Abstract
The purpose of the study was to develop tumor specific, water dispersible superparamagnetic iron oxide nanoparticles (SPIONs) and evaluate their efficacy as a contrast agent in magnetic resonance imaging (MRI). We have developed SPIONs capped with citric acid/2-bromo-2-methylpropionic acid which are compact, water dispersible, biocompatible having narrow range of size dispersity (8–10 nm), and relatively high T2 relaxivity (R2 = 222L · mmol−1 · sec−l). The targeting efficacy of unconjugated and folic acid-conjugated SPIONs (FA-SPIONS) was evaluated in a folic acid receptor overexpressing and negative tumor cell lines. Folic acid receptor-positive cells incubated with FA-SPIONs showed much higher intracellular iron content without any cytotoxicity. Ultrastructurally, SPIONs were seen as clustered inside the various stages of endocytic pathways without damaging cellular organelles and possible mechanism for their entry is via receptor mediated endocytosis. In vitro MRI studies on tumor cells showed better T2-weighted images in FA-SPIONs. These findings indicate that FA-SPIONs possess high colloidal stability with excellent sensitivity of imaging and can be a useful MRI contrast agent for the detection of cancer.
Introduction
Super paramagnetic iron oxide nanoparticles (SPIONs) have garnered increased attention for their role in biomedical applications such as targeted delivery, localized therapy, stem cell labeling, tracking, and as contrast agents for magnetic resonance imaging (MRI).Citation1–Citation3 Currently, a number of SPIONs are either in early stage clinical trials or in preclinical studies.Citation1,Citation4 Some examples are: Lumiren for bowel imaging,Citation5 Feridex 4® for liver and spleen imaging,Citation6 Combidex for lymph node metastases imaging,Citation7 and most recently, Ferumoxytol for iron replacement therapy.Citation8 The physicochemical profiles of these SPIONs provide passive targeting, but not the higher level targeting offered by bioligands. Attachment of bioactive molecules to the SPION surface can potentially increase the targeting specificity of nanoparticles,Citation4,Citation9,Citation10 producing contrast agents that specifically illuminate targeted tissue and drug carriers that do not interact with healthy tissue.Citation6,Citation10
The co-precipitation method is widely used for the synthesis of iron oxide nanoparticles,Citation11 but nanoparticles obtained by this method have a broad range of size distribution and are also not highly stable.Citation12 Currently, significant work on the development and characterization of SPIONs is underway. The creation of next generation SPIONs (Fe3O4 and γ-Fe2O3) can be obtained by thermal decomposition methods. These SPIONs can be used as molecular imaging probes due to their high crystallinity and monodispersity. However they are only soluble in organic solvents due to the hydrophobic alkyl ligands on their surface.Citation9,Citation13–Citation15 Water solubility and surface functionality are the two critical parameters necessary for interaction with biological systems. This includes surface coating or ligand exchange to make the SPIONs water dispersible. These surface coatings directly affect the biological activities of nanoparticles; in particular, cellular uptake,Citation15 bio-distribution,Citation16 blood circulation,Citation17 and metabolism.Citation18 Depending on the application, the efficacy of nanoparticles can be directly influenced by the surface coatings. All of these surface coatings, besides giving the SPIONs water solubility, also provide functional groups for the attachment of various ligands for targeted delivery or concentrating the resulting complexes to the desired locale. Targeting the SPION contrast agents to specifically accumulate in regions of interest greatly enhances their clinical utility.Citation11,Citation19,Citation20 Other studies have demonstrated various approaches for active targeting of SPIONs including peptides, hormones, aptamers, antibodies, proteins, therapeutics, and nutrients. Antibodies are inherently immunogenic and are also bulky; both properties are believed to inhibit internalization of attached structures.Citation21 The nutrient pathways are attractive since they are directly linked to proliferation and thus in principle will cause increased uptake of the imaging agent and therefore give greater signals for most tumor types. The folic acid receptor (FAR) is overexpressed in a wide variety of human tumors, as folic acid (FA) is a key precursor in DNA base synthesis and is thus required for tumor cell proliferation.Citation22–Citation24
In the present study, we demonstrate that combining more than one ligand, as effective phase transfer agents for SPIONs to transfer them from the organic phase to the water phase, is crucial for their colloidal stability and clinical utility. These coatings act as stabilizers for the magnetite nanoparticles and also provide a functionality to attach biological moiety to target the particles to cells. Additionally, we have also studied the stability of water soluble FA-conjugated SPIONs. We have successfully demonstrated selective binding, effective uptake, and the kinetics of FA-conjugated SPIONs into various tumor cell lines. Furthermore, we have also demonstrated the magnetic resonance (MR) properties of FA-conjugated SPIONs. Our studies were directed towards increasing the concentration of SPIONs into tumor cells by conjugating SPIONs with a targeted ligand, folic acid (FA). Internalization kinetics studies were performed in detail to optimize the maximum uptake of SPIONs into the tumor cells. The main aim of the present study was to design SPIONs, which can be used as a potential candidate for specific and sensitive cancer diagnosis.
Materials and methods
Materials
Iron (3) chloride hexahydrate (FeCl3 · 6H2O), 1-octadecene (ODE), oleic acid (99.5%), folic acid, N-hydroxy succinamide (NHS), 1-ethyl-3-(-3-diethylaminopropyl)-carbodiimide (EDC), Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Sigma Aldrich (St Louis, MO). Sodium hydroxide, methanol (HPLC grade), toluene, potassium ferrocynide, DPX mount, and hydrochloric acid were purchased from Qualigens Fine Chemical (Mumbai, India).
Methods
Synthesis of SPIONs
The nanoparticles were synthesized according to protocol by Jana et alCitation25 ligand exchange of oleic coated SPION with tetramethyl ammonium hydroxide (TMAOH) was done according to protocol by Salgueirino-Maceira et alCitation26 and dimercaptosuccinic acid (DMSA) was done according to Chen et al.Citation27
Preparation of citric acid coated SPION
Ligand exchange of oleic coated SPION with citric acid was done according to the procedures given by Lattuda and HattonCitation28 with slight modification. Oleic acid coated nanoparticles (120 mg) prepared as described above were dispersed in a 1:1 mixture of 1,2-dichlorobenzene and N,N-dimethyl formamide (30 mL of total volume), to which 0.1 g of citric acid was added. The mixture was then stirred at 90°C for ~12 hours. The particles were subsequently precipitated by the addition of ethanol (40 mL) and separated using a magnet. The particles were redispersed in acetone and again separated by means of a magnet 3 to 4 times to remove all traces of free citric acid. The particles were then dried in a vacuum oven for 20 minutes at 80°C.
Preparation of citric acid/2-bromo-2-methylpropionic acid coated SPION (CA/BMPA-SPIONs)
The procedure used here is identical to that described in citric acid coating of magnetic nanoparticles,Citation28 with slight modification, wherein a mixture of 0.050 g of citric acid and 0.4 g of 2-bromo-2-methylpropionic acid was used for the ligand exchange reaction. Bio conjugation of CA/BMPA coated SPION with folic acid was done using the same procedures as You et al.Citation29
Prussian blue staining
To demonstrate Fe in the target cells (MCF-7, HepG2, A549), 5 × 104 cells were seeded on coverslips in twelve well culture plates and grown for 24 hours. Then the cells were incubated with FA-SPION and non-conjugated SPIONs with different concentrations of SPION (25–200 μg/mL). After incubation of 6 hours, the coverslips were removed followed by washing with phosphate buffer saline (PBS) solution and finally fixed with 4% paraformaldehyde for 20 minutes at room temperature. After removing the fixing agent, the cells were washed and stained with Prussian blue iron stain. The SPIONs were labeled and the control cells were incubated with a 1:1 mixture of 4% potassium ferrocyanide and 4% hydrochloric acid for 20 minutes, and washed with distilled water several times. To stain the nuclei the cells were treated with nuclear fast red solution (Polysciences, Inc, Warrington, PA) for 5 minutes and then rinsed in running tap water for 1 minute. After drying the cells, a cover slip was mounted by using a mounting medium (Dako, Carpenteria, CA) and then the cells were observed using light microscopy (Olympus, Fluoview FV1000 Microscope, Japan).
Transmission electron microscopy (TEM) study
MCF-7 cells were grown in twelve well cell culture plates. After 48 hours FA-SPION (100 μg/mL) were added and incubated for different time intervals (1, 3, and 6 hours) and the rest of the procedure was performed according to Zhou et al.Citation30 Finally, samples were viewed under an electron microscope (Morgagni 268; Philips, Amsterdam, Netherlands).
XTT assay
A cytotoxicity test of the SPIONs was performed according to the manufacturer’s protocol (Cayman Chemical Company, Ann Arbor, MI) as follows. A total of 5 × 104 cells were seeded on each well of a 96-well plate and cultured for 24 hours. The cells were treated with different concentrations of SPION’s (62.5, 125, 250, 500, and 1000 μg Fe/mL) in a serum-free medium for 24 hours and 48 hours. After incubation, the medium containing SPION’s was exchanged with a fresh medium, and 10 μL of the reconstitute XTT mixture kit reagent were added to each well. After culturing for 4 hours, the absorbance of the sample was measured by using a microtiter plate reader (Bio-Rad, Hercules, CA) at 450 nm. The viability of the cells was determined as the percentage of viable cells of the untreated control and analyzed in triplicate.
Cellular uptake study
To study the intracellular uptake of surface-modified nanoparticles, 1 × 105 cells were initially plated and grown to 60% confluence in twelve well culture plates. Cells were then washed twice with PBS and cultured in 10 mL of DMEM containing 10% FBS and 1% penicillin/streptomycin for 24 hours. The growth medium was then aspirated and the cells were washed twice with PBS. The effect of surface coating on the uptake of nanoparticles by MCF-7 and HepG2 cells was evaluated by culturing these cells with FA-SPION and unconjugated SPION, each dispersed in DMEM at a concentration range of 10–400 μg Fe/mL for 6 hours. While in time-dependent study, cells were incubated with a fixed concentration of 100 μg Fe/mL for different time intervals ranging from 0.5 to 12 hours. A plate of control cells was prepared in a similar manner without the addition of nanoparticles. At the end of incubation, cells were washed three times with PBS, detached with trypsin-EDTA, and resuspended in 1 mL of PBS containing 5% FBS. Cell concentrations were determined by hemocytometer using trypan blue dye. The specific uptake of FA-SPION conjugates by MCF-7 and HepG2 cells was further examined in a competitive uptake inhibition assay, where FA-SPION was incubated with 10 mM of free folic acid. To evaluate the specificity of cellular targeting of the nanoparticle conjugates, the uptake of FA-SPION conjugates by MCF-7 and HepG2 cells was compared with the uptake by A549 cells that do not overexpress the folate receptor. A549 cells were cultured with different concentrations (10 to 400 μg Fe/mL) and for different time periods (0.5–12 hours) with FA-SPION and unconjugated SPION in a similar manner as with the MCF-7 and HepG2 cells. The cells were then detached and counted as mentioned above. Intracellular uptake of the nanoparticles was quantified by atomic absorption spectroscopy (AAS), where a known number of cells from each sample were analyzed for iron content. AAS samples were prepared by centrifuging down the cells and dissolving the cell pellet in 37% hydrochloric acid at 70°C for 3 hours. The samples were then diluted to a volume of 3 mL for analysis. Intracellular uptake experiments were performed in triplicate with percent error determined by calculating the standard deviation of these measurements.
Magnetic resonance imaging
Preparation of phantom agar gels for imaging
Suspensions of FA-SPION in the concentration range of 0–20 μg/mL of Fe were prepared in PBS. A 2.0% w/v agar solution was prepared by heating 200 mg of agar in 10 mL of PBS at 80°C for 20 minutes. For preparing phantom gels, 160 μL of the above agar solution was mixed thoroughly with vigorous shaking with 840 μL of SPION suspension at each concentration at 60°C to prevent gelation while mixing and poured in 1.5 mL microcentrifuge tubes, followed by the cooling of the these tubes at room temperature.
A total of 5 × 104 cells were seeded on each well of a twelve-well plate and grown for 24 hours. The cells were then incubated with a medium containing different concentrations (40–160 μg/mL) of FA-SPION and unconjugated SPIONs. After 6 hours, the medium was removed and then the cells were washed with a PBS solution to remove free SPION. The labeled cells (FA-SPION and unconjugated SPION) were detached from the well using a trypsin/EDTA solution and harvested by centrifugation at 3000 rpm for 5 minutes. The cells were resuspended in a PBS solution containing 1% paraformaldehyde followed by incubation at 4°C for 2 hours. The cells were subsequently washed with PBS solution and again harvested at 3000 rpm for 3 minutes and finally counted with a hemocytometer. Thereafter, the cells were transferred in 1.5 mL of microcentrifuge tubes at fixed number (~50,000 cells per tube) for each concentration of FA conjugated SPION and unconjugated SPION.
All the MRI experiments were performed using a Bruker-Biospec 4.7 T MRI scanner (BrukerBioSpin Corporation, Billerica, MA) within a 72 mm transmit/receive coil with a homogeneous B1 field to obtain the MR images and the rest of the parameters used in the experiment were similar as used by Jain et al.Citation31
Statistical analysis
Statistical analyses were performed using the Student’s t-test for unpaired data, and P values of 0.05 were considered statistically significant. Data are presented as means ± standard error of the mean.
Results and discussion
Synthesis and characterization of hydrophobic SPIONs
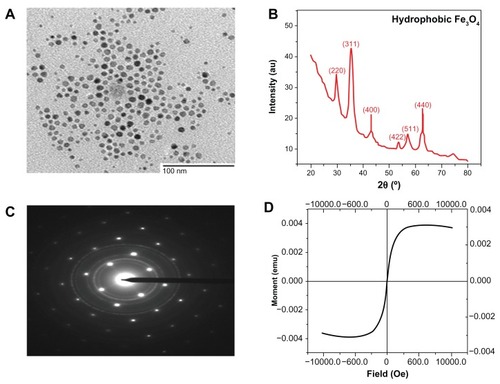
Before one can utilize magnetic nanocrystals for biomedical applications, the utmost requirement is to develop well-defined magnetic nanocrystals. The key requirement for this model system is the fabrication of high-quality magnetic nanocrystals in terms of the size, crystalline phase, and stoichiometry, as these characteristics can affect the properties of SPIONs.Citation13–Citation15 In the present study, we synthesized magnetic nanocrystals through the thermal decomposition method in organic solvent to get high quality nanocrystals. TEM images demonstrated that the monodisperse SPIONs were formed in organic solvent (). The crystal structure information from an assembly of Fe3O4 nanoparticles was also obtained from both X-ray diffraction (XRD) and selected area electron diffraction (). The peaks were labeled with the indexed Bragg reflections of the magnetite structure and the particles were found to be highly crystalline. Using the Debye–Scherrer formula, the average size of the crystallite was determined to be 10 nm, which was in good agreement with the average diameter of 8–10 nm measured from TEM images. These findings indicated that the particles were single crystalline.Citation32 The selected area electron diffraction pattern taken from the area consisting of many particles represented Fe3O4 polycrystal-line diffraction rings, in accordance with the XRD result. The magnetic properties of the SPIONs examined at room temperature by using a superconducting quantum interference device magnetometer indicated that the particles are super paramagnetic in nature. Furthermore, the net magnetization of the particle assemblies in the absence of an external field was zero. No hysteresis was seen when magnetization studies were performed on 10 nm Fe3O4 nanoparticles at room temperature (). Under a large external field, the magnetization of the particles aligned with the field direction and reached its saturation value (saturation magnetization, σs). Fourier-transform infrared (FTIR) spectra of SPION were recorded to confirm the presence of a coating layer consisting of oleic acid on the surface of the nanoparticles (data not shown). The FTIR spectrum of oleic acid showed strong characteristic peaks, assigned to the CH2 asymmetric and symmetric stretching at 2893 cm−1, 2841 cm−1, and scissoring at 1460 cm−1. A peak corresponding to the C=O asymmetric stretching of ester of oleic acid around 1720 cm−1 was observed in iron oxide nanoparticles capped with oleic acid, which confirmed the capping of iron oxide nanoparticles by oleic acid.
Generation and characterization of SPIONs suitable for biomedical applications by ligand exchange
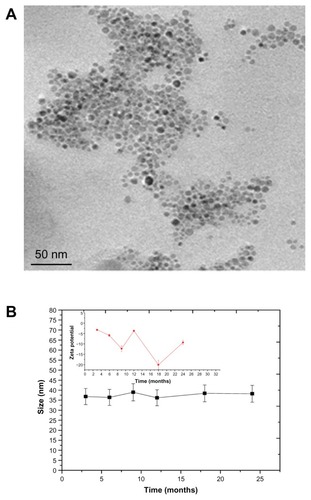
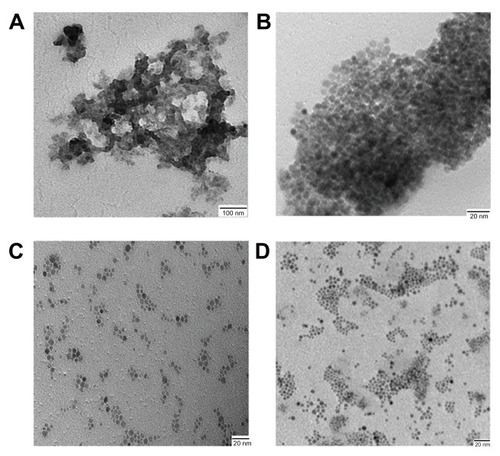
To make the SPION’s suitable for biomedical application, SPIONs are synthesized in aqueous media wherein nanoparticles coated with hydrophilic dextranCitation11 or polyvinyl alcohol.Citation33 But this process leads to large size and is limited in its ability to get uniform and monodispersed nanoparticles. SPIONs synthesized in organic solvent are highly hydrophobic and do not disperse well in water and thus cannot be used for biological application. Hydrophobic SPION’s were subjected to ligand exchanges to produce hydrophilic SPIONs. The ligand exchange process was carried out at different temperatures for different ligands with continuous stirring, wherein hydrophobic ligand was exchanged by hydrophilic ligand. We carried out ligand exchange reactions with four different ligands: TMAOH at 30°C, DMSA at 60°C, citric acid (CA, 90°C), and a combination of CA/2-Bromo2-methyl propionic acid (BMPA, 90°C). TMAOH functionalized SPIONs were found to be aggregated as shown in TEM images (). For DMSA capped SPIONs dispersion was unstable over a period of time as evident from TEM images (). Citric acid functionalized SPION () showed better dispersion stability but over a period of time it settled at the bottom due to aggregation. To enhance the dispersion stability, a combination of CA/BMPA was used. CA/BMPA capped SPIONs showed excellent dispersion stability as shown by TEM images (), which clearly indicated that there was no aggregation of nanoparticles upon transfer from organic to aqueous phase. CA/BMPA capped SPIONs were also characterized by XRD which clearly indicated that there was no alteration in crystal structure after ligand exchange. The FTIR spectra of CA and CA/BMPA showed complete ligand exchange while TMAOH and DMSA still had residual –CH2 peaks indicating incomplete removal of oleic acid from the surface of the SPIONs. This could be the possible cause of aggregation in these two systems. After ligand exchange, it was observed that CA/BMPA-SPIONs were more stable as compared to citric acid capped SPIONs (CA-SPIONs). CA/BMPA-SPIONs were stable up to 250 mM NaCl solution and between pH 6 to 10. However, these SPIONs were aggregated below pH 6.0. Furthermore, CA-SPIONs were found to have stable dispersion in PBS (pH 7.4) up to 4–5 weeks while CA/BMPA-SPIONs were stable for 2 years as confirmed by TEM and dynamic light scattering (). CA/BMPA showed better dispersion stability of SPIONs for a longer period of time. Hence, all further studies were performed using CA/BMPA-SPIONs unless otherwise stated. CA/BMPA-SPIONs were successfully conjugated to FA using EDC/NHS chemistry. Excess FA was removed by thoroughly washing the sample with PBS (pH 7.4). Attenuated total reflectance-FTIR spectroscopy was performed to confirm the conjugation of FA with SPIONs. The results demonstrated additional peaks at 1645 cm−1 and 1566 cm−1 in FA-conjugated SPIONs but the additional peaks were absent in unconjugated SPIONs, which indicate the –C=O stretch in FA-SPION due to –COOH group (data not shown). The smaller size of the ligands in our nanocrystals is extremely advantageous in physiological conditions. Retaining the small probe size has been shown to be critical for successful in vivo applications since large-sized probes significantly reduce their bio-stability, diffusion, and circulation processes.Citation14 Large size probes were shown to increase undesired nonspecific binding and reticuloendothelial system uptake.Citation34 Moreover, small sized probes can be potentially useful for enhanced biological targeting efficiency and specificity.Citation35 Since the folate-receptor (FAR) is over expressed in multiple tumor types,Citation22–Citation24 specific targeting of the nanoparticles with FA would be potentially useful for biological functions.
Figure 2 TEM micrograph of hydrophilic SPION coated with different ligands (A) TMAOH (B) DMSA (C) CA (D) CA/BMPA.
Abbreviations: TEM, transmission electron microscopy; SPION, superparamagnetic iron oxide nanoparticles; TMAOH, tetramethyl ammonium hydroxide; DMSA, dimercaptosuccinic acid; CA, citric acid; BMPA, 2-Bromo2-methyl propionic acid.
Uptake of FA-conjugated SPIONs in FAR overexpressing tumor cells
Prussian blue staining
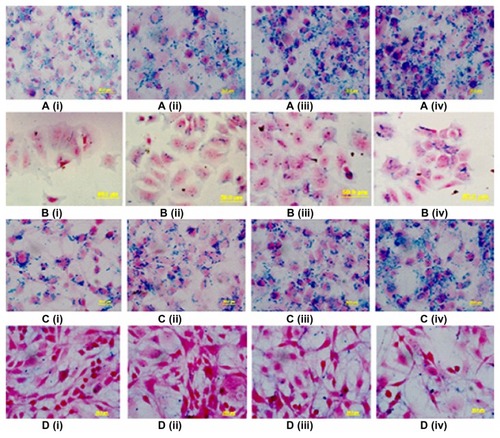
Earlier studies have shown that FAR is overexpressed in multiple tumor types.Citation22–Citation24 We generated SPIONs that were conjugated with FA for tumor specific uptake and characterized them for appropriate biomedical applications. To demonstrate uptake of FA-conjugated SPIONs by FAR-positive tumor cells, further experiments were performed in hormone-dependent breast carcinoma cells, MCF-7 cells, and hepato-carcinoma cells HepG2. As a control, uptake of FA-conjugated SPIONs was also studied in FAR-negative cell lines, such as non-small cell lung carcinoma, A549. The results demonstrated that there was a clear increase of intracellular iron, as visualized by blue granules in Prussian blue staining of MCF-7 cells (). Similar results were obtained when the HepG2 cell line was used (). Additionally, we also compared the uptake of FA-conjugated verses unconjugated SPIONs in MCF-7 and HepG2 cells. In this regard, by contrast to unconjugated SPIONs, the uptake of FA-conjugated SPIONs by MCF-7 and HepG2 cells was significantly increased (). Histological analysis of MCF-7 and HepG2 cells demonstrated significant reduction in blue granules by cells treated with unconjugated SPIONs. While the increase in intracellular unconjugated SPION granules was somewhat visible, however, the staining was considerably less intense as compared to that with the FA-conjugated SPIONs. Furthermore, substantially less staining was observed when FAR-negative A549 cells were treated with FA-conjugated SPIONs (data not shown). Taken together, these findings indicated that conjugating the SPIONs with a tumor targeting agent, FA specifically, increased targeting and internalization of SPIONs in tumor cells.
Figure 3 Prussian blue histochemical reaction of MCF-7 and HepG2 cells following incubation with different concentrations (25–200 μg/mL) of FA-SPION and SPION. A(i–iv) MCF-7 with different concentrations of FA-SPION B(i–iv) MCF-7 with different concentrations of unconjugated SPION. C(i–iv) HepG2 with different concentrations of FA-SPION D(i–iv) HepG2 with different concentrations of unconjugated SPION.
Abbrevations: MCF-7, hormone dependent breast carcinoma cells; HepG2, Hepato-carcinoma cells; FA-SPIONs, folic acid conjugated superparmagnetic iron oxide nanoparticles; SPION, superparamagnetic iron oxide nanoparticles.
TEM studies
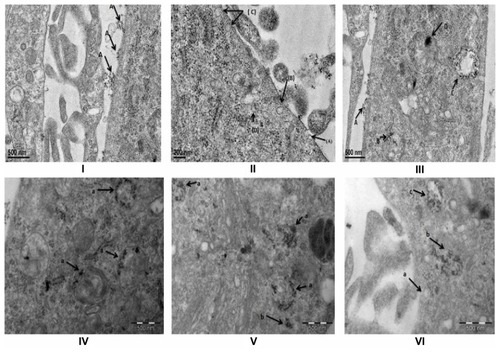
FA-specific tumor targeting and internalization of FA-conjugated SPIONs was further confirmed using TEM analysis. Earlier studies have indicated that the uptake of nanoparticles is mediated via endocytosis.Citation36 Endocytosis is considered to be the prime mechanism for the uptake of <150 nm sized extracellular materials. Moreover, clathrin coated pits, the prime plasma membrane components, are shown to be involved in the uptake of a wide variety of molecules by receptor-mediated endocytosis.Citation37,Citation38 To define internalization and cellular trafficking of SPIONs, MCF-7 cells were treated with FA-conjugated SPIONs and followed for different time intervals and analyzed by TEM. FA-conjugated SPIONs were initially seen on the MCF-7 cell membranes demonstrating interactions of FA with its receptors before internalization into the cells (). At different time points, internalized FA-SPIONs were localized throughout the endocytic-degradative pathways (ie, endocytic vesicles, endosomal compartment, and lysosome). Endosomes appeared as electron lucent multivescicular bodies with varying numbers of internal vesicles, probably representing the transition from early to late endosome (). We also observed numerous enlarged endosomes in which FA-conjugated SPIONs were accumulated. Moreover, FA-conjugated SPIONs were also seen in the cytosol. This study also indicated that other cellular organelles did not show any structural abnormality by accumulation of SPIONs. Taken together, the results indicated that the internalization of nanoparticles is dependent on the nature of the nanoparticle, incubation time, and the targeting molecule. These findings further indicate that the possible mechanism of uptake of FA-conjugated SPIONs, at least in part, is via receptor-mediated endocytosis. However, further in-depth studies are needed to clearly define the mechanism of internalization of FA-conjugated SPIONs in tumor cells.
Figure 4 TEM Studies of interaction of FA-SPION with MCF-7 cells. (I) (A) Attachment of FA-SPION on the cell membrane of MCF-7. (II) (A) Pit formation started (B) Clathrin Pit almost completed (C) multiple pits on the membrane (D) SPION inside the lysosome and start of coalescing of multiple vesicles. (III) (A) SPION in the pit (B) SPION inside the lysosome (C) Multi vesicular body (MVB) filled with SPION (D) aggregated SPION inside the mature lysosomes. (IV) (a) SPIONs inside the different stages of lysosomes. (V) (a) Aggregation of SPION in lysosomes (b) SPION in the cytoplasm (outside lysosomes). (VI) (a) clathrin pit (b) ruptured MVB’s with SPIONS (possible entry of SPIONs to cytoplasm) (c) Multi Vesicular Body (MVB’s).
Abbreviations: MCF-7, hormone dependent breast carcinoma cells; FA-SPIONs, Folic acid conjugated superparamagnetic iron oxide nanoparticles; MVB’s, multi vesicular bodies; FA-SPIONs, folic acid conjugated superparmagnetic iron oxide nanoparticles; SPION, superparamagnetic iron oxide nanoparticles.
XTT assays
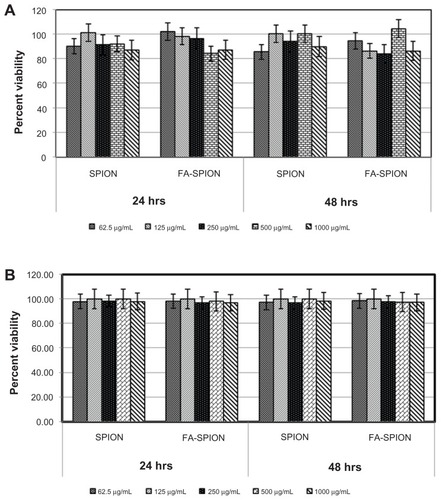
To assess any potential cytotoxicity of these FA-conjugated SPIONs on cells, further studies were performed using MCF-7 and HepG2 cells. These cell lines were treated with different concentrations of FA-SPIONs for 24 or 48 hours and analyzed by XTT assays for cell viability. The results demonstrated no toxicity of FA-conjugated SPIONs, even at the highest concentration tested ().
Concentration dependent cellular uptake studies
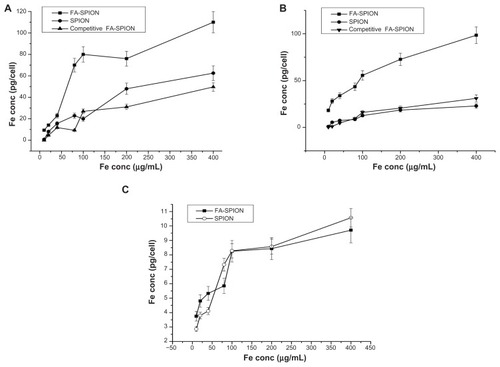
Earlier studies have shown that iron oxide nanoparticles biodegrade in lysosomes over a period of time.Citation18 In this context, the FA-conjugated SPIONs described in the present study could be useful in multiple biomedical applications. To further define the practical implications of FA-conjugated SPION-cell interaction, we next studied the potency of cellular uptake of these nanoparticles. The detailed cellular uptake studies were conducted as a function of concentration and time. These cellular iron quantification studies were performed using FAR-positive (MCF-7, HepG2) and (A549) tumor cell lines. Investigations were also carried out to define the role of FA as a targeting agent and the surface coating efficacy on internalization of SPIONs. The results demonstrated that the Fe content per cell, as determined by AAS, significantly varied among different cell types (). We found an accumulation of iron oxide in MCF-7 cells with the mean iron content ranges from 9.37 + 0.8 to 110 + 9.9 pg Fe/cell (). HepG2 cells also showed an intense uptake of FA-SPION, reaching an intracellular iron oxide concentration range of 18.18 + 1.6 to 98.48 + 6.81 pg Fe/cell (). Moreover, in concert with the results shown above, the uptake of FA-conjugated SPIONs is significantly higher than that of non-conjugated SPIONs. The accumulated iron oxide concentration range for MCF-7 and HepG2 cells treated with non-conjugated SPIONs was 0.83 + 0.9 to 62.5 + 6.9 pg Fe/cell and 0.91 + 1.01 to 22.85 + 2.5 pg Fe/cell respectively. In comparison, MCF-7 and HepG2 cell uptake of FA-conjugated SPIONs was 2–15 times higher than that obtained with non-conjugated SPIONs at different concentrations. To further define the targeting specificity of FA-SPIONs, uptake studies of FA-SPIONs were compared between FAR-positive and FAR-negative tumor cell lines. The results demonstrated that uptake of FA-SPIONs by HepG2 or MCF-7 cells was 10–12 times more than that compared with A549 (). The increased uptake by A549 cells at higher concentrations of nanoparticles is likely due to increased availability of the nanoparticles and internalization through nonspecific mechanisms. In this regard, the results from our present study are in agreement with the previously published findings regarding the uptake of non-conjugated SPIONs at very high concentrations.Citation39–Citation42
Figure 5 Intracellular Fe concentration in MCF-7, HepG2 and A549. Iron concentration in MCF-7 (A), HepG2 (B) FAR positive and A549 (C) FAR negative cells that were treated with different concentrations ranging from 10 μg/mL–400 μg/mL of FA-SPION, SPION and competitive inhibition by FA (10 mM) of competitive FA-SPION for 6 hrs.
Abbreviations: FAR, folic acid receptor;FA, folic acid; MCF-7, hormone dependent breast carcinoma cells; HepG2, Hepato-carcinoma cells; A549, non-small cell lung carcinoma cells;FA-SPIONs, folic acid conjugated superparmagnetic iron oxide nanoparticles; SPION, superparamagnetic iron oxide nanoparticles.
To further assess the competitive and specific uptake of FA-conjugated CA/BMPA-SPIONs, we treated MCF-7 or HepG2 cells with FA-SPIONs in the presence or absence of free FA in molar excess. The results demonstrate that the uptake of FA-SPIONs by MCF-7 cells was significantly decreased when the cells were grown in the presence of an excess amount of free FA (). Similar results were obtained in HepG2 cells (). These findings strongly demonstrated that free FA competed with the FA-SPIONs for uptake by the cells and thereby confirmed that FA-SPIONs were specifically targeted at folate receptors.
Time dependent cellular uptake studies
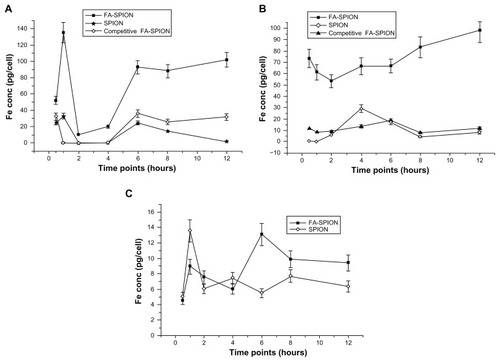
Additional studies were carried out to determine the internalization kinetics of FA-conjugated and non-conjugated SPIONs into MCF-7 and HepG2 cells at different time intervals. In these studies, MCF-7 cells were treated with 100 μg of FA-conjugated or non-conjugated SPIONs and internalization kinetics were studied over a period of time. shows the internalization kinetics of conjugated and unconjugated SPIONs in MCF-7 cells. The results convincingly indicated that the uptake of FA-conjugated SPIONs was much higher compared to non-conjugated SPIONs (). However, internalization of FA-conjugated SPIONs over a period of time was not uniform. A unique pattern was observed in the uptake of FA-conjugated SPIONs by MCF-7 cells. Initially there was an increase in SPIONs concentration (134 + 12.14 pg Fe/cell) followed by a decline at 2 to 4 hours (~10–20 pg Fe/cell) and then again a sharp increase to 92.67 + 8.34 pg Fe/cell by 6 hours which was maintained through long intervals (). Similarly, HepG2 cells have also shown higher uptake at 30 minutes followed by a small decline at 2 hours and finally reached to a concentration of 75.75 + 9.17 pg Fe/cell (). In comparison to unconjugated SPIONs, the FA-conjugated SPIONs showed a prolonged retention of internalized nanoparticles, which may be helpful for MRI detection of FA receptor expressing cells. The initial decline in the uptake of FA-conjugated SPIONs observed in this study may in part be explained by the probable phenomenon of exocytosis. Nanoparticle endocytosis and exocytosis are dynamic and energy-dependent processes, which are proceeding simultaneously in eukaryotic cells.Citation43–Citation45
Figure 6 Intracellular Fe concentration in different cell lines. Fe concentration in MCF-7 (A), HepG2 (B) FAR positive and A549 (C) FAR negative cells that were treated with 100 μg/mL concentration of FA-SPION, SPION and competitive inhibition by FA (10 mM) of competitive FA-SPION for different period of time ranging from 30 minutes to 12 hours.
Abbreviations: FAR, folic acid receptor; FA, folic acid; MCF-7, hormone dependent breast carcinoma cells; HepG2, Hepato-carcinoma cells; A549, non-small cell lung carcinoma cells; FA-SPIONs, folic acid conjugated superparmagnetic iron oxide nanoparticles; SPION, superparamagnetic iron oxide nanoparticles.
MRI characteristics of magnetic nanoparticles in phantom agar gel and MCF-7 cells
The T1 and T2-weighted MRI of FA-SPIONs in water was examined at 4.7T using various concentrations in phantom gel and MCF-7 cells. The results demonstrated that as the SPION concentration was increased, the signal intensity of spin–spin relaxation time T2-weighted MRI image decreased. The T2 and T1 relaxivity coefficient (R2 and Rl values), which are the measures of the spin–spin relaxation rate (T2 −1) and spin lattice relaxation rate (T1 −1) per unit Fe concentration, were calculated at 4.7T to be 222 L mmol−1 sec−1 and 4.11 L mmol−1 sec−l, respectively ( (R1 Inset graph)). For the commercial products, Feridex and Resovist, the R2values were 105 L mmol−1 sec−1 and 176 L mmol−1 sec−1, respectively, and the R1 values were 2.3 L mmol−1 sec−1 and 2.8 L mmol−1 sec−1, respectively, at 4.7T.Citation46 Therefore, the R2 of FA-SPIONs was higher and the R1 of FA-SPIONs was almost equivalent to those of commercial products, respectively. It is well known that the relaxivity ratio, R2/R1, is usually an important parameter to estimate the efficiency of negative contrast agents. In our work R2/R1 was calculated to be 54, which was higher than Feridex and a little less than Resovist coated with higher molecular weight ligands. shows T2-weighted MR images in untreated MCF-7 cells or treated with FA-conjugated-SPION at three different Fe concentrations. Results were also compared to that with MCF-7 cells treated with unconjugated SPIONs. The results demonstrate that T2-weighted MR images of FA-SPION treated MCF-7 cells were darker than those of either the unconjugated SPION or the untreated control at all the Fe concentrations used in this study (). T2 relaxation time of the FA-SPION-treated cells decreased from 777.78 to 163.66 ms−1 in comparison to non-conjugated SPION which showed a decrease from 1015.13 to 493 ms−1 at Fe concentrations ranging from 40 μg Fe/mL to 160 μg Fe/mL. The R2 value of cells treated with FA-SPION was higher as compared to nonconjugated SPIONs (). As expected, the FA conjugated SPIONs offered a specific targeting ability in MCF-7 cells that overexpress FAR.
Figure 7 Magnetic resonance imaging properties of FA-SPIONs: (A) signal intensity weighted images (TR 1/4 10,000 ms, TE 1/4 10 ms) of FA-SPIONs in phantom agar gel at various iron concentrations at 25°C, blank phantom agar gel was taken as a control (data as mean intensity within region of interest (ROI)); (B) T2-weighted MR images of FA-SPION in phantom gel with various concentrations at 4.7 T. (C) Graphs of R2 (R1 inset) against the iron concentration in FA-SPION.
Abbreviations: TR, repetition time; TE, echo time; T2, transverse relaxation time; R2, transverse relaxation rate; R1, longitudinal relaxation rate; ROI, region of interest; FA-SPIONs, folic acid conjugated superparmagnetic iron oxide nanoparticles.
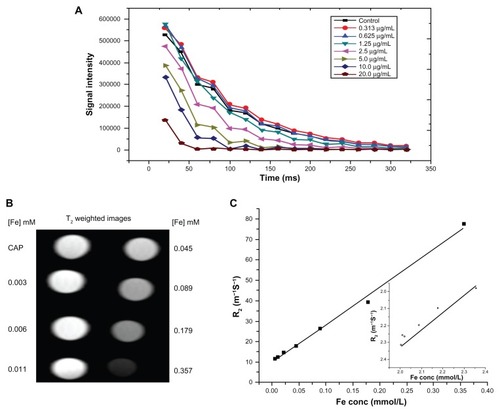
Figure 8 Magnetic resonance imaging properties of MCF-7 cells labeled with SPIONs: (A) signal intensity weighted images (TR 1/4 10,000 ms, TE 1/4 10 ms) of MCF-7 cells labeled FA-SPIONs and SPIONs in DMEM media at various iron concentrations at 25°C, blank phantom agar gel was taken as a control (data as mean intensity within region of interest (ROI)); (B) T2-weighted MR images of MCF-7 cancer cells treated with different concentrations FA-SPION and SPION (C) Graph of R2 of MCF-7 cancer cells treated with different concentrations FA-SPION and SPION.
Abbreviations: TR, repetition time; TE, echo time; T2, transverse relaxaxtion time; R2, transverse relaxation rate; ROI, region of interest; MCF-7, hormone-dependent breast carcinoma cells; FA-SPIONs, folic acid conjugated superparmagnetic iron oxide nanoparticles; SPION, superparamagnetic iron oxide nanoparticles.
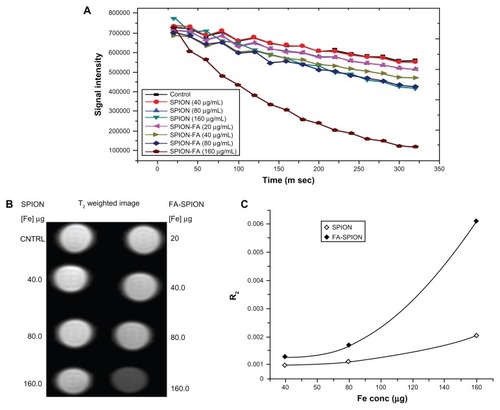
Figure 9 Cytotoxicity (XTT assay) to cells exposed to different concentrations (62.5–1000 μg/mL) of FA-SPION and SPION for different time points 24 and 48 hours. (A) MCF-7 cells; (B) HepG2 cells.
Abbreviations: XTT, (2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide); FA-SPION, folic acid conjugated superparamagnetic iron oxide nanoparticles; SPION, superparamagnetic iron oxide nanoparticles; MCF-7, hormone dependent breast carcinoma cells; HepG2, Hepato-carcinoma cells.
Conclusion
FA conjugated, CA/BMPA capped iron oxide nanoparticles were synthesized and characterized for MR imaging of breast cancer MCF-7 cells. The hydrophilic SPIONs capped with CA/BMPA and conjugated with FA were found to be monodispersed, highly stable in PBS and have shown dispersion stability for more than 2 years as observed from dynamic light scattering and TEM studies. FA-SPIONs showed no cellular cytotoxicity and were expected to be completely degraded by enzymes present in physiological conditions. The cellular uptake of FA-SPIONs was mediated through FAR mediated endocytosis and a possible mechanism, at least in part, is a clathrin mediated pathway. As verified by Prussian blue staining, iron content and in vitro MR imaging, FA-SPIONs had better targeting tropism, showed better cellular internalization in FAR-positive MCF-7 and HepG2 cells and maintained for longer periods in these cells than did their non-targeted counterparts. The relaxivity of FA-SPIONs was much greater than that of the existing commercial products. T2-weighted images of MCF-7 breast cancer cells that overexpressed FAR showed significantly enhanced signal intensities. The high intracellular uptake of FA-conjugated SPIONs may help to detect the collection of a small number of cancer cells (early and mirometastasis) which is difficult to detect by conventional MRI imaging. Furthermore the excellent long term colloidal stability may enhance its translational potential. Therefore, the FA-SPIONs, possessing good colloidal stability and affording excellent sensitivity of imaging, are proposed as a potential MRI contrast agent for the detection of breast cancer and other cancer cells overexpressing the folate receptor.
Acknowledgment
The authors gratefully acknowledge Lockheed Martin Corporation (NJ, USA), Department of Science and Technology, Govt of India and Council of Scientific and Industrial Research for financial assistance and Supported Accommodation Innovation Fund, SAIF, AIIMS for TEM studies.
Disclosure
The authors report no conflicts of interest in this work.
References
- LaurentSForgeDPortMMagnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applicationsChem Rev200810862064211018543879
- BrunsOTIttrichHPeldschusKReal-time magnetic resonance imaging and quantification of lipoprotein metabolism in vivo using nanocrystalsNat Nanotechnol3200943193201 Epub January 25, 200919265850
- FangCZhangMMultifunctional magnetic nanoparticles for medical imaging applicationsJ Mater Chem2009196258626620593005
- McCarthyJRWeisslederRMultifunctional magnetic nanoparticles for targeted imaging and therapyAdv Drug Deliv Rev8172008601112411251 Epub April 10, 200818508157
- WangYXHussainSMKrestinGPSuperparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imagingEur Radiol200111112319233111702180
- BonnemainBSuperparamagnetic agents in magnetic resonance imaging: physicochemical characteristics and clinical applications. A reviewJ Drug Target1998631671749888302
- HarisinghaniMGBarentszJHahnPFNoninvasive detection of clinically occult lymph-node metastases in prostate cancerN Engl J Med2003348252491249912815134
- SinghAPatelTHertelJBernardoMKauszABrennerLSafety of ferumoxytol in patients with anemia and CKDAm J Kidney Dis112008525907915 Epub September 27, 200818824288
- LeeJHHuhYMJunYWArtificially engineered magnetic nanoparticles for ultra-sensitive molecular imagingNat Med120071319599 Epub December 24, 200617187073
- GoyaGFGrazúVIbarraMRMagnetic nanoparticles for cancer therapyCurr Nanosci200841116
- ChoiHChoiSRZhouRKungHFChenIWIron oxide nanoparticles as magnetic resonance contrast agent for tumor imaging via folate receptor-targeted deliveryAcad Radiol2004119996100415350580
- LuAHSalabasELSchüthFMagnetic nanoparticles: synthesis, protection, functionalization, and applicationAngew Chem Int Ed Engl20074681222124417278160
- QinJLaurentSJoYSA high-performance magnetic resonance imaging T-2 contrast agentAdv Mater2007191418741878
- HuhYMJunYWSongHTIn vivo magnetic resonance detection of cancer by using multifunctional magnetic nanocrystalsJ Am Chem Soc200512735123871239116131220
- CliftMJRothen-RutishauserBBrownDMThe impact of different nanoparticle surface chemistry and size on uptake and toxicity in a murine macrophage cell lineToxicol Appl Pharmacol11120082323418427 Epub July 1, 200818708083
- ChoulyCPouliquenDLucetIJeuneJJJalletPDevelopment of superparamagnetic nanoparticles for MRI: effect of particle size, charge and surface nature on biodistributionJ Microencapsul19961332452558860681
- MoghimiSMHunterACMurrayJCLong-circulating and target-specific nanoparticles: theory to practicePharmacol Rev200153228331811356986
- LunovOSyrovetsTRöckerCLysosomal degradation of the carboxydextran shell of coated superparamagnetic iron oxide nanoparticles and the fate of professional phagocytesBiomaterials122010313490159022 Epub August 23, 201020739059
- ArtemovDMolecular magnetic resonance imaging with targeted contrast agentsJ Cell Biochem200390351852414523986
- LeuschnerCKumarCSHanselWSoboyejoWZhouJHormesJLHRH-conjugated magnetic iron oxide nanoparticles for detection of breast cancer metastasesBreast Cancer Res Treat92006992163176 Epub June 3, 200616752077
- ZhangYKohlerNZhangMSurface modification of superparamagnetic magnetite nanoparticles and their intracellular uptakeBiomaterials20022371553156111922461
- WeitmanSDLarkRHConeyLRDistribution of the folate receptor GP38 in normal and malignant cell lines and tissuesCancer Res19925212339634011596899
- RossJFChaudhuriPKRatnamMDifferential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implicationsCancer1994739243224437513252
- ParkerNTurkMJWestrickELewisJDLowPSLeamonCPFolate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assayAnal Biochem2005338228429315745749
- JanaNRChenYFPengXGSize- and shape-controlled magnetic (Cr, Mn, Fe, Co, Ni) oxide nanocrystals via a simple and general approachChem Mater2004162039313935
- Salgueirino-MaceiraVLiz-MarzánLMFarleMWater-based ferrofluids from FexPt1-x nanoparticles synthesized in organic mediaLangmuir200420166946695015274608
- ChenZPZhangYZhangSPreparation and characterization of water-soluble monodisperse magnetic iron oxide nanoparticles via surface double-exchange with DMSAColloids Surf A Physicochem Eng Asp20083161–3210216
- LattuadaMHattonTAFunctionalization of monodisperse magnetic nanoparticlesLangmuir20072342158216817279708
- YouJLiXde CuiFDuYZYuanHHuFQFolate-conjugated polymer micelles for active targeting to cancer cells: preparation, in vitro evaluation of targeting ability and cytotoxicityNanotechnology1302008194045102 Epub January 4, 200821817496
- ZhouJLeuschnerCKumarCHormesJSoboyejoWOA TEM study of functionalized magnetic nanoparticles targeting breast cancer cellsMaterials Science and Engineering: C200626814511455
- JainTKRicheyJStrandMLeslie-PeleckyDLFlaskCALabhasetwarVMagnetic nanoparticles with dual functional properties: drug delivery and magnetic resonance imagingBiomaterials102008292940124021 Epub July 22, 200818649936
- SunSZengHRobinsonDBMonodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticlesJ Am Chem Soc2004126127327914709092
- WuWHeQJiangCMagnetic iron oxide nanoparticles: synthesis and surface functionalization strategiesNanoscale Res Lett200831139741521749733
- PouliquenDLe JeuneJJPerdrisotRErmiasAJalletPIron oxide nanoparticles for use as an MRI contrast agent: pharmacokinetics and metabolismMagn Reson Imaging1991932752831881245
- YokoyamaMSatohASakuraiYIncorporation of water-insoluble anticancer drug into polymeric micelles and control of their particle sizeJ Control Release1998552–32192299795065
- MarshMMcMahonHTThe structural era of endocytosisScience1999285542521522010398591
- PearseBMCrowtherRAStructure and assembly of coated vesiclesAnnu Rev Biophys Biophys Chem19871649682885011
- HeuserJEAndersonRGHypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formationJ Cell Biol198910823894002563728
- LandmarkKJDiMaggioSWardJSynthesis, characterization, and in vitro testing of superparamagnetic iron oxide nanoparticles targeted using folic acid-conjugated dendrimersACS Nano20082477378319206610
- KohlerNSunCWangJZhangMMethotrexate-modified superparamagnetic nanoparticles and their intracellular uptake into human cancer cellsLangmuir200521198858886416142971
- KohlerNSunCFichtenholtzAGunnJFangCZhangMMethotrexate-immobilized poly(ethylene glycol) magnetic nanoparticles for MR imaging and drug deliverySmall20062678579217193123
- KimJSYoonTJYuKNCellular uptake of magnetic nanoparticle is mediated through energy-dependent endocytosis in A549 cellsJ Vet Sci20067432132617106221
- PanyamJLabhasetwarVDynamics of endocytosis and exocytosis of poly(D,L-lactide-co-glycolide) nanoparticles in vascular smooth muscle cellsPharm Res200320221222012636159
- ChithraniBDChanWCElucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapesNano Lett620077615421550 Epub April 28, 200717465586
- GuJXuHHanYThe internalization pathway, metabolic fate and biological effect of superparamagnetic iron oxide nanoparticles in the macrophage-like RAW264.7 cellSci China Life Sci92011549793805 Epub September 16, 201121922429
- RohrerMBauerHMintorovitchJRequardtMWeinmannHJComparison of magnetic properties of MRI contrast media solutions at different magnetic field strengthsInvest Radiol2005401171572416230904