?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
Therapy for central nervous system disease is mainly restricted by the blood–brain barrier. A drug-delivery system is an effective approach to overcome this barrier. In this research, the potential of polymeric micelles for brain-targeting drug delivery was studied.
Methods
Stearic acid–grafted chitosan (CS-SA) was synthesized by hydrophobic modification of chitosan with stearic acid. The physicochemical characteristics of CS-SA micelles were investigated. bEnd.3 cells were chosen as model cells to evaluate the internalization ability and cytotoxicity of CS-SA micelles in vitro. Doxorubicin (DOX), as a model drug, was physically encapsulated in CS-SA micelles. The in vivo brain-targeting ability of CS-SA micelles was qualitatively and quantitatively studied by in vivo imaging and high-performance liquid chromatography analysis, respectively. The therapeutic effect of DOX-loaded micelles in vitro was performed on glioma C6 cells.
Results
The critical micelle concentration of CS-SA micelles with 26.9% ± 1.08% amino substitute degree was 65 μg/mL. The diameter and surface potential of synthesized CS-SA micelles in aqueous solution was 22 ± 0.98 nm and 36.4 ± 0.71 mV, respectively. CS-SA micelles presented excellent cellular uptake ability on bEnd.3 cells, the IC50 of which was 237.6 ± 6.61 μg/mL. DOX-loaded micelles exhibited slow drug-release behavior, with a cumulative release up to 72% within 48 hours in vitro. The cytotoxicity of DOX-loaded CS-SA micelles against C6 was 2.664 ± 0.036 μg/mL, compared with 0.181 ± 0.066 μg/mL of DOX · HCl. In vivo imaging results indicated that CS-SA was able to transport rapidly across the blood–brain barrier and into the brain. A maximum DOX distribution in brain of 1.01%/g was observed 15 minutes after administration and maintained above 0.45%/g within 1 hour. Meanwhile, free DOX · HCl was not detected in brain. In other major tissues, DOX-loaded micelles were mainly distributed into lung, liver, and spleen, with a reduction of DOX accumulation in heart.
Conclusion
The CS-SA micelles were able to be used as a promising carrier for a braintargeting drug delivery system.
Introduction
There has been an increasing population worldwide suffering various central nervous system disorders, such as Alzheimer’s disease, Parkinson’s, cerebral ischemia, and brain tumor. The treatment for these diseases is still confronting a big challenge due to the existence of the blood–brain barrier (BBB), which prevents most drug molecules from entering the brain. The BBB controls substance flow in and out of the brain with precision and strictness, so as to maintain the internal environment of the brain. Besides the physical barrier characterized by the tight junction, the brain capillary endothelial cells highly express the active efflux system.Citation1 The system that is representative of P-glycoprotein (member of adenosine triphosphate-binding cassette P-gp) greatly reinforces BBB function by effectively removing drugs from the brain and pumping them back into blood.Citation2 Therefore, the development of brain-targeting drug-delivery systems has become an urgent matter.
A number of attempts have been made to overcome the barrier. Various nanoparticle delivery systems were designed for brain targeting, including liposomes, solid lipid nanoparticles, and polymeric micelles. Among these, polymeric micelles have emerged as an ideal candidate for brain-targeting delivery. The amphiphilic copolymer can self-assemble into micelles in aqueous solution with a core-shell structure. The hydrophobic core acts as the drug-loading depot, with the surrounding hydrophilic block to increase stabilization. Incorporation of low molecules into micelles can effectively enhance drug solubility and improve drug pharmacokinetics and biodistribution.Citation3 Polymeric micelles possess many advantageous properties: small particle size that allows escaping from the reticuloendothelial system, stability in plasma, and flexibility of modification with targeting ligands. Compared to liposomes, polymeric micelles require less excipients and are easier to formulate, with high physical stability and sustained drug release.Citation4 Various biodegradable polymers such as poly(lactic acid), poly(lactic-co-glycolic acid), poly(butylcyanoacrylate), chitosan (CS), and polylysine have been exploited as brain-targeting drug-delivery systems.Citation5–Citation7 These carrier systems have successfully delivered poorly diffused drugs into the brain, including doxorubicin (DOX), loperamide, and dalargin.Citation8–Citation12
It is widely accepted that free DOX is not able to transport across the BBB, which is mainly associated with the active efflux system on the BBB.Citation13–Citation15 Known as a biodegradable and biocompatible material, CS with its low molecular weight is studied widely as a drug carrier due to its good solubility in physiological conditions.Citation16 Studies have shown that CS can transiently open tight junctions, resulting in a paracellular pathway of material through the epithelial barrier.Citation17 In our previous studies, stearic acid–grafted CS (CS-SA) was synthesized using low molecular–weight CS, which was able to form micelle-like nanoparticles in the aqueous phase, and has been used to deliver geneCitation18 and antitumor agents like doxorubicinCitation19,Citation20 and paclitaxel.Citation21 All the results demonstrated the excellent biomembrane penetration ability of CS-SA, indicated by rapid in vitro cellular uptake and comparable in vivo therapeutic effect with a commercial agent. Moreover, the micelles were able to reserve multidrug resistance by avoiding recognition by P-gp. The ability of CS-SA to open transiently biobarriers and bypass the P-gp system by application on oral drug delivery was further confirmed.Citation22 The purpose of this study was to explore the potential of CS-SA micelles as a brain-targeting delivery system. Stearic acid (SA) was conjugated onto CS with 18-KDa weight average molecular weight using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)as the coupling reagent.Citation18,Citation19 The physicochemical characteristics of CS-SA were then investigated. In vitro cellular uptake and cytotoxicity were performed on bEnd.3 cells. In vivo imaging was applied to track the route of micelles after administration. DOX was chosen as the model drug and encapsulated into CS-SA micelles to prepare DOX-loaded micelles. The in vivo distribution of DOX was conducted in a quantitative way.
Materials and methods
Materials
CS with an average molecular weight of 18 kDa was obtained by enzymatic degradation from CS (Mw = 450.0 kDa, 95% deacetylated degree; Yuhuan Marine Biochemistry, Zhejiang, China). SA was purchased from Chemical Reagent (Shanghai, China). EDC, 2,4,6-trinitrobenzene sulfonic acid (TNBS), and 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) came from Sigma (St Louis, MO). DiR dye was a product of Molecular Probes (Eugene, OR). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and trypsin-ethylenediaminetetraacetic acid (EDTA) were purchased from Gibco (Gaithersberg, MD). DOX hydrochloride DOX · HCl was gifted by Zhejiang Hisun Pharmaceutical (Taizhou, China). All other chemicals were of analytical or chromatographic grade.
ICR mice and Sprague Dawley (SD) rats (180–200g, male) were obtained from Experimental Animal Center of Zhejiang University and maintained at 22 ± 2°C on a 12-hour light–dark cycle with access to food and water. The animals used for the experiment were treated according to the protocols evaluated and approved by the ethical committee of Zhejiang University.
Synthesis of CS-SA copolymer
The CS-SA conjugate was synthesized via the reaction of carboxyl groups of stearic acid (SA) with amine groups of CS in the presence of EDC.Citation23 It mainly followed two steps. Firstly, SA (0.7 g) with EDC (SA:EDC = 1:10, mol:mol) were dissolved in 80 mL of ethanol and reacted at 60°C by stirring in a water bath for 30 minutes. Secondly, 1 g of CS was dissolved in 120 mL of pure water and incubated to 80°C. The organic mixture was added into the CS solution under intensive stirring and kept reacting for 6 hours. The resultant mixture was dialyzed against deionized (DI) water for 48 hours using a dialysis membrane (MWCO 7 kDa; Spectrum Laboratories, Laguna Hills, CA) followed by lyophilization. The lyophilized product was further washed with ethanol to remove the unreacted SA. Finally, the product CS-SA was redispersed in DI water and lyophilized.
Characterization of CS-SA
The 1H NMR spectra of CS-SA were obtained by a nuclear magnetic resonance spectrometer (AC-80; Bruker BioSpin, Rheinstetten, Germany). CS-SA was dissolved in D2O at a concentration of 20 mg/mL before testing.
The critical micelle concentration of CS-SA in aqueous medium was estimated by spectroscopy using pyrene as a probe.Citation18 The fluorescence emission spectrum of pyrene was obtained by a fluorometer (F-2500; Hitachi, Tokyo, Japan). The excitation wavelength was 337 nm, and the slits were set at 2.5 nm (excitation) and 10 nm (emission), respectively. The concentrations of CS-SA solution with 5.93 × 10−7 M pyrene were varied from 5.0 × 10−3 to 1.0 mg/mL. The intensity ratio of the first peak (I1, 374 nm) to the third peak (I3, 385 nm) in the emission spectra of pyrene was calculated.
The degrees of amino substitution of CS-SA were determined by 2,4,6-trinitrobenzene sulfonic acid (TNBS) method. Citation19 Briefly, 3.0 mL of CS-SA solution at a concentration of 0.1 mg/mL was incubated with 2.0 mL of 4% NaHCO3 and 2.0 mL of 0.1% TNBS at 37°C for 2 hours. Following addition of 2.0 mL of HCl (2.0 mol/L), the mixture was measured at 344 nm by a ultraviolet spectrophotometer (TU-1800PC; Beijing Purkinje General Instrument, Beijing, China).
The hydrodynamic diameter and zeta potential of the micelles of CS-SA (1.0 mg/mL) were measured by dynamic light scattering using a Zetasizer (3000 HS; Malvern Instruments, Malvern, UK). Each sample determination was done in triplicate.
The morphological observation of CS-SA micelles was performed by transmission electron microscopy (TEM) (JEM-1230; Jeol, Tokyo, Japan). The samples were stained with 2% (w/v) phosphotungstic acid and placed on copper grids with films for viewing by TEM.
Preparation of DOX-loaded CS-SA micelles
DOX was obtained by the alkalization of DOX · HCl with twice the molar numbers of triethylamine in dimethyl sulfoxide (DMSO) overnight and used for the preparation of DOX-loaded CS-SA (CS-SA/DOX) micelles.Citation24 To prepare CS-SA/DOX micelles, 200 mg of CS-SA was dissolved in 100 mL of DI water, followed by the addition of 30 mL of DOX-DMSO solution (1.0 mg/mL) in drops. The mixture was dialyzed against DI water using a dialysis membrane (MWCO 7 kDa) for 24 hours. It was centrifuged at 4000 rpm for 10 minutes to remove unentrapped drug, and the final product was obtained by lyophilization.
Characterization of DOX-loaded CS-SA micelles
Micellar size and zeta potential of CS-SA/DOX micelles with the CS-SA concentration of 1.0 mg/mL were performed using the Zetasizer analyzer. All measurements were performed in triplicate.
The DOX content encapsulated in CS-SA micelles was measured by the F-2500 spectrophotometer. The excitation wavelength and emission wavelength were set at 505 nm and 565 nm, respectively, both with slit openings of 5 nm. The drug encapsulation efficiency (EE) and drug loading capacity (DL) of CS-SA/DOX were estimated by centrifugal ultrafiltration method. Briefly, a part of CS-SA/DOX micelle solution was centrifuged in ultrafiltration tubes (Microcon YM-10, MWCO 3000 kDa; Millipore, Billerica, MA). The DOX concentration (C, μg/mL) of ultrafiltrate was determined to evaluate the amount of the free drug. Another part of CS-SA/DOX solution was diluted 100-fold by DMSO aqueous solution (DMSO:H2O = 9:1, v:v), the DOX concentration (C0, μg/ml) of which was considered as the total drug amount. The EE (%) and DL (%) of CS-SA/DOX was calculated by the following equations:
where Ma is the charged amount of drug, mg; and V is the total volume of CS-SA/DOX solution, mL.
In vitro release study
In vitro release studies of DOX from CS-SA micelles were performed in phosphate-buffered saline (PBS) (pH 7.2). The saturated solubility of DOX in PBS (pH 7.2) was measured to be 76 μg/mL. Briefly, 1.0 mL of CS-SA/DOX micelle solution (containing 75 μg of DOX) was added into the dialysis filter (MWCO 7 kDa) and put into a plastic tube containing 20 mL of PBS (pH 7.2) solution. The plastic tube was then placed in an incubator shaker (HZ-8812S; Scientific and Educational Equipment, Taicang, China), which was maintained at 37°C and shaken horizontally at 65 rpm. At predetermined time intervals, sample solution was withdrawn and release medium was totally replaced with fresh PBS solution. The drug concentration was determined by fluorescence spectrophotometer. All drug-release tests were performed in triplicate.
Cell culture
bEnd.3 cells (immortalized mouse brain endothelial cell line) and murine C6 glioma cells were donated by the Pharmacolgy Department, College of Medicine, Zhejiang University (Hangzhou, China). For both bEnd.3 and C6, cells were maintained in DMEM supplemented with 10% (v/v) FBS and penicillin/streptomycin (100 U/mL,100 U/mL) at 37°C in a humidified atmosphere containing 5% CO2. Cells were subcultured regularly using trypsin-EDTA.
In vitro cellular uptake and cytotoxicity
To visualize cellular uptake of micelles, CS-SA was labeled with fluorescein isothiocyanate (FITC). FITC was conjugated onto CS-SA through the reaction between the amino group of CS-SA and the isothiocyanate of FITC.Citation20 Briefly, 10 mg of CS-SA was dissolved in 5.0 mL of DI water and an aliquot of FITC ethanol solution (CS-SA:FITC = 1:1, mol:mol) was added in drops. The resultant solution was kept stirring overnight at room temperature. The final product was obtained after dialysis against DI water for 24 hours.
bEnd.3 cells were seeded at 2 × 104 cells/well in a 24-well plate (Nalge Nunc, Naperville, IL) and grown for 24 hours. Aliquots of FITC-CS-SA (containing 100 μg of CS-SA) were added, and the cells were further incubated for predetermined time intervals. At the end of the experiment, cells were washed with PBS three times and cellular uptake was observed by fluorescence microscopy (Olympus America, Melville, NY).
bEnd.3 was used to evaluate the cytotoxicity of CS-SA in vitro by MTT assay. Briefly, 1 × 104 of bEnd.3 cells were planted in 96-well plates (Nalge Nunc) and grown for 24 hours. The cells were exposed to a series of concentrations of CS-SA micelles at 37°C for another 48 hours. At the end of incubation, 20 μL of MTT solution with a concentration of 5 mg/mL was added and incubated for further 4 hours at 37°C. Each well was then washed with PBS after the medium was removed, and then 100 μL of DMSO was added to dissolve MTT formazan crystals. Finally, the plate was shaken for 10 minutes, and the absorbance of formazan product was measured at 570 nm in a microplate reader (Model 680; Bio-Rad, Hercules, CA). Cell viability was expressed as percentage of absorbance in comparison with that of the control, which comprised the cells without exposure to the samples. All the experiments were performed in triplicate. The cytotoxicity of CS-SA/DOX micelles against C6 cells was obtained in a similar process, except that C6 cells were exposed to a series of DOX concentrations. Free DOX · HCl was set as positive control.
In vivo imaging
The fluorescent dye DiR was physically entrapped into micelles to keep track of micelles in vivo. In brief, 10 mg of CS-SA was dissolved in 2.0 mL of DI water, followed by the addition of 20 μL of DiR-DMSO solution (5.0 mg/mL) in drops. The mixture was subjected to ultrasonic treatment in a water bath for 30 minutes, followed by dialysis against DI water using a dialysis membrane (MWCO 7 kDa) for 24 hours. The DiR-loaded CS-SA micelle solution (CS-SA/DiR) was finally obtained.
Before the experiment, the animals were anesthetized. Then, the CS-SA/DiR solution was injected via tail vein of nude mice at a dose of 25 mg CS-SA/kg. Images were taken by Maestro EX in vivo imaging system (CRI, Woburn, MA) at predetermined time points after injection. Brain was extirpated 0.5 hours after injection for fluorescence image. Blank brain served as negative control.
Biodistribution study
The pharmacokinetics of DOX-loaded micelles was studied in Sprague Dawley rats. Rats were divided into two groups, each group containing five rats. DOX · HCl solution and DOX-loaded micelles were administered intravenously, both at a dose of 1 mg DOX/kg. Blood samples were collected from retro-orbital sinus into heparinized tubes at each time point (5 minutes, 15 minutes, 30 minutes, 1 hour, 2 hours, and 4 hours) after administration. The blood was centrifuged at 3000 rpm for 10 minutes, and plasma was obtained for determination of DOX concentration.
Biodistribution study of CS-SA/DOX micelles was carried out in ICR mice in comparison with DOX HCl. CS-SA/DOX micelles and DOX · HCl solution were injected via tail vein of ICR mice, both at a dose of 2.5 mg DOX/kg. At 5 minutes, 15 minutes, 30 minutes, 1 hour, 2 hours, and 4 hours after administration, brains were taken out and rinsed with PBS. Brain samples were carefully handled to remove residual blood and capillaries and dried with filter paper. All samples were weighed and kept at −80°C before analysis. Other major tissues (including heart, liver, spleen, lung, and kidney) were used for distribution study at 0.5 and 2 hours after administration. Three mice were used for each time point.
Processing of tissues for HPLC analysis
Plasma samples were prepared according to the following procedure. In brief, 1.5 mL of chloroform/methanol (1:2, v:v) was added to 0.2 mL of plasma, followed by vortexing and centrifugation (8000 rpm, 10 minutes). The supernatant was transferred into a plastic tube and dried at 37°C under a stream of N2. The residual was redissolved in methanol by ultrasound treatment in an ice bath for 15 minutes. After centrifugation (15,000 rpm, 4°C and 10 minutes) the supernatant was subjected to high-performance liquid chromatography (HPLC) analysis. In the case of tissue samples, these were homogenized with PBS (pH 7.6) in a high-speed tissue homogenizer under an ice bath (JY92; Scientz, Ningbo, China), and 0.4 mL of the homogenate was subjected to the same procedure as for plasma samples. Blank plasma or homogenates were spiked with appropriate DOX-loaded micelle solutions. Control samples of plasma or tissues for drug analysis were prepared in the same manner.
HPLC analysis for doxorubicin was performed under a modular liquid chromatograph system (Agilent, Santa Clara, CA) with a fluorescence detector. A Diamonsil TM C18 (Dikma Technologies, Beijing, China) column (250 mm × 4.6 mm, 5 μm) was used. The mobile phase consisted of acetonitrile and water containing 10 mM phosphoric acid and 20 mM lauryl sodium sulfate (80:20, v:v) and the flow rate was kept at 1.0 mL/min. The column temperature was set to be 30°C. The effluent was monitored at an excitation of 505 nm and an emission of 555 nm.
DOX · HCl was extracted from tissue samples as previously reported.Citation25 Before processing, blood samples were added with same volume of borate buffer solution (pH 9.6) and tissues were homogenized with PBS solution (pH 7.6). Tissue homogenate or blood samples was extracted by chloroform/methanol (4:1, v:v). The extracts were then subjected to HPLC analysis according to the method of Yu et al.Citation25
Results
Synthesis of CS-SA
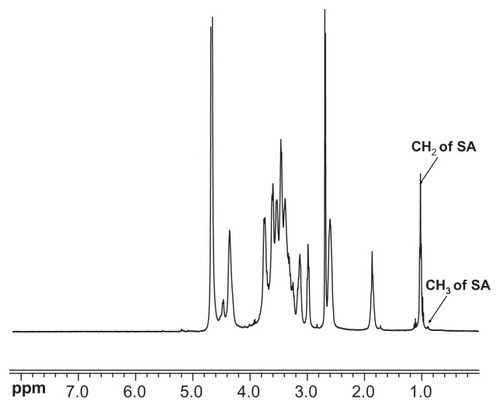
The CS-SA was obtained by the reaction between the carboxyl group of SA and the amino group of CS oligosaccharide using EDC as the coupling reagent. The chemical structure of CS-SA was confirmed by 1H NMR spectra (). The 0.9 ppm and 1.0 ppm of chemical shift could be respectively attributed to the methyl hydrogen and methylene hydrogen of the stearate group. Therefore, it was assumed that the SA had been successfully grafted onto CS. According to the TNBS test, the amino substitute degree of CS-SA was determined to be 26.9% ± 1.08%.
Characterization of CS-SA micelles
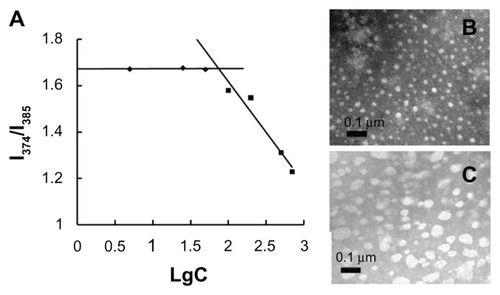
The synthesized CS-SA was able to self-assemble to form micelles in aqueous solution. As seen in , CS-SA micelles exhibited an average number diameter of 22 ± 0.98 nm, consistent with the TEM observation results (), which showed a regular spherical morphology of CS-SA micelles. The relatively high zeta potential of 36.4 ± 0.71 mV helped to increase the stability of micelles by repulsion interaction. The concentration of CS-SA plotted against I1/I3 is presented in . The critical micelle concentration of CS-SA measured by fluorescence was 65 μg/mL, which indicated that CS-SA micelles had good self-assembling ability.
Table 1 Characteristics of CS-SA micelles and DOX loaded CS-SA micelles
In vitro cellular uptake and cytotoxicity
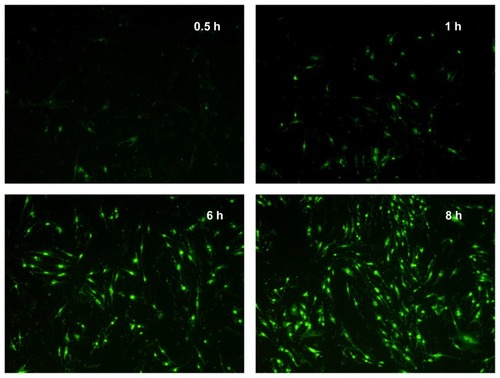
The FITC-CS-SA was synthesized and the cellular uptake was observed using fluorescence microscopy. As shown in , distribution of FITC-CS-SA micelles in cells was observed after 0.5 hour’s incubation. Fluorescence intensity inside cells increased gradually and significant fluorescence was observed after 6 hours’ incubation. The CS-SA micelles displayed an excellent ability to transport across cell membrane. It was assumed that endocytosis mainly occurs in the internalization.Citation22 The IC50 of CS-SA against bEnd.3 was 237.6 ± 6.61 μg/mL, determined by MTT assay.
Figure 3 Fluorescence images of bEnd.3 after incubation with fluorescein isothiocyanate–labeled stearic acid–grafted chitosan micelles for 0.5, 1, 6, and 8 hours.
The cytotoxicity of CS-SA/DOX micelles against C6 was 2.664 ± 0.036 μg/mL, compared with 0.302 ± 0.069 μg/mL of DOX · HCl. The relatively lower toxicity of CS-SA/DOX micelles might be relevant with slow release of DOX from micelles.
Characteristics of CS-SA/DOX micelles
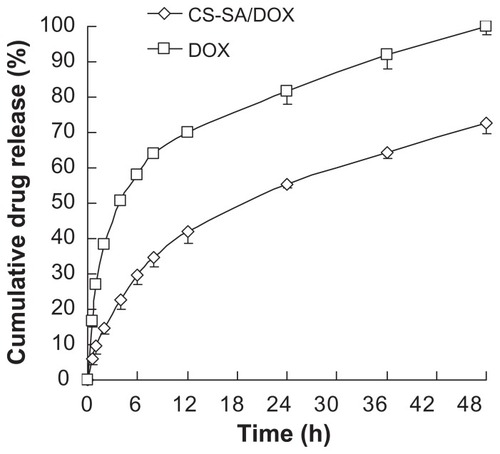
DOX was physically entrapped into the hydrophobic core of CS-SA micelles. As seen in , the size of micelles increased after drug loading, while the surface potential presented no significant change. The EE and DL were determined to be 81.23% and 10.65%, respectively. In vitro release profiles of DOX from CS-SA micelles in PBS (pH 7.2) are shown in . CS-SA/DOX micelles exhibited relatively slow release behavior in vitro. As shown in , the cumulative DOX release percentage was 34.7% in the first 8 hours and reached 72% in 48 hours.
In vivo imaging
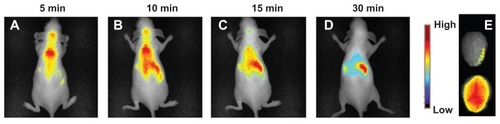
Nude mice were injected with NiR-loaded CS-SA (CS-SA/DiR) micelles and were subjected to observation using the CRI Maestro in vivo imaging system. Images were taken at different time points within 30 minutes after injection. As shown in , CS-SA/DiR was strongly accumulated in brain 5 minutes after injection. The fluorescence gradually decreased and was still detectable 30 minutes after injection by observing ex vivo brain samples. Taking into account that fluorescence was not seen in brain treated with free DiR (not shown), the fast distribution of DiR in brain could be mainly ascribed to the permeability of CS-SA across BBB.
In vivo distribution study
In vivo distribution study of CS-SA/DOX micelles was performed in comparison with free DOX · HCl. The HPLC analysis system for in vivo DOX detection was established. Baseline separation of DOX was achieved. Recoveries for different tissues were all above 60% (data not shown), which could be acceptable in the analysis of DOX in vivo.Citation26
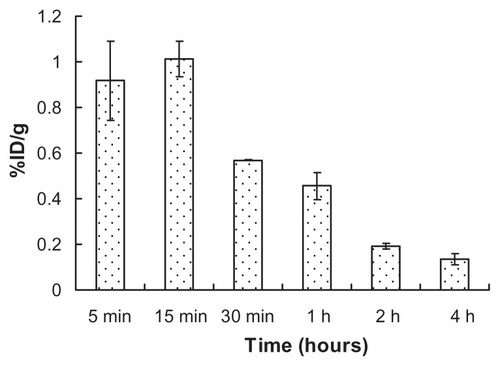
The DOX level in brain was detected at different time points, as presented in . It was found that DOX was able to distribute rapidly throughout brain, with the highest drug level of 1.01%/g achieved at 15 minutes after injection. Then drug level gradually decreased. It maintained above 0.45%/g for 1 hour and did not arrive at 0.2%/g until 2 hours after administration.
Figure 6 Brain distribution after injection of doxorubicin-loaded stearic acid–grafted chitosan at a dose of 2.5 mg/kg (equivalent of doxorubicin).
Note: Data represent the mean ± standard deviation. n = 3.
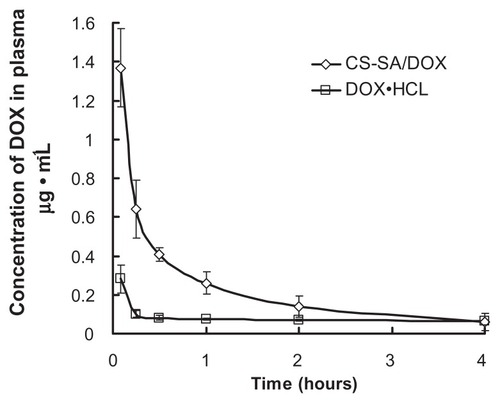
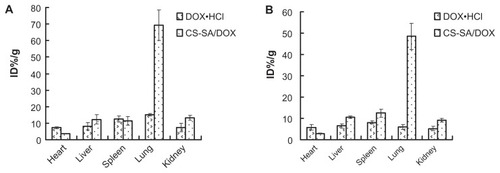
The plasma concentration versus time profiles of CS-SA/DOX micelles and free DOX · HCl is shown in . According to analysis, the area under the curve was increased 1.97-fold by using CS-SA/DOX micelles, compared to DOX · HCl. The pharmacokinetics parameters were obtained by DAS 2.0 analysis and listed in in detail. Biodistribution of CS-SA/DOX in other tissues was also investigated. illustrates the distribution of DOX at 0.5 and 2 hours following administration. It was found that drug was mainly located in lung, liver, and spleen. It is worthy of note that the amount of DOX in heart was reduced by half by CS-SA/DOX, compared with DOX · HCl.
Table 2 Pharmacokinetic parameters in rat after intravenous administration of CS-SA/DOX and DOX · HCl
Discussion
In vitro uptake study showed that CS-SA displayed excellent internalization ability on bEnd.3. The result was consistent with previous studies, in which CS-SA exhibited excellent cellular uptake on other tumor cells as well.Citation19,Citation22 In vitro toxicity study indicated that CS-SA could be used as a relatively safe carrier for brain-targeting drug delivery.
The rapid distribution of DOX in brain by quantification study agreed well with the in vivo imaging results. Meanwhile, no drug signal was detected in brain for the DOX · HCl group. Therefore, high drug levels in brain could be virtually attributed to BBB transport ability exerted by CS-SA micelles. The large amount of DOX accumulated in brain demonstrated the superior advantage of CS-SA micelles for brain targeting in contrast with the other kinds of nanoparticles whose average drug level in brain was merely 0.1%/g–0.2%/g.Citation27–Citation31
Transport across BBB for micelles might be related to the high lipophicity increased by SA with a substitute degree of 26%. Moreover, another important contribution to the successful transport of DOX into brain could be bypassing the P-gp effect. The efflux system (eg, P-gp), highly expressed on endothelial cells, effectively pumps exogenous agents from brain back to blood.Citation2 Being the substrate of P-gp prevented the entry of DOX to brain. It was assumed that CS-SA/DOX micelles enhanced distribution of incorporated DOX in brain by masking physicochemical properties of DOX and thus bypassing P-gp recognition. However, it was noted that a dramatic decrease of DOX in brain was observed. The specific mechanism remained unknown and requires further investigation.
The large amount of DOX in lung might be associated with the particle size. The uptake of micelles by macrophages in the reticuloendothelial system might explain the drug distribution in the liver and the spleen. Cardiac toxicity was one major concern in clinical application of adriamycin. CS-SA/DOX greatly lowered drug distribution in heart, thus reducing cardiac toxicity.
Conclusion
The synthesized amphipathic polymer (CS-SA) was able to self-assemble into micelles. These CS-SA micelles exhibited excellent internalization capability and relatively low cytotoxicity on bEnd.3. In vivo imaging displayed a rapid distribution of CS-SA in brain. DOX was entrapped into CS-SA micelles with a high drug-loading capacity, and the DOX-loaded micelles exhibited slow release behavior in vitro. CS-SA/DOX micelles showed a therapeutic effect on C6 in vitro. A relatively high amount of DOX was found in brain, while a lower amount of drug was accumulated in heart. A longer retention time in brain would be achieved in treatment of brain tumor through the EPR effect of a nanosized micellar delivery system. It was confirmed that CS-SA possessed unique ability to transport across BBB both in vitro and in vivo. In conclusion, CS-SA could be exploited as a potential carrier to effectively deliver drugs across BBB and into brain.
Acknowledgments
We appreciate the financial support from the National Basic Research Program of China (973 Program) under Contract 2009CB930300, Key Project of Science and Technology of Zhejiang Province (grant no 2009C13037) and Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents.
Disclosure
The authors declare no conflicts of interest in this work.
References
- BegleyDJDelivery of therapeutic agents to the central nervous system: the problems and the possibilitiesPharmacol Ther20041041294515500907
- KusuharaHSugiyamaYEfflux transport systems for drugs at the blood–brain barrier and blood-cerebrospinal fluid barrier (Part 1)Drug Discov Today20016315015611165188
- BatrakovaEVKabanovAVPluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiersJ Control Release200813029810618534704
- OlivierJCDrug transport to brain with targeted nanoparticlesNeuroRx20052110811915717062
- GrefRCouvreurPBarrattGSurface-engineered nanoparticles for multiple ligand couplingBiomaterials200324244529453712922162
- CalvoPRemunan-LopezCVila-JatoJLDevelopment of positively charged colloidal drug carriers: chitosan-coated polyester nanocapsules and submicron-emulsionsColloid Polym Sci199727514653
- CouvreurPVauthierCPolyalkylcyanoacrylate nanoparticles as drug carrier: present state and perspectivesJ Control Release1991172187198
- GulyaevAEGelperinaSESkidanINSignificant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticlesPharm Res199916101564156910554098
- SteinigerSCJKreuterJKhalanskyASChemotherapy of glioblastoma in rats using doxorubicin loaded nanoparticlesInt J Cancer2004109575976714999786
- TosiGCostantinoLRivasiFTargeting the central nervous system: in vivo experiments with peptide-derivatized nanoparticles loaded with Loperamide and Rhodamine-123J Control Release200712211917651855
- AlyautdinRGothierDPetrovVAnalgesic activity of the hexapeptide dalargin adsorbed on the surface of polysorbate 80-coated poly (butyl cyanoacrylate) nanoparticlesEur J Pharm Biopharm19954114448
- KreuterJAlyautdinRNKharkevichDAPassage of peptides through the blood–brain barrier with colloidal polymer particles (nanoparticles)Brain Res199567411711747773690
- KusuharaHSuzukiHSugiyamaYThe role of P Glycoprotein and canalicular multispecific organic anion transporter in the hepatobiliary excretion of drugsJ Pharm Sci1998879102510409724551
- LoeDWDeeleyRGColeSPBiology of the multidrug resistance-associated protein, MRPEur J Cancer19963269458763335
- HipfnerDRDeeleyRGColeSPCStructural, mechanistic and clinical aspects of MRP1Biochim Biophys Acta19991461235937610581367
- ParkJHSaravanakumarGKimKTargeted delivery of low molecular drugs using chitosan and its derivativesAdv Drug Deliver Rev20106212841
- MistryAStolnikSIllumLNanoparticles for direct nose-to-brain delivery of drugsInt J Pharm2009379114615719555750
- HuFQZhaoMDYuanHA novel chitosan oligosaccharide-stearic acid micelles for gene delivery: properties and in vitro transfection studiesInt J Pharm20063151–215816616632285
- HuFQLiuLNDuYZSynthesis and antitumor activity of doxorubicin conjugated stearic acid-g-chitosan oligosaccharide polymeric micellesBiomaterials200930366955696319782395
- HuFQWuXDuYZCellular uptake and cytotoxicity of shell crosslinked stearic acid-grafted chitosan oligosaccharide micelles encapsulating doxorubicinEur J Pharm Biopharm200869111712517997293
- HuFQRenGFYuanHShell cross-linked stearic acid grafted chitosan oligosaccharide self-aggregated micelles for controlled release of paclitaxelColloids Surf B Biointerfaces20065029710316759840
- YuanHLuLDuYZStearic acid-g-chitosan polymeric micelle for oral drug delivery: in vitro transport and in vivo absorptionMol Pharm20118122523821138243
- HuangMMaZKhorEUptake of FITC-chitosan nanoparticles by A549 cellsPharm Res200219101488149412425466
- KohoriFYokoyamaMSakaiKProcess design for efficient and controlled drug incorporation into polymeric micelle carrier systemsJ Control Release2002781–315516311772457
- YuJMLiYJQiuLYPolymeric nanoparticles of cholesterol modified glycol chitosan for doxorubicin delivery: preparation and in vitro and in vivo characterizationJ Pharm Pharmacol200961671371919505361
- StefanieWAlexanderKSvetlanaGKinetics of transport of doxorubicin bound to nanoparticles across the blood–brain barrierJ Control Release2011154110310721616104
- LodeJFichtnerIKreuterJInfluence of surface-modifying surfactants on the pharmacokinetic behavior of 14C-poly (methylmethacrylate) nanoparticles in experimental tumor modelsPharm Res200118111613161911758771
- AmbruosiAKhalanskyASYamamotoHBiodistribution of polysorbate 80-coated doxorubicin-loaded [14C]-poly(butyl cyanoacrylate) nanoparticles after intravenous administration to glioblastoma-bearing ratsJ Drug Target20061429710516608736
- BriggerIMorizetJLaudaniLNegative preclinical results with stealth nanospheres-encapsulated doxorubicin in an orthotopic murine brain tumor modelJ Control Release20041001294015491808
- BriggerIMorizetJAubertGPoly(ethylene glycol)-coated hexadecylcyanoacrylate nanospheres display a combined effect for brain tumor targetingJ Pharmacol Exp Ther2002303392893612438511
- CalvoPGouritinBChacunHLong-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain deliveryPharm Res20011881157116611587488