U/μL) to provide a final total volume of 25 μL. To obtain cDNA, the conditions of reverse transcriptase were 42°C for one hour, 85°C for 5 minutes, and then 5°C for 5 minutes. The designed PCR primers included MDR1 primer (sense primer 5′-AACGGAAGCCAGAACATTCC- 3′, antisense primer 5′-AGGCTTCCTGTGGCAAAGAG-3′) and β-actin primer (sense primer 5′-GCTCGTCGT CGACAACG GCTC-3′, antisense primer 5′-CAAACATGATCTGGGT CATCTTCTC-3′). The amplified PCR products were 353 base pairs for β-actin and 180 base pairs for MDR1. The newly synthesized cDNA was amplified by PCR, each cycle comprising denaturation at 95°C for 40 seconds, annealing at 52°C for 30 seconds, and elongation at 72°C for 35 seconds. Predenaturing was performed at 95°C for 2 minutes and final extension at 72°C for 10 minutes. RT-PCR products were analyzed by ScnImage software (Scion Corporation, Frederick, MD) with ethidium bromide-stained 1.5% agarose gels.
Western blotting assay
After treatment as before, total protein was isolated on ice and subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis using a modified radioimmunoprecipitation assay buffer, and then transferred to a polyvinylidene difluoride membrane (65421, Pall Corporation, Port Washington, NY). Nonspecific binding sites were blocked with 5% nonfat milk for one hour at room temperature. The blots were stained with mouse monoclonal antihuman P-glycoprotein (1:200) or β-actin (1:400) antibodies overnight at 4°C, and then followed by horseradish peroxidase-labeled rabbit-mouse immunoglobulin G (1:5000) as a secondary antibody. The blots were visualized by enhanced chemiluminescence (ECL system, Amersham, UK), and β-actin was used as the internal control.
Statistical analysis
All data were expressed as the mean ± standard deviation, and analyzed using SPSS software (version 18.0, SPSS Inc, Chicago, IL). Differences between the groups were evaluated using one-way analysis of variance. A value of P < 0.05 was considered to be statistically significant.
Results
Nanoparticles located in the endosome vesicles of the cytoplasm were observed by transmission electron microscopy, and nearly 100% of the cells were labeled with Prussian blue stain after incubation for 48 hours. The diameter of a single blank magnetic nanoparticle was 16.72 ± 1.37 nm.
Cell survival
As the results of the MTT assay show, daunorubicin, a combination of daunorubicin + wogonin, daunorubicin magnetic nanoparticles, and daunorubicin-wogonin magnetic nanoparticles showed antiproliferative activity against drug-resistant cells in a dose and time-dependent manner (). Meanwhile, 0.1–0.4 (v/v) magnetic nanoparticles alone and 0–40 μmol/L wogonin alone did not have a significant influence on cell proliferation (survival faction > 90%,Citation10 ), and neither 20 μmol/L wogonin nor 0.1 (v:v) magnetic nanoparticles induced significant cell inhibition at 24, 48, and 72 hours (). After incubation for 48 hours, the reversible effect of daunorubicin-wogonin magnetic nanoparticles (IC50 daunorubicin/IC50 daunorubicin-wogonin magnetic nanoparticles) was 8.87-fold, which was higher than that of daunorubicin + wogonin (IC50 daunorubicin/IC50 daunorubicin + wogonin, 4.85-fold) and daunorubicin-magnetic nanoparticles (IC50 daunorubicin/IC50 daunorubicin-magnetic nanoparticles, 3.9-fold). The difference was statistically significant (P < 0.05).
Apoptosis assay by flow cytometry
After incubation for 48 hours, the apoptotic rates of K562/A02 cells treated with the control, daunorubicin, magnetic nanoparticles, wogonin, daunorubicin + wogonin, daunorubicin magnetic nanoparticles, and daunorubicin-wogonin magnetic nanoparticles were 7.80% ± 0.36%, 9.08% ± 0.33%, 8.23% ± 0.47%, 8.71% ± 0.54%, 33.65% ± 1.96%, 28.47% ± 2.28%, and 41.04% ± 2.63%, respectively. Compared with the control group, apoptotic rates in the daunorubicin + wogonin, daunorubicin magnetic nanoparticle, and daunorubicin-wogonin magnetic nanoparticle groups were significantly increased (P < 0.05). Although the combination of daunorubicin + wogonin induced significant apoptosis, daunorubicin- wogonin magnetic nanoparticles showed much higher induction of apoptosis (P < 0.05, ).
Cellular accumulation of daunorubicin
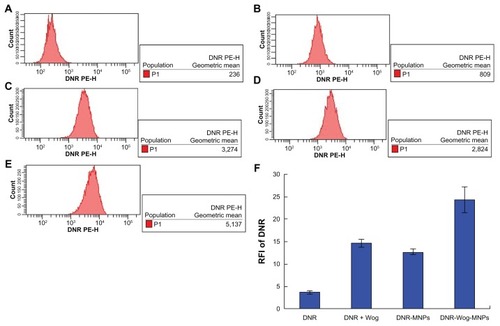
The relative fluorescence intensity (fluorescence intensity-treated group/fluorescence intensity control group) was 3.74 ± 0.34 for K562/A02 cells incubated with 2 μmol/L of daunorubicin for 48 hours, 14.71 ± 0.84 for daunorubicin + wogonin, 12.71 ± 0.65 for daunorubicin magnetic nanoparticles, and 24.31 ± 2.82 for daunorubicin-wogonin magnetic nanoparticles. The daunorubicin + wogonin, daunorubicin magnetic nanoparticle, and daunorubicin-wogonin magnetic nanoparticle groups showed higher daunorubicin accumulation than did the daunorubicin alone group (P < 0.05). Notably, daunorubicin-wogonin magnetic nanoparticles led to an increase in intracellular daunorubicin concentration compared with the daunorubicin + wogonin and daunorubicin magnetic nanoparticle groups (P < 0.05).
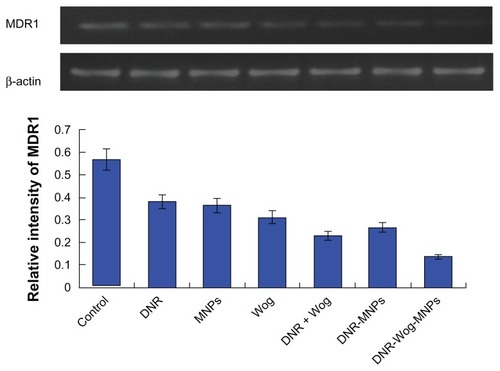
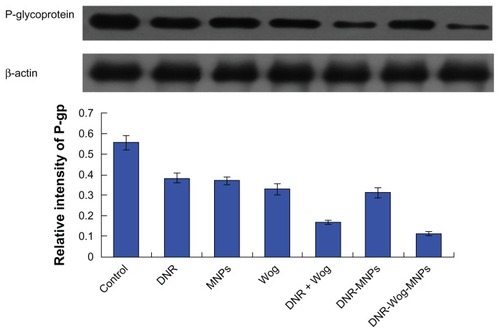
Expression of MDR1/P-glycoprotein in K562/A02 cells
MDR1 mRNA was not detected in drug-sensitive K562 cells (data not shown), and was overexpressed in drug-resistant K562/A02 cell lines. Both daunorubicin and wogonin groups can downregulate transcription of MDR1 mRNA to some extent, and both were strengthened by the addition of magnetic nanoparticles. Compared with the control group, MDR1 mRNA transcriptions were significantly inhibited by nearly 44.85% ± 3.89% in the wogonin group, by 59.13% ± 3.48% in the daunorubicin + wogonin group, and by 75.80% ± 4.32% in the daunorubicin-wogonin magnetic nanoparticle group (P < 0.05, ). The expression of P-glycoprotein were significantly downregulated by nearly 40.62% ± 2.57% in the wogonin group, by 69.93% ± 4.63% in the daunorubicin + wogonin group, and by 79.51% ± 4.48% in the daunorubicin-wogonin magnetic nanoparticle group, respectively, when compared with the control group (P < 0.05, ).
Discussion
A novel copolymer containing a nanoparticle, chemotherapeutic agent, and chemosensitizer with high efficiency and high potency has been devised in this work. Although there have been reports of sequential or concurrent administration of cytotoxic drugs and P-glycoprotein inhibitors, none could guarantee that the two drugs will have activity in the same cancer cells due to their different pharmacokinetics and tissue disposition. Since over expression of P-glycoprotein is the main cause of multidrug resistance in leukemia systemic chemotherapy,Citation2 we choose this combination.
Daunorubicin is an anthracycline and a substrate for P-glycoprotein,Citation17 and the current standard of care for induction chemotherapy of acute myeloid leukemia includes 3 days of daunorubicin.Citation18 Although intensification of the dose of daunorubicin (90 mg/m2) improves the complete remission rate in acute myeloid leukemia,Citation19,Citation20 it will result in hematologic toxicity or adverse events.Citation18 A low dose exposes some populations of tumor cells to sublethal doses of the chemotherapeutic agent used, resulting in onset of the multidrug-resistant phenotype.Citation21 If one agent having the same or higher cytotoxicity can lower the dose of drug we utilize nowadays, it might be a promising solution for multidrug resistance. The copolymer in our research can lower the dose of daunorubicin used by 8.87-fold and owned higher fluorescence of daunorubicin compared with daunorubicin used alone. Even daunorubicin loaded with magnetic nanoparticles can lower the dose of daunorubicin needed by 3.9-fold, its reversal power was significantly lower than that of daunorubicin-wogonin magnetic nanoparticles.
Wogonin, a flavone originating from the root of a Chinese herb, Scutellaria baicalensis Georgi, can impair the function of P-glycoprotein and increase the cellular content of the chemotherapeutic agent entering multidrug-resistant cells.Citation8 In addition, the inhibitory potency of wogonin was nearly equal to that of the first-generation P-glycoprotein inhibitor,Citation3 verapamil.Citation8 We had taken the lead to investigate the influence of wogonin, and the results show that it did downregulate transcription of MDR1 mRNA and expression of P-glycoprotein in K562/A02 cells. Because this flavone does not generate significant cytotoxicity, increase apoptosis in multidrug-resistant cancer cells, or decrease induction of apoptosis in normal cells,Citation9 wogonin could be an ideal P-glycoprotein inhibitor with high efficiency and low toxicity.
Magnetic nanoparticles, a promising biocompatible material, have the features of satisfactory water solubilization, biocompatibility, and easy functionalization.Citation10 A previous study in our laboratory showed that magnetic nanoparticles could improve the sensitivity of anticancer drugs and increase their effectiveness.Citation12,Citation13 We also found that magnetic nanoparticles have the capability to load single or multiple drugs with a variety of properties.Citation11,Citation12 The hydrodynamic diameter of ideal nanoparticles ranges from 10 nm to 100 nm, as reported. Citation22 If the diameter is less than 10 nm, most of them will undergo extravasation in tissue and be cleared by the kidney.Citation23 However, if the diameter exceeds 100 nm, the nanoparticles will soon be eliminated from the circulation by the reticuloendothelial system.Citation23 In the present study, the mean size of the blank magnetic nanoparticles was 16.72 ± 1.37 nm, indicating that citric acid-functionalized magnetic nanoparticles were suitable for biological application and drug delivery. When the concentration of magnetic nanoparticles was 0.1 (v/v), they showed no obvious toxicity () or apoptosis () to K562/A02 cells. Transmission electron microscopy suggested that the magnetic nanoparticles are taken up by the cell via membrane-bound vesicles, and shuttled to the cytosol in K562/A02 cells. The magnetic nanoparticles were stained using potassium ferrocyanide (blue), while the cells were stained using nuclear fast red. There were no blue granules in the controls; on the contrary, nearly 100% of multidrug resistant cells were labeled. Thus, the ability to overcome efflux pumps in the cell membrane and transport an active drug into the cell might be one of the proposed mechanisms that allows magnetic nanoparticles to be a potential strategy to overcome multidrug resistance.
Although inhibitors of P-glycoprotein have been developed as a way to overcome multidrug resistance, rapid drug metabolism may be one of the factors influencing the effect of treatment.Citation24 It should be noticed that there were no significant differences in IC50 of daunorubicin between the daunorubicin + wogonin and daunorubicin-wogonin magnetic nanoparticle groups at 24 hours, but this situation changed at 48 hours and 72 hours, when the IC50 of daunorubicin in the daunorubicin-wogonin magnetic nanoparticle group was obviously lower than that in the daunorubicin + wogonin group. Possible reasons for this phenomenon might be that a large proportion of wogonin in the daunorubicin + wogonin group may have been metabolized to a residue which could inhibit P-glycoprotein further, and there is some evidence in the literature to support this,Citation25 and the sustained-release properties of daunorubicin-wogonin magnetic nanoparticles might enable continuous release of the chemosensitizing agent, instead of rapid metabolism, and much more daunorubicin was released continuously from the copolymer after 24 hours. Also, a previous study in our laboratory showed that drug-loaded magnetic nanoparticles were able to release daunorubicin in a sustained manner for 25 days, and less than 20% of daunorubicin was released from the magnetic nanoparticles in 24 hours.Citation11 This sustained release might lead to an effective dose between two cycles of chemotherapy with maximal killing of malignant cells.
A copolymer with the power to reverse multidrug resistance might enable particles to be taken up by membrane-bound vesicles into cells such that their cargo becomes distal to the cell membrane and is inaccessible to the effects of ABC transporter-mediated drug efflux (), raise the daunorubicin level in multidrug-resistant cells by releasing the copolymer from the vesicles (), and downregulate expression of P-glycoprotein () at the same time. We believe that downregulation of P-glycoprotein and passive targeting of nanoparticles raise the intracellular concentration of daunorubicin, and the copolymer of daunorubicin-wogonin magnetic nanoparticles can induce the cell apoptosis rate to a higher degree as a result. We did not investigate sequential or concurrent administration of separate P-glycoprotein inhibitors and anticancer drug-loaded nanoparticles, but there have been reports demonstrating chemosensitizer coloading with an anticancer drug in the same nanoparticle resulting in higher drug uptake than found with coadministration of nanoparticles loaded with a single agent.Citation26,Citation27
Figure 1 Distribution of nanoparticles in K562/A02 cells incubated with and without 10% (v/v) magnetic nanoparticles for 48 hours.
Notes: (A) Transmission electron micrograph for control, (B) transmission electron micrograph for 10% (v/v) magnetic nanoparticles, red arrows direct nanoparticles in the endosome vesicles, and the upper left and upper right pictures were the magnifications (10×), (C) Prussian blue staining for control, and (D) Prussian blue staining for 10% (v/v) magnetic nanoparticles.
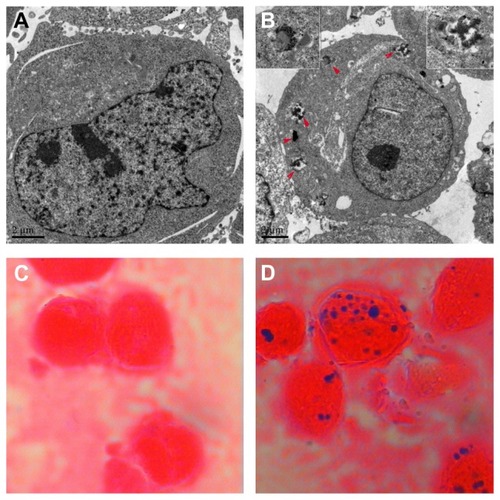
Figure 2 (A) Viability of K562/A02 cells treated with different concentrations of magnetic nanoparticles, (B) viability of K562/A02 cells treated with different concentrations of wogonin, (C) surviving fraction of K562/A02 cells treated with 20 μmol/L wogonin or 0.1 (v/v) magnetic nanoparticles for 24, 48, and 72 hours, (D) inhibition rates of K562/A02 cells treated with different concentrations of daunorubicin with and without magnetic nanoparticles for 48 hours, (E) inhibition rates of K562/A02 cells treated with coadministered daunorubicin + wogonin with and without magnetic nanoparticles for 48 hours, (F) IC50 of daunorubicin treated with daunorubicin, daunorubicin + wogonin, daunorubicin magnetic nanoparticles, and daunorubicin-wogonin magnetic nanoparticles for 24, 48, and 72 hours.
Abbreviations: DNR, daunorubicin; Wog, wogonin; MNPs, magnetic nanoparticles; IC50, inhibitory concentration at 50%.
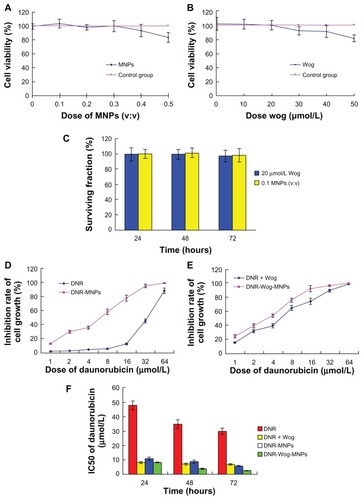
Figure 3 Apoptotic rates of K562/A02 cells incubated for 48 hours by flow cytometry. (A) control, (B) 2 μmol/L daunorubicin, (C) 0.1 (v/v) magnetic nanoparticles, (D) 20 μmol/L wogonin, (E) combination of 2 μmol/L daunorubicin and 20 μmol/L wogonin, (F) 2μmol/L daunorubicin magnetic nanoparticles (at a molar ratio of 2:100), (G) 2 μmol/L daunorubicin-wogonin magnetic nanoparticles (at a molar ratio of 2:20:100).
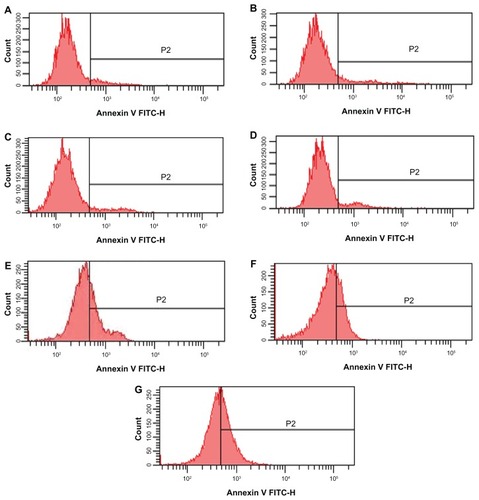
Figure 4 Intracellular accumulation of daunorubicin in K562/A02 cells cultured with different drugs for 48 hours. (A) Control, (B) 2 μmol/L daunorubicin, (C) combination of 2 μmol/L daunorubicin combined with 20 μmol/L Wog, (D) 2 μmol/L daunorubicin magnetic nanoparticles (at a molar ratio of 2:100), (E) 2 μmol/L daunorubicin-Wog magnetic nanoparticles (at a molar ratio of 2:20:100), (F) relative fluorescence intensity of daunorubicin in treated cells.
Abbreviations: DNR, daunorubicin; Wog, wogonin; MNPs, magnetic nanoparticles; RFI, relative fluorescence intensity.
Conclusion
These results indicate that the unique properties of daunorubicin-wogonin magnetic nanoparticles can reverse multidrug resistance in K562/A02 cells, which would be a promising strategy for overcoming multidrug resistance in the future.
Acknowledgments
This work was supported financially by the National Key Basic Research Program 973 of China (2010CB732404), National Natural Science Funds of People’s Republic of China (81170492), and Key Medical Disciplines of Jiangsu Province.
Disclosure
The authors report no conflicts of interest in this work.
References
- ParasrampuriaRMehvarRDivergent effects of nitric oxide donors on the biliary efflux transporters in isolated perfused rat livers: nitric oxide-independent inhibition of ABC transporters by sodium nitroprussideDrug Metab Lett201151647221198435
- LeeCACookJAReynerELSmithDAP-glycoprotein related drug interactions: clinical importance and a consideration of disease statesExpert Opin Drug Metab Toxicol20106560361920397967
- McDevittCACallaghanRHow can we best use structural information on P-glycoprotein to design inhibitors?Pharmacol Ther2007113242944117208306
- BinkhathlanZHamdyDABrocksDRLavasanifarAPharmacokinetics of PSC 833 (valspodar) in its Cremophor EL formulation in the ratXenobiotica2010401556119903013
- LuurtsemaGSchuitRCKlokRPEvaluation of taniquidar as a tracer of P-glycoprotein: radiosynthesis and biodistribution in ratsNucl Med Biol200936664364919647170
- FriedenbergWRTallmanMSBrodskyIModified VAD and PSC-833 in the treatment of resistant or relapsing chronic lymphocytic leukemia (E4996): a trial of the Eastern Cooperative Oncology GroupLeuk Res200428881381915203279
- AbrahamJEdgerlyMWilsonRA Phase I study of the P-glycoprotein antagonist tariquidar in combination with vinorelbineClin Cancer Res200915103574358219417029
- IshiiKTanakaSKagamiKEffects of naturally occurring polymethyoxyflavonoids on cell growth, p-glycoprotein function, cell cycle, and apoptosis of daunorubicin-resistant T lymphoblastoid leukemia cellsCancer Invest201028322022919863351
- EnomotoRSuzukiCKoshibaCWogonin prevents immunosuppressive action but not anti-inflammatory effect induced by glucocorticoidAnn N Y Acad Sci2007109541241717404053
- ChengJWuWChenBAEffect of magnetic nanoparticles of Fe3O4 and 5-bromotetrandrine on reversal of multidrug resistance in K562/A02 leukemic cellsInt J Nanomedicine2009420921619918367
- ChengJWangJChenBA promising strategy for overcoming MDR in tumor by magnetic iron oxide nanoparticles co-loaded with daunorubicin and 5-bromotetrandrinInt J Nanomedicine201162123213122114476
- XiaGChenBDingJEffect of magnetic Fe3O4 nanoparticles with 2-methoxyestradiol on the cell-cycle progression and apoptosis of myelodysplastic syndrome cellsInt J Nanomedicine201161921192721931487
- WangLZhangHChenBEffect of magnetic nanoparticles on apoptosis and cell cycle induced by wogonin in Raji cellsInt J Nanomedicine2012778979822359456
- CollnotEMAliHLehrCMNano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosaJ Control Release1252012 [Epub ahead of print.]
- PhilchenkovAAZavelevichMPMikhailenkoVMKuyavaLMApoptosis and content of mobile lipid domains in human leukemia K-562 cells induced to differentiate by quercetin or dimethyl sulfoxideUkr Biokhim Zh2010822104110 Ukrainian20684251
- JiangZChenBAXiaGHThe reversal effect of magnetic Fe3O4 nanoparticles loaded with cisplatin on SKOV3/DDP ovarian carcinoma cellsInt J Nanomedicine2009410711419516889
- BattistiRFZhongYFangLModifying the sugar moieties of daunorubicin overcomes P-gp-mediated multidrug resistanceMol Pharm20074114015317274671
- EsteyEHAcute myeloid leukemia: 2012 update on diagnosis, risk stratification, and managementAm J Hematol2012871899922180162
- LöwenbergBOssenkoppeleGJvan PuttenWHigh-dose daunorubicin in older patients with acute myeloid leukemiaN Engl J Med2009361131235124819776405
- FernandezHFSunZYaoXAnthracycline dose intensification in acute myeloid leukemiaN Engl J Med2009361131249125919776406
- HueberAEsserJMKociokNMitomycin C induces multidrug resistance in glaucoma surgeryGraefes Arch Clin Exp Ophthalmol2008246229730417934748
- GuptaAKWellsSSurface-modified superparamagnetic nanoparticles for drug delivery: preparation, characterization, and cytotoxicity studiesIEEE Trans Nanobioscience200431667315382647
- ColeAJYangVCDavidAECancer theranostics: the rise of targeted magnetic nanoparticlesTrends Biotechnol201129732333221489647
- HoPCGhoseKSavilleDWanwimolrukSEffect of grapefruit juice on pharmacokinetics and pharmacodynamics of verapamil enantiomers in healthy volunteersEur J Clin Pharmacol2000569–1069369811214778
- PengJQiQYouQSubchronic toxicity and plasma pharmacokinetic studies on wogonin, a natural flavonoid, in Beagle dogsJ Ethnopharmacol2009124225726219397969
- WongHLBendayanRRauthAMWuXYSimultaneous delivery of doxorubicin and GG918 (Elacridar) by new polymer-lipid hybrid nanoparticles (PLN) for enhanced treatment of multidrug-resistant breast cancerJ Control Release2006116327528417097178
- XiongXBMaZLaiRLavasanifarAThe therapeutic response to multifunctional polymeric nano-conjugates in the targeted cellular and subcellular delivery of doxorubicinBiomaterials20103175776819818492