Abstract
Gold nanoparticles synthesized via sodium citrate reduction of chloroauric acid (HAuCl4) were functionalized with either various concentrations of thiol-terminated Bodipy® FL L-cystine (0.5, 1.0, 1.5, and 2.0 μg/mL) or Bodipy-poly(ethylene glycol) at concentrations of 0.5–18.75, 1.0–12.50, and 1.5–6.25 μg/mL to form a mixed monolayer of BODIPY-PEG. Thiol-terminated Bodipy, a fluorescing molecule, was used as the model drug, while PEG is widely used in drug-delivery applications to shield nanoparticles from unwanted immune responses. Understanding the influence of PEG-capping on payload release is critical because it is the most widely used type of nanoparticle functionalization in drug delivery studies. It has been previously reported that glutathione can trigger release of thiol-bound payloads from gold nanoparticles. Bodipy release from Bodipy capped and from Bodipy-PEG functionalized gold nanoparticles was studied at typical intracellular glutathione levels. It was observed that the addition of PEG capping inhibits the initial burst release observed in gold nanoparticles functionalized only with Bodipy and inhibits nanoparticle aggregation. Efficient and controlled payload release was observed in gold nanoparticles cofunctionalized with only a limited amount of PEG, thus enabling the coattachment of large amounts of drug, targeting groups or other payloads.
Introduction
Gold nanoparticles have shown great potential application in the biomedical fields of immunostaining,Citation1,Citation2 phagokinetics,Citation3 in single particle tracking,Citation4 as contrast agents,Citation3 and in drug delivery.Citation5,Citation6 Gold nanoparticles can be readily synthesized in sizes of 1–150 nm from gold salt solutions, using reducing agents such as sodium citrate or sodium borohydride. It has been reported that both the payload delivery and toxicity of nanoparticles depend to a significant degree on particle size.Citation7 Gold nanoparticles can be easily functionalized with a wide range of drugs, targeting and shielding groups using thiol linkers.Citation8 Due to the noncovalent interaction of DNA with gold nanoparticles, DNA-gold complexes have been also been used in gene delivery,Citation9 biosensing, diagnostics, finding complementary DNA sequences, and in nanocrystal formation.Citation8 Numerous studies have found that gold nanoparticles can be internalized in cells; and with appropriate surface modification using membrane-bound transferrin ligands, specific cellular uptake is possible.Citation10,Citation11 For example, gold nanoparticles which are surface modified with folate receptors have been used for targeting cancer cells,Citation12 and with the presence of peptides on the surface, direct translocation to the nucleus has been observed.Citation13
As noted, the surface of gold nanoparticles can be readily cofunctionalized with a wide range of groups for different functions. The stability of metallic nanoparticles in a range of media and their circulation time in vivo can be increased using capping agents such as polyethylene glycol (PEG),Citation14–Citation16 mercaptosuccinic acid,Citation17 mercaptopropionic acid,Citation14,Citation18 and various block polymers,Citation19–Citation22 leading to improved stability at various pH levels and at physiological salt concentrations.Citation5,Citation18 These antifouling coatings can be combined with other groups, such as drug payloads and targeting agents. For example, Shenoy functionalized gold nanoparticles with coumarin as a fluorescent dye using PEG as a spacer,Citation23 while modification with a mixed PEG-peptide conjugate has been used to target the cytoplasm of HeLa cells.Citation24 Meanwhile, Dreaden et al used gold nanoparticles capped with both PEG and tamoxifen to target and deliver the drug to breast cancer cells using the estrogen receptor.Citation15 In another study, PEG-functionalized gold nanoparticles were conjugated with monoclonal F19 antibodies to target human pancreatic carcinoma tissues.Citation25
The formulation CYT-6091 which is tumor necrosis factor and PEG on gold nanoparticles, has been tested for the treatment of tumors in a mouse model.Citation26 It was reported that these gold nanoparticles accumulated in tumors, resulting in reduced tumor size and an increased mean survival time, and this work is now proceeding to clinical trials in humans. The studies have shown that PEGylated nanoparticles can overcome rapid removal by the reticuloendothelial system, and after payload release, the nanoparticles were extracted from the systemic circulation by cells in the liver.Citation26,Citation27
Release of the payload at the required site can be achieved using photoexcitation or by means of intracellular glutathione levels. The intracellular concentration of glutathione is 1–10 mM, whereas extracellular levels are 2 μM, which can be used to break the thiol linker, releasing the payload inside the cell.Citation28–Citation30 For example, an eight-fold increase in the release of Bodipy® from gold nanoparticles cofunctionalized with tetra (ethylene glycol)-lyated cationic ligand was observed in the presence of typical intracellular levels of glutathione.Citation28
While the influence of PEG on the stability, circulation time, and biological fate of gold nanoparticles has been widely studied, the effect of such cofunctionalization on payload release is not fully understood. The presence of large PEG molecules on the surface of gold nanoparticles will likely influence any interactions with other groups on the surface through steric effects, altering payload release. In this study, we use the quenching properties of gold nanoparticlesCitation31 to study the release of a thiol-terminated fluorescent dye (Bodipy)Citation32 from gold nanoparticles with and without PEG capping in glutathione over a period of 5 days using photoluminescence spectroscopy. Such data would be of interest in the development of mixed monolayer surface coatings for gold nanoparticle drug-delivery systems.
Materials and methods
Materials
Chloroauric acid (HAuCl4 · xH20), sodium hydroxide (NaOH), trisodium citrate (Na3C6H5O7), O-(2-(3-mercaptopropionylamino)ethyl)-O′-methylpolyethylene glycol [thiol-terminated poly(ethylene glycol)], referred to as PEG (molecular weight 5000), glutathione (C10H17N3O6S), and dithiothreitol (DTT, C4H10O2S2) were purchased from Sigma Aldrich (Dorset, UK). Chloroauric acid, trisodium citrate, and sodium hydroxide stock solutions were prepared using distilled water (15 MΩ cm) at concentrations of 0.2 wt%, 1%, and 0.1 M, respectively. Freshly prepared solutions of glutathione (5 mM and 10 mM) and DTT (7 mg/mL) were made in distilled water before the release experiments. Bodipy FL L-cystine (C34H38B2F4N6O6S2) was purchased from Invitrogen (Paisley, UK) and stored at −20°C. The 0.5 mg/mL solution used to functionalize gold nanoparticles was prepared in distilled water using DTT and stored at −4°C.
Synthesis of gold nanoparticles
Gold nanoparticles were prepared using a modified Turkevich and Frens method as previously described.Citation32,Citation33 Briefly, 4.5 mL of 1 wt% sodium citrate solution was added to 200 mL of boiling 0.01 wt% chloroauric acid solution, refluxed for one hour, and then allowed to cool to room temperature with continuous stirring, yielding colloidal gold with a concentration of 0.058 mg/mL or 0.29 mM. NaOH solution (0.1 M, 500 μL) was added to 20 mL batches of gold nanoparticle colloid under stirring in order to adjust the pH to 8.0. Nanoparticles at pH 8.0 were used for all subsequent experimentation, including surface modification by Bodipy and Bodipy-PEG.
Bodipy-PEG conjugate formation
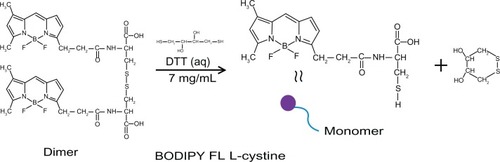
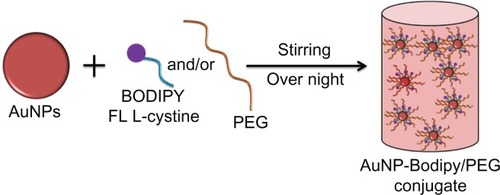
Bodipy FL L-cystine 1 mg comes in a lyophilized dimer form, and 2 mL of DTT 7 mg/mL was added to that to make a 0.5 mg/mL solution of Bodipy. This solution was then vortexed in an Eppendorf tube for about 20 minutes at room temperature to form Bodipy monomer, as shown in . The Bodipy-DTT solution was dialyzed three times with water changes to remove the DTT from the Bodipy solution. The dialyzed thiol-terminated Bodipy solution was taken in precalculated concentrations (0.5, 1.0, 1.5, and 2.0 μg per mL of colloidal gold) and added to the gold nanoparticles (in 20 mL batch sizes). Next, 0.5, 1.0, and 1.5 μg/mL of Bodipy were added with various amounts of PEG (6.25, 12.50, and 18.75 μg/mL) to the gold nanoparticles in 20 mL batches and stirred overnight to prepare cofunctionalized nanoparticles with 0.5–18.75, 1.0–12.50, and 1.5–6.25 μg/mL of Bodipy-PEG, as shown in . The Bodipy and Bodipy-PEG functionalized gold nanoparticles were then further dialyzed to remove any residual reactants, and stored at 4°C.
Characterization of gold nanoparticles alone and those conjugated with Bodipy and Bodipy-PEG
The gold nanoparticles were characterized using ultraviolet-visible spectroscopy, dynamic light scattering, zeta potential measurement, and Fourier transform infrared spectroscopy. A PerkinElmer Lambda 35 ultraviolet-visible spectrometer was used for collecting the absorbance spectra of all samples from 400 nm to 700 nm at 0.1 nm intervals. For size and zeta potential measurements, a Malvern Zetasizer Nano-ZS series (Worcestershire, UK) was used with disposable cuvettes of 2 mL and 700 μL, respectively, for dynamic light scattering and zeta measurements. The size data are reported as the intensity average measured from three samples, with error bars of ±1 standard error. Fourier transform infrared absorbance spectra were taken of the dried gold nanoparticle samples prepared into pellets with KBr using a Varian 640-IR Fourier transform infrared spectrometer in diffuse reflectance mode at 4 cm−1 resolution between 400 cm−1 and 4000 cm−1. Transmission electron microscopy was used to characterize the size and shape of the gold nanoparticles. Transmission electron microscopy images were collected using a Philips TECNAI system (Eindhoven, The Netherlands) at 200 kV and the samples were dried at room temperature onto carbon-coated copper grids.
Stability of gold nanoparticles conjugated with Bodipy and Bodipy-PEG in glutathione
The stability of “as-synthesized” gold nanoparticles and gold nanoparticles capped with 0.5 and 2.0 μg/mL of Bodipy and with 0.50–18.75, 1.0–12.5, and 1.50–6.25 μg/mL of Bodipy-PEG were examined using ultraviolet-visible spectroscopy in both 5 mM and 10 mM glutathione solutions.
Glutathione-mediated Bodipy release
To investigate Bodipy release from gold nanoparticles functionalized with Bodipy and Bodipy-PEG, 0, 5, and 10 mM glutathione solutions were made up using 1 mL of functionalized colloidal gold. Next, 1 mL of toluene was added over the gold suspension, and Bodipy release into the toluene layer was studied by photoluminescence for 5 days.Citation28–Citation30,Citation34 Use of the toluene layer was necessary because the hydrophobic Bodipy tends to aggregate in water. Photoluminescence was recorded using an inhouse system. The software used was Solis 4.19.30 with acquisition mode DDG on, accumulation number 20, slit 500 μm, gain 50, transistor-transistor logic width 400, and a system temperature of −10°C. Bodipy release over a period of 5 days in 5 mM and 10 mM glutathione was characterized by photoluminescence counts at 513 nm as a result of excitation at 340 nm. The release experiment was maintained at 37°C using a water bath (schematic representation, Supplementary Figure 11).
Results and discussion
Ultraviolet-visible spectroscopy
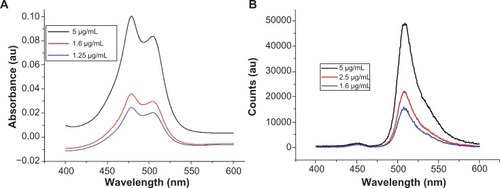
shows ultraviolet-visible and photoluminescence spectra for the free Bodipy molecule in water, which has not previously been reported in the literature. The ultraviolet-visible spectra for the three concentrations measured show two characteristic absorbance peaks at 479.3 nm and 503.5 nm. Photoluminescence (PL) measurements of the aqueous solutions excited at 340 nm displayed a characteristic emission peak at 513 nm.
Figure 3 (A) Absorbance spectra of BODIPY® with Lambda Max values at 479.3 nm and 503.5 nm. (B) Photoluminescence spectra of BODIPY with emission at 513 nm (340 nm excitation).
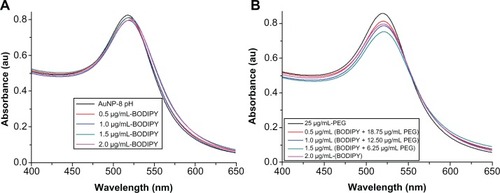
shows the ultraviolet-visible absorbance spectra of gold nanoparticles conjugated with Bodipy and with Bodipy-PEG. The Lambda Max and absorbance for each sample is given in . With increasing amounts of Bodipy functionalization from 0.0 μg/mL to 2.0 μg/mL, the position of Lambda Max increased from 518.1 nm to 520.7 nm, which is indicative of an increase in diameter resulting from attachment of Bodipy. In the case of the Bodipy-PEG cofunctionalized gold nanoparticles, the position of Lambda Max increased from 518.1 nm for as-synthesized gold nanoparticles to 520.6–521.5 nm for gold nanoparticles conjugated with Bodipy-PEG. Peaks for free Bodipy were not observed in the spectra of the functionalized gold nanoparticles due to the quenching properties of the gold nanoparticles. The ultraviolet-visible spectra of the functionalized gold nanoparticles prepared with more than 2.0 μg/mL of Bodipy displayed peaks at 479.3 nm and at 503.5 nm as a result of free Bodipy in the solution. These peaks were not visible after washing by dialysis.
Table 1 Ultraviolet-visible characteristics of AuNPs samples modified with BODIPY and or PEG with corresponding Lambda Max and absorbance values
Dynamic light scattering and zeta potential measurement
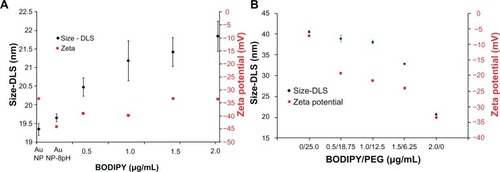
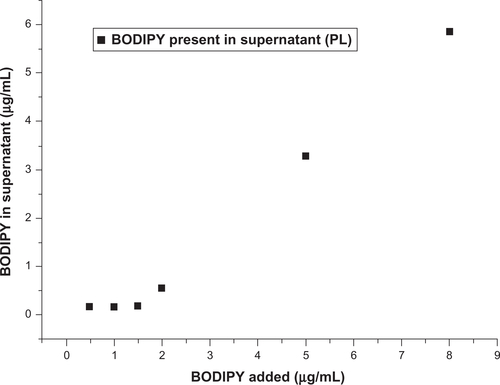
The dynamic light scattering results shown in show a slight increase in hydrodynamic radius from 19.3 for the as-synthesized nanoparticles to 19.6 nm with pH adjustment to 8.0, and to 20.4–21.9 nm with increasing amounts of Bodipy functionalization up to 2 μg/mL. Photoluminescence measurements of the supernatant remaining after the gold nanoparticles have been removed using centrifugation showed that the gold nanoparticle surface begins to saturate with Bodipy at an addition rate of 1.5 μg/mL. At higher addition levels, it was found that excess Bodipy remained in the supernatant (see Supplementary Figure 10). A diameter of about 40.6 nm was measured for gold nanoparticles functionalized only with 25.0 μg/mL of PEG. This level of PEG has been previously shown to equate to almost complete surface saturation, with the PEG monolayer adopting an extended random coil conformation.Citation35 A relatively small reduction in diameter compared with the fully PEG-capped gold nanoparticles of 38–39 nm was measured for the 0.5–18.75 and 1.0–12.5 μg/mL gold nanoparticles cofunctionalized with Bodipy-PEG. This demonstrates that reducing the density of PEG functionalization by some 50% and attaching Bodipy to the gold nanoparticle surface does not dramatically alter the PEG conformation. Gold nanoparticles functionalized with 6.25 μg/mL of PEG, which is equivalent to approximately 25% surface coverage and 1.25 μg/mL of Bodipy, displayed a diameter of about 32 nm. This low packing density results in the PEG adopting a somewhat less extended conformation producing a smaller hydrodynamic radius.Citation36 Dynamic light scattering measurements recorded a surface charge (zeta potential) of −45 mV for the pH-adjusted citrate-capped gold nanoparticles, rising to −34 mV with increasing amounts of Bodipy capping. This compared with −7 mV for the 25.0 μg/mL PEG-capped nanoparticles. Gold nanoparticles cofunctionalized with both Bodipy and PEG display intermediate levels of surface charge in the range of −25 mV to −19 mV with increasing amounts of PEG.
Figure 5 Dynamic light scattering and zeta potential of gold nanoparticles coated with (A) BODIPY® and (B) BODIPY-PEG.
Abbreviations: DLS, dynamic light scattering; PEG, poly(ethylene glycol); AuNPs, gold nanoparticles.
Transmission Electron Microscopy
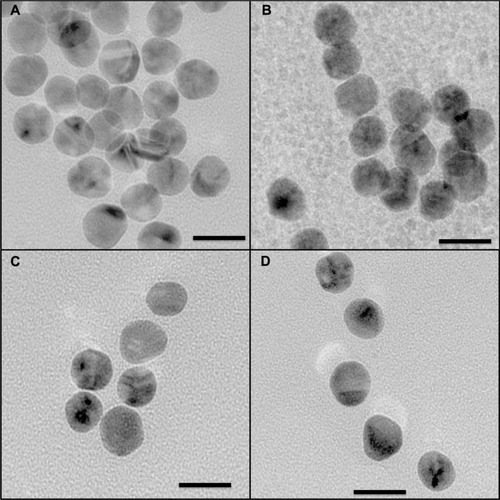
Transmission electron microscopic images for as-prepared gold nanoparticles and those functionalized with Bodipy and/or PEG are shown in . The somewhat faceted spherical particles show a largely monodispersed size distribution with an average (± standard deviation) size of 15.5 ± 1.53 nm for the as-synthesized gold nanoparticles. As expected, the size of the functionalized gold nanoparticles measured using transmission electron microscopy was not significantly different to the as-synthesized gold nanoparticles because transmission electron microscopy is not sensitive to the organic coating at the acceleration voltage used. For each sample type, some 80–120 nanoparticles were counted in order to calculate size and standard deviation using Image J software and the statistics option in Origin 7.0.
Figure 6 Transmission electron microscopic images for gold nanoparticles (A). Gold nanoparticles conjugated with BODIPY® (2.0 μg/mL), (B). Gold nanoparticles conjugated with BODIPY-PEG (1.5–6.25 μg/mL), (C). And gold nanoparticles conjugated with PEG (25.0 μg/mL) (D).
Note: Scale is 20 nm at 145,000×.
Abbreviation: PEG, poly(ethylene glycol).
Fourier transform infrared spectroscopy
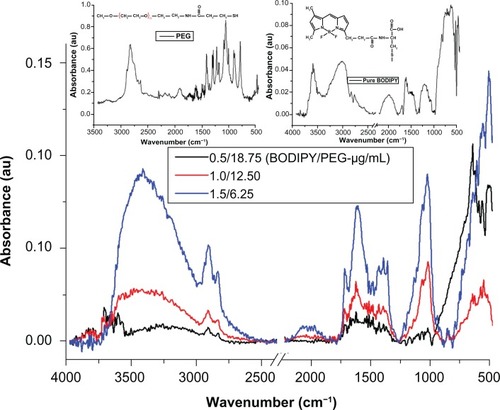
The presence of PEG on the surface of the gold nanoparticle conjugated with PEG is confirmed by the bands at 3000–2850 cm−1 (–CH2 stretching), 1631 cm−1 (N–H bending), 1660 cm−1 (C=O stretching), 1380 cm−1 (C–H bending; –CH2 and –CH3), 1100 cm−1 (C–O–C stretching), and 600–900 cm−1 (N–H wagging)Citation35,Citation37 (see Supplementary Figure 1). In the case of gold nanoparticles conjugated with Bodipy-PEG, peaks at 3190 cm−1 [ν(N–H, O–H)], 2969–2850 cm−1 [ν(C–H)], about 1710, 1604–1400 cm−1 [νas (C=O), νs (COO–)], 1350–1250 cm−1 [ν(C–N)], about 1625 (C=C), 3100–3010 (C–H, ring), 3550–2500 (carboxylic O–H, broad) and in the 2969–2850 cm−1 region ν(C–H), as shown in , confirm the presence of both Bodipy and PEG on the gold nanoparticle surface. The bonding of both groups to the gold nanoparticle surface in a mixed monolayer is further demonstrated by the peaks missing at 2550 cm−1 and 945 cm−1 (–SH) in the spectra for gold nanoparticles conjugated with Bodipy-PEG, which are present in the spectra of pure Bodipy and PEG.Citation38 The absence of these peaks is attributed to the breakup of the –SH group, which results from the bonding of thiolated Bodipy and PEG with the gold nanoparticle surface. Fourier transform infrared spectra for gold nanoparticles capped solely with PEG (25 μg/mL) and Bodipy (1.0 and 2.0 μg/mL) are given in Supplementary Figures 1 and 2.
Stability of gold nanoparticles conjugated with Bodipy and Bodipy-PEG
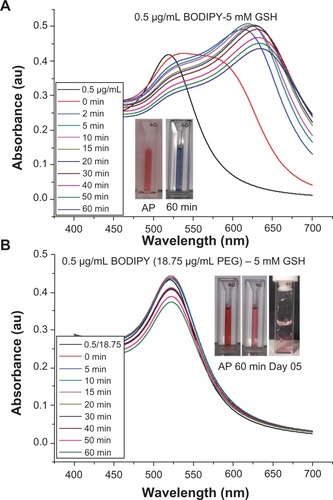
It is widely reported in the literature that as-synthesized citrate-capped gold nanoparticle colloids are stable for extended periods at room temperature. However, the addition of PEG-capping also renders the gold nanoparticles stable in a wide range of media, including organic solvents, and at physiological salt concentrations.Citation35 The stability of 0.5 μg/mL Bodipy-capped and 0.5–18.75 μg/mL Bodipy-PEG-capped gold nanoparticles were evaluated in 5 mM and 10 mM glutathione. The black trace in denotes the ultraviolet-visible spectra prior to addition of glutathione. The large upshift in peak position observed in the gold nanoparticles conjugated with Bodipy as a result of addition of glutathione is indicative of significant aggregation. This is confirmed by the insert image in , with a blue color observed with in minutes after the addition of glutathione, while the insert images of Bodipy-PEG-conjugated gold nanoparticle solutions in still show the characteristic red color of colloidal gold after addition of glutathione. The Bodipy-PEG-conjugated gold nanoparticles show excellent stability, with no upshift in ultraviolet-visible peak position and only a slight decrease in absorbance over time. The stability graphs of ultraviolet-visible absorbance spectra for as-synthesized gold nanoparticles and those functionalized with Bodipy and/or PEG formulations in 5 mM and 10 mM of glutathione are shown in Supplementary Figures 3–9, and confirm the effect of PEG on stability.
Figure 8 UV-Visible spectra of gold nanoparticles capped with 0.5 μg/ml of BODIPY (A) and 0.5–18.75 μg/ml of BODIPY-PEG (B) in 5 mM glutathione (GSH) solution. The inset images showing the color of as prepared (AP) nanoparticles and after 60 minutes and at day 5 in 5 mM GSH.
Abbreviation: PEG, poly(ethylene glycol).
Glutathione-mediated Bodipy release
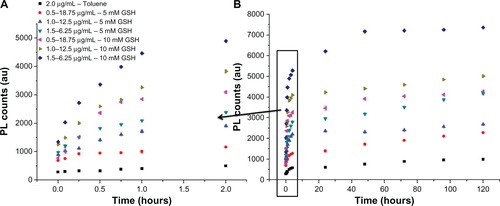
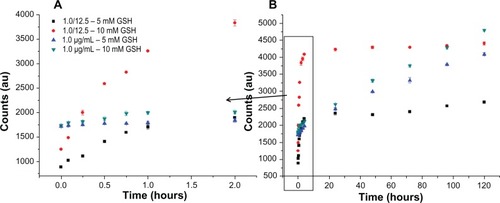
shows the release of Bodipy from gold nanoparticles conjugated with Bodipy (1.0 μg/mL) and gold nanoparticles conjugated with Bodipy-PEG (1.0–12.5 μg/mL) in the presence of 5 mM and 10 mM glutathione. Glutathione contains a thiol group and acts as a reducing agent in cells to prevent damage caused by reactive oxygen species, such as free radicals.Citation39 The photoluminescence of the released Bodipy displayed peaks equivalent to those of free Bodipy, demonstrating that attachment and release of the dye from the gold nanoparticle surface has not altered its fluorescent behavior. Comparing the release profile from gold nanoparticles functionalized with 1.0 μg/mL of Bodipy with and without 12.5 μg/mL of PEG cofunctionalization, it is apparent that the presence of PEG has a significant influence on Bodipy release. Gold nanoparticles conjugated with Bodipy show an initial burst release in the presence of 5 mM and 10 mM glutathione, whereas gold nanoparticles conjugated with Bodipy-PEG show controlled release. Without PEG functionalization, the gold nanoparticles display an initial burst release followed by a prolonged period of sustained release over a period of 120 hours. It is postulated that the initial burst release occurs during aggregation of the gold nanoparticles, and that this aggregation slows the subsequent release. However, with the addition of PEG capping no initial burst release is observed, with gradual release over an approximately 2-hour period. While the release profile differs, the total amount of Bodipy released over a 120-hour period in the presence of 10 mM glutathione is similar for the gold nanoparticles with and without addition of PEG. The slight reduction in the total amount of Bodipy released from the PEG cofunctionalized gold nanoparticles may be a result of steric shielding. This effect is more evident at a glutathione concentration of 5 mM, with the total amount of Bodipy released over 120 hours significantly reduced by PEG cofunctionalization.
Figure 9 Gold Au nanoparticles conjugated with BODIPY®-PEG and with BODIPY (1.0 μg/mL) released in 5 mM and 10mM glutathione (GSH). First 2 hours of release (A) and release until day 5 (B) expressed as photoluminescence (PL) counts.
Abbreviation: PEG, poly(ethylene glycol).
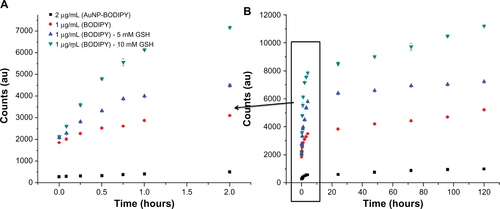
shows release of Bodipy expressed as a percentage of the initial amount attached from 0.5–18.75 μg/mL, 1.0–12.5 μg/mL, and 1.5–6.25 μg/mL gold nanoparticles cofunctionalized with Bodipy-PEG in 5 mM and 10 mM glutathione. also includes the Bodipy release profile from gold nanoparticles functionalized with 2.0 μg/mL of Bodipy in the absence of glutathione as a control. The limited release of Bodipy from gold nanoparticles conjugated with Bodipy in the absence of glutathione confirms the critical role that the tripeptide plays in mediating the release of thiol-bound payloads at typical intracellular levels.
Figure 10 Release of BODIPY® over 2 hours (A) and 5 days (B) from gold nanoparticles conjugated with BODIPY-PEG expressed as wt%. Release in 0 mM glutathione (GSH) for control (AuNP-BODIPY) and 5 and 10 mM GSH for AuNP-BODIPY-PEG samples.
Abbreviation: AuNP, Gold nanoparticles; PEG, poly(ethylene glycol).
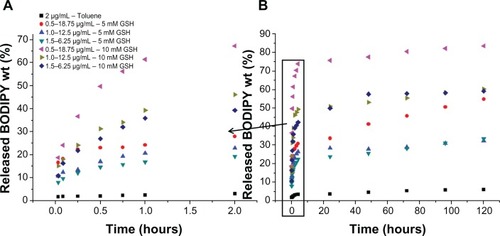
In , the release is expressed in wt% of the original amount of attached Bodipy calculated using a photoluminescence calibration curve of free Bodipy. As with the Bodipy release expressed as counts shown in , no initial burst phase is observed for the cofunctionalized gold nanoparticles. Over the first 2 hours, 27.9, 22.8, and 19.2 wt% of the attached Bodipy is released from the nanoparticles in 5 mM glutathione for the 0.5–18.75, 1.0–12.5, and 1.5–6.25 μg/mL samples. This compares with 67.2, 46.1 and 39.2 wt% over the same period for 10 mM glutathione. shows the release profiles over a 5-day period, with up to 83.3% of the attached Bodipy released in the case of 0.5–18.75 μg/mL BODIPY-PEG functionalized gold nanopartilces. Experiments conducted with free Bodipy (see Supplementary Figure 13), by adding a layer of toluene to solutions containing 1.0 μg/mL of Bodipy in the presence of 0, 5, and 10 mM glutathione were also undertaken, and it was observed that glutathione not only helps in dissociation of Bodipy from the gold nanoparticle surface but also assists in phase transfer of the Bodipy to the toluene layer. As compared with release in 0 mM glutathione (control), 2.3, 3.8, and 4.7 times higher Bodipy release was observed after 2 hours in 5 mM glutathione for 0.5–18.75, 1.0–12.50, and 1.5–6.25 μg/mL samples of gold nanoparticles functionalized with Bodipy-PEG, respectively.
Conclusion
The study has demonstrated cofunctionalization of gold nanoparticles with thiolated PEG and Bodipy, with the presence of both molecules confirmed via Fourier transform infrared spectroscopy. It was observed that the addition of Bodipy caused an increase in gold nanoparticle diameter, measured by dynamic light scattering, from 19.6 to 20–22 nm, compared with 32–39 nm for Bodipy and PEG cofunctionalization. For gold nanoparticles cofunctionalized with Bodipy-PEG, addition of PEG rendered the gold nanoparticles stability at intracellular glutathione levels, while the gold nanoparticle samples conjugated only with Bodipy displayed significant aggregation. The glutathione-mediated release studies showed that the addition of PEG to gold nanoparticles stops the initial burst release of Bodipy observed in samples without PEG. However, addition of PEG reduced the total amount of Bodipy released, especially at a glutathione concentration of 5 mM. It was observed that up to about 83% of the attached Bodipy was released from the cofunctionalized gold nanoparticles in the presence of 10 mM glutathione. The study confirms the role of PEG in stabilizing gold nanoparticles and describes the payload release behavior of gold nanoparticles capped with a mixed monolayer. Varying the amount of PEG functionalization and glutathione concentration enables control of the loading capacity and release profile of Bodipy.
Acknowledgements
We acknowledge Somak Mitra and Davide Mariotti for the photoluminescence spectroscopy and Isha Mutreja and Susmita Mitra for their support and guidance. Department for Employment and Learning and Cross Border Research Funding are also thanked for funding this project.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary figures
Figure S1 Fourier transform infrared absorbance spectra for 25 μg/mL PEG capped gold nanoparticles.
Abbreviations: AuNPs, gold nanoparticles; PEG, poly(ethylene glycol).
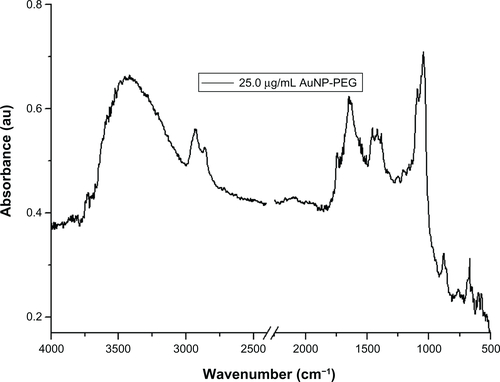
Figure S2 Fourier transform infrared absorbance spectra for 1.0 μg/mL and 2.0 μg/mL of BODIPY® on the surface of gold nanoparticles.
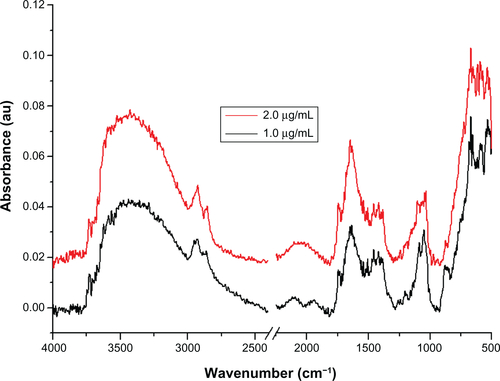
Figure S3 Ultraviolet-visible absorbance spectra for gold nanoparticles conjugated with PEG (25.0 μg/mL), stability in 10 mM glutathione (GSH).
Abbreviations: PEG, poly(ethylene glycol); AP, as prepared sample and in GSH after 60 minutes.
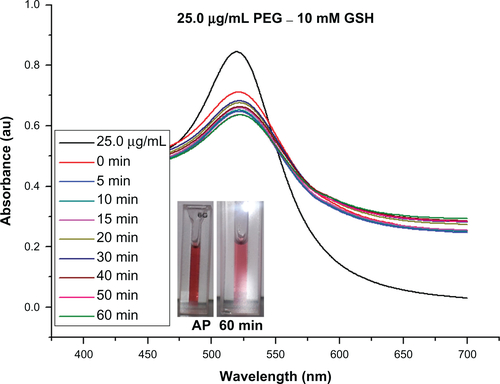
Figure S4 Ultraviolet-visible absorbance spectra for gold nanoparticles capped with BODIPY® (2.0 μg/mL), and their stability in 5 mM glutathione (GSH) (samples show as prepared [AP] and GSH in 60 minutes).
![Figure S4 Ultraviolet-visible absorbance spectra for gold nanoparticles capped with BODIPY® (2.0 μg/mL), and their stability in 5 mM glutathione (GSH) (samples show as prepared [AP] and GSH in 60 minutes).](/cms/asset/cfc5fd10-1fe3-4a7b-b729-ec91980489af/dijn_a_33726_sf0004_c.jpg)
Figure S5 Ultraviolet-visible absorbance spectra for stability of as-prepared gold nanoparticles in 5 mM and 10 mM glutathione solution (samples show as prepared [AP] and in GSH after 60 minutes).
Abbreviations: AuNPs, gold nanoparticles; PEG, poly(ethylene glycol).
![Figure S5 Ultraviolet-visible absorbance spectra for stability of as-prepared gold nanoparticles in 5 mM and 10 mM glutathione solution (samples show as prepared [AP] and in GSH after 60 minutes).Abbreviations: AuNPs, gold nanoparticles; PEG, poly(ethylene glycol).](/cms/asset/b1966899-109b-4b59-a338-43676b6a9063/dijn_a_33726_sf0005_c.jpg)
Figure S6 Ultraviolet-visible absorbance spectra for stability of gold nanoparticles conjugated with 0.5 and 2.0 μg/ml of BODIPY® in 10 mM glutathione. (samples show as prepared [AP] and in GSH after 60 minutes).
Abbreviation: GSH, glutathione.
![Figure S6 Ultraviolet-visible absorbance spectra for stability of gold nanoparticles conjugated with 0.5 and 2.0 μg/ml of BODIPY® in 10 mM glutathione. (samples show as prepared [AP] and in GSH after 60 minutes).Abbreviation: GSH, glutathione.](/cms/asset/931efb53-27e1-4758-8a88-01cbfe712ae3/dijn_a_33726_sf0006_c.jpg)
Figure S7 Ultraviolet-visible absorbance spectra for stability of gold nanoparticles conjugated with 1.0–12.5 μg/ml of BODIPY®-PEG in 5 and 10 mM glutathione. (AP-As Prepared Sample and in GSH after 60 minute and on day 5).
Abbreviation: GSH, glutathione.
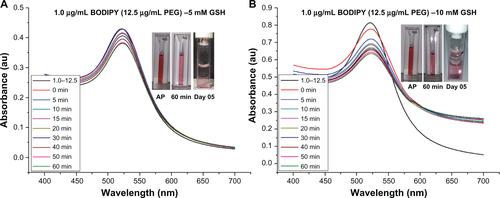
Figure S8 Ultraviolet-visible absorbance spectra for stability of gold nanoparticles conjugated with 1.5–6.25 μg/ml of BODIPY®-PEG in 5 mM and 10 mM glutathione (GSH). As prepared (AP) sample and in GSH after 60 minutes and on day 5.
Abbreviations: PEG, poly(ethylene glycol).
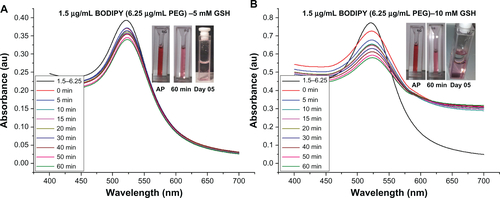
Figure S9 Ultraviolet-visible absorbance spectra for stability of gold nanoparticles conjugated with BODIPY®-PEG (0.5–18.75 μg/mL), and stability in 10 mM glutathione (GSH). As prepared (AP) sample and in GSH after 60 minutes and on day 5.
Abbreviations: PEG, poly(ethylene glycol).
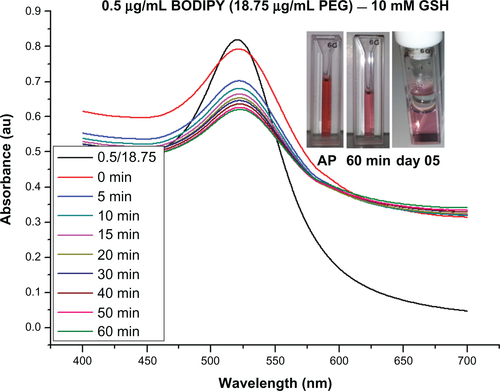
Figure S10 Amount of free BODIPY® present in supernatant after AuNP-BODIPY nanoparticles centrifugation with increasing concentration of BODIPY.
References
- FaulkWPTaylorGMAn immunocolloid method for the electron microscopeImmunochemistry1971811108110834110101
- GeuzeHJSlotJWvan der LeyPASchefferRCUse of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sectionsJ Cell Biol19818936536656166621
- SperlingRAGilPRZhangFZanellaMParakWJBiological applications of gold nanoparticlesChem Soc Rev20083791896190818762838
- YangYHNamJMSingle nanoparticle tracking-based detection of membrane receptor – ligand interactionsAnal Chem20098172564256819228043
- De JongWHBormPJADrug delivery and nanoparticles: applications and hazardsInt J Nanomed200832133149
- CaiWGaoTHongHSunJApplications of gold nanoparticles in cancer nanotechnologyNanotechnol Sci Appl200811732
- OhEDelehantyJBSapsfordKECellular uptake and fate of PEGylated gold nanoparticles is dependent on both cell-penetration peptides and particle sizeACS Nano.2011586434644821774456
- DanielMCAstrucDGold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnologyChem Rev2004104129334614719978
- KuriyamaSMitoroATsujinoueHParticle-mediated gene transfer into murine livers using a newly developed gene gunGene Ther20007131132113610918480
- ZaunerWOgrisMWagnerEPolylysine-based transfection systems utilizing receptor-mediated deliveryAdv Drug Deliv Rev1998301–39711310837605
- LiHQianZMTransferrin/transferrin receptor-mediated drug deliveryMed Res Rev200222322525011933019
- DixitVVan den BosscheJShermanDMThompsonDHAndresRPSynthesis and grafting of thioctic acid-PEG-folate conjugates onto Au nanoparticles for selective targeting of folate receptor-positive tumor cellsBioconjug Chem200617360360916704197
- JesusMBerryCCTat peptide as an efficient molecule to translocate gold nanoparticles into the cell nucleusBioconjug Chem20051651176118016173795
- TsaiDHDelRioFWMacCuspieRIChoTJZachariahMRHackleyVACompetitive adsorption of thiolated polyethylene glycol and mercaptopropionic acid on gold nanoparticles measured by physical characterization methodsLangmuir20102612103251033320465235
- DreadenECMwakwariSCSodjiQHOyelereAKEl-SayedMATamoxifen-poly(ethylene glycol)-thiol gold nanoparticle conjugates: enhanced potency and selective delivery for breast cancer treatmentBioconjug Chem200920122247225319919059
- ChoWSChoMJeongJAcute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticlesToxicol Appl Pharm200923611624
- ZhuTVasilevKKreiterMMittlerSKnollWSurface modification of citrate-reduced colloidal gold nanoparticles with 2-mercaptosuccinic acidLangmuir2003192295189525
- LiJWangXWangCThe enhancement effect of gold nanoparticles in drug delivery and as biomarkers of drug-resistant cancer cellsChem Med Chem20072337437817206735
- MollerMSpatzJPRoescherAGold nanoparticles in micellar poly(styrene)-β-poly(ethylene oxide) films – size and interparticle distance control in monoparticulate filmsAdv Mater199684337340
- SakaiTAlexandridisPSpontaneous formation of gold nanoparticles in poly(ethylene oxide)poly(propylene oxide) solutions: solvent quality and polymer structure effectsLangmuir200521178019802516089415
- LiuSWeaverJVMSaveMArmesSPSynthesis of pH-responsive shell cross-linked micelles and their use as nanoreactors for the preparation of gold nanoparticlesLangmuir2002182283508357
- AzzamTEisenbergAMonolayer-protected gold nanoparticles by the self-assembly of micellar poly(ethylene oxide)-b-poly(ɛ-caprolactone) block copolymerLangmuir20072342126213217279704
- ShenoyDFuWLiJSurface functionalization of gold nano-particles using hetero-bifunctional poly (ethylene glycol) spacer for intracellular tracking and deliveryInt J Nanomed2006115157
- LiuYShiptonMKRyanJKaufmanEDFranzenSFeldheimDLSynthesis, stability, and cellular internalization of gold nanoparticles containing mixed peptide-poly(ethylene glycol) monolayersAnal Chem20077962221222917288407
- EckWCraigGSigdelAPEGylated gold nanoparticles conjugated to monoclonal F19 antibodies as targeted labeling agents for human pancreatic carcinoma tissueACS Nano.20082112263227219206392
- GoelRShahNVisariaRPaciottiGFBischofJCBiodistribution of TNF-α-coated gold nanoparticles in an in vivo model systemNanomedicine20094440141019505243
- PaciottiGFMyerLWeinreichDColloidal gold: a novel nanoparticle vector for tumor directed drug deliveryDrug Deliv200411316918315204636
- HongRHanGFernandezJMKimBForbesNSRotelloVMGlutathione-mediated delivery and release using monolayer protected nanoparticle carriersJ Am Chem Soc200612841078107916433515
- VermaASimardJMWorrallJWERotelloVMTunable reactivation of nanoparticle-inhibited β-galactosidase by glutathione at intracellular concentrationsJ Am Chem Soc200412643139871399115506760
- HanGChariNSVermaAHongRMartinCTRotelloVMControlled recovery of the transcription of nanoparticle-bound DNA by intracellular concentrations of glutathioneBioconjug Chem20051661356135916287230
- DulkeithERinglerMKlarTFeldmannJJavierAMParakWGold nanoparticles quench fluorescence by phase induced radiative rate suppressionNano Lett20055458558915826091
- VermaAUzunOHuYSurface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticlesNat Mater20087758859518500347
- KumarDMeenanBJDixonDControlling the size and size distribution of gold nanoparticles: a design of experiment studyInt J Nanoscience20122111250023
- NagaiAYoshiiROtsukaTKokadoKChujoYBODIPY-based chain transfer agent: reversibly thermoswitchable luminescent gold nanoparticle stabilized by BODIPY-terminated water-soluble polymerLangmuir20102619156441564920815359
- MansonJKumarDMeenanBJDixonDPolyethylene glycol functionalized gold nanoparticles: the influence of capping density on stability in various mediaGold Bull201144299105
- WangMThanouMTargeting nanoparticles to cancerPharm Res20106229099
- AsadishadBVossoughiMAlamzadehIIn vitro release behavior and cytotoxicity of doxorubicin-loaded gold nanoparticles in cancerous cellsBiotechnol Lett201032564965420131082
- ChenYChenZHeYL-cysteine-capped CdTe QD-based sensor for simple and selective detection of trinitrotolueneNanotechnology2010211212550220203361
- HalliwellBFree radicals and antioxidants: a personal viewNutr Rev19945282532657970288