Abstract
Biomaterials are commonly applied in regenerative therapy and tissue engineering in bone, and have been substantially refined in recent years. Thereby, research approaches focus more and more on nanoparticles, which have great potential for a variety of applications. Generally, nanoparticles interact distinctively with bone cells and tissue, depending on their composition, size, and shape. Therefore, detailed analyses of nanoparticle effects on cellular functions have been performed to select the most suitable candidates for supporting bone regeneration. This review will highlight potential nanoparticle applications in bone, focusing on cell labeling as well as drug and gene delivery. Labeling, eg, of mesenchymal stem cells, which display exceptional regenerative potential, makes monitoring and evaluation of cell therapy approaches possible. By including bioactive molecules in nanoparticles, locally and temporally controlled support of tissue regeneration is feasible, eg, to directly influence osteoblast differentiation or excessive osteoclast behavior. In addition, the delivery of genetic material with nanoparticulate carriers offers the possibility of overcoming certain disadvantages of standard protein delivery approaches, such as aggregation in the bloodstream during systemic therapy. Moreover, nanoparticles are already clinically applied in cancer treatment. Thus, corresponding efforts could lead to new therapeutic strategies to improve bone regeneration or to treat bone disorders.
Introduction
Biomaterials such as polymers, ceramics, and metals are widely used in bone for regenerative therapies, including in bone grafts and in tissue engineering as well as for temporary or permanent implants to stabilize fractures or replace joints.Citation1 In recent years, biomaterials in general and bone-related implant materials in particular have been considerably refined,Citation2 with the objective of developing functionalized materials, so-called smart materials, containing bioactive molecules to directly influence cell behavior.Citation3
In this context, nanoparticles that are in the same size range as integral parts of natural bone, such as hydroxyapatite crystals or cellular compartments,Citation4 are promising candidates for local applications. They form the basis of modular systems, which provide the opportunity to elicit cell responses in a spatially and temporally controlled manner by the defined release of physiologically active substances.Citation5 Alternatively, nanoparticles can be immobilized and applied as coatings on implant surfaces or can be used for transmembrane transport for cell labelingCitation6 or gene therapy.Citation7 In bone, locally applied nanoparticles may be suitable for numerous potential uses with respect to the improvement of tissue regeneration, the enhanced osseointegration of implants, and the prevention of infections.
Moreover, systemic application of nanoparticles in bone in a way analogous to cancer therapy is conceivable. However, tissue-specific targeting still represents a great challenge. Specific cell targeting of nanoparticles has been successfully developed for hyperthermia treatment of cancerCitation8 as well as for drug delivery of paclitaxel.Citation9,Citation10 Similarly, nanoparticle treatment of systemic bone diseases such as osteoporosis might be feasible in the future. To date, several molecules to target bone have been identified, such as bisphosphonates and their derivatives,Citation11–Citation13 as well as oligopeptides targeting specifically bone-resorptionCitation14 or bone-formation surfaces.Citation15
Summarizing, to achieve the desired effect, nanoparticles should fulfill the following criteria: they should be nontoxic for cells, ie, bioinert or biodegradable; they should effectively carry the molecule of interest, eg, labeling agent or drug; and they should exert their actions specifically on their target, without evoking side effects in other tissues. Here, the application of targeting molecules or local application of nanoparticles in the form of bioactive coatings or cements is very promising.
This review focuses on nanoparticles and their potential with regard to bone. In this field of research, no clinical trials as yet have been initiated. However, large numbers of in vitro and in vivo studies emphasize the great potential for nanoparticle applications in bone. Nanoparticles feature various modifiable facets, such as particle chemistry, functionalization, and attachment to certain surfaces. In this review, recent research approaches concerning cell labeling, drug delivery, and gene therapy to influence bone cells, as well as the interactions of different types of nanoparticles with bone cells, will be highlighted.
Applications of nanoparticles in bone
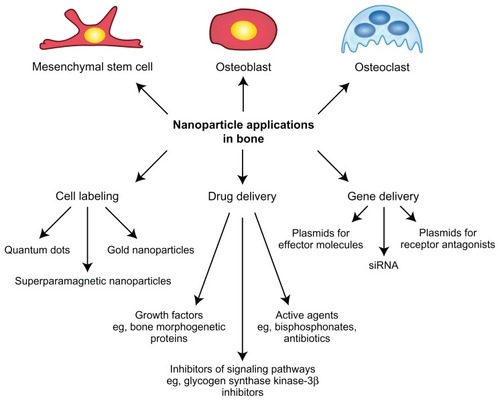
Increasingly refined nanoparticles are being developed for a wide range of applications (). These include cell labeling to broaden research possibilities as well as to improve and noninvasively monitor cell therapy approaches.Citation6 Moreover, drug delivery systems with improved pharmacologic characteristics are being developed. They promote enhanced therapeutic outcome by providing controlled release of bioactive molecules, such as growth factors or anticancer drugs.Citation16 In addition, gene therapy concepts with good prospects are required for future treatment options based on intracellular manipulation.Citation17 Due to the great potential for nanoparticles in the applications indicated here, these topics are discussed against the background of bone cells and tissue.
Cell labeling
Nanoparticles offer great potential for cell labeling during regenerative therapies. Depending on the therapeutic approach, labeling agents are applied in vivo or the cells are labeled ex vivo and are subsequently applied locally or systemically. Thereby, cell labeling allows for the practicable detection of transplanted cells, eg, via magnetic resonance imaging (MRI).Citation18 Thus, cell labeling provides the opportunity to visualize and track cell transport to the area of the defect in vivo and to assess the fate and participation of the transplanted cells in tissue regeneration. This is essential for a reliable evaluation of cell therapy outcome.
Due to their exceptional regenerative potential, mesenchymal stem cells (MSCs) are thought to support tissue regeneration in stem cell therapy. The promising fields for MSC application range from promoting bone regenerationCitation19,Citation20 to improving fracture healing.Citation21–Citation24 To monitor these processes, MSCs have been labeled with diverse nanoparticles, such as quantum dots, which are small semiconductor nanocrystals,Citation25,Citation26 fluorescence-labeled mesoporous silica nanoparticles,Citation27 gold nanoparticles,Citation28 or superparamagnetic iron oxide (SPIO) nanoparticles.Citation29–Citation31 Several types of SPIO nanoparticles have already been clinically approved for use as contrast agents in MRI, eg, of bowel or liver.Citation32 It should be noted that bone represents a formidable target organ, which poses a particular challenge with regard to cell labeling, due to its high mineralization grade, making the visualization of labeled cells in MRI difficult.
For application as cell-labeling agents, nanoparticles need to fulfill certain criteria. On the one hand, the number of nanoparticles per cell has to be high enough to be detectable. On the other hand, this number should be low enough to avoid any interference with cellular functions. With respect to bone cells, and particularly MSCs, the particles ideally should not compromise the differentiation potential. In vitro analyses of MSC differentiation capacity in the presence of nanoparticles demonstrated the innocuousness of several SPIO nanoparticles,Citation29–Citation31,Citation33 as well as of certain gold nanoparticlesCitation28 that were optimized for efficient MSC labeling and MRI visualization. As the MSC differentiation potential in vitro does not necessarily correlate with the in vivo situation, a study investigating the stemness of MSCs exposed to SPIO nanoparticles went one step further by verifying the differentiation capacity in vivo based on ossicle formation by labeled human MSCs in immunocompromised mice.Citation34 However, depending on the experimental setting, quantum dotsCitation35 as well as SPIO nanoparticlesCitation36–Citation38 were observed to alter osteogenic differentiation of MSCs in vitro and in vivo. For SPIO nanoparticles, the iron component was found to be one of the factors responsible for the impairment of the differentiation potential, possibly due to its increased intracellular content caused by the presence of the particles.Citation36,Citation38 With regard to clinical application, various SPIO nanoparticles were applied subcutaneously in mice, eg, via the incorporation of labeled MSCs in collagen scaffolds, and were demonstrated to be suitable for efficient in vivo long-term labeling and for producing convincing visualization by MRI.Citation30,Citation31 However, one has to keep in mind that with this technique, primarily the particles themselves are tracked and not the cells.
Further efforts are being made to expand the possibilities for detecting nanoparticle-labeled cells. For a considerable time, mostly bifunctional systems such as fluorescent, iron-containing nanoparticles were used.Citation30 By adding a third functionality via the incorporation of gold for the visualization of the nanoparticles through computer tomography, a refinement of cell-labeling nanoparticles could be achieved.Citation39
As it appears, biocompatibility poses a great challenge regarding stem cell labeling due to the natural differentiation potential of the cells and essential preservation in vivo. Another issue to be addressed in the near future will be the successful applicability of cell labeling in bone, as visualization of labeled MSCs in mineralized tissue is still challenging. However, ongoing research will further improve and widen the application of nanoparticles for cell labeling, seeking the best possible way to image and track cells in musculoskeletal therapy.
Drug delivery
Due to the unsatisfactory performance of several biomaterials currently used for bone replacement or tissue engineering, improvements such as the introduction of bioactive molecules are being determinedly pursued.Citation40 Proteins of interest can either be directly injected at the respective site or adsorbed to a biomaterial surface. In contrast to injected proteins, which are usually rapidly cleared from the body, locally adsorbed proteins are released by desorption or diffusion and may be retained longer.Citation41 Increasingly, nanoparticles are being explored as finely adjustable delivery systems with regard to the location and time period of drug release. Local drug delivery is favorable in comparison with systemic application to minimize unwanted side effects.Citation42 Moreover, adequate tuning of the nanoparticles allows for a temporally controlled, sustained delivery according to requirements.Citation42,Citation43 The delivery of inhibitory factors of signaling pathways has been reported as a promising tool to elucidate so far unknown pathway functions for research purposes.Citation44 In addition, nanoparticle refinement with respect to clinical application in cancer treatment has been demonstrated by loading particles with the drug paclitaxel.Citation9,Citation10
The following paragraph focuses on the use of nanoparticles as an effective protein or drug delivery system to support bone tissue regeneration. For this purpose, several nondegradable particles, such as silica, lipid, dendrimer, hydroxyapatite, or gold nanoparticles,Citation43–Citation50 as well as degradable particles made of poly(L-lactide)Citation51,Citation52 or poly(L-lactide-co-glycolide) (PLGA),Citation11,Citation42,Citation52–Citation56 have been used. Frequently, nanoparticles were combined with scaffolds such as proteinaceous hydrogels or degradable polymeric matrixes to facilitate application in bone.Citation47,Citation48,Citation53,Citation55,Citation56
As bone contains bone-forming cells, the osteoblasts, and bone-resorbing cells, the osteoclasts, which act in concert to guarantee bone homeostasis,Citation57,Citation58 different strategies can be envisioned that could promote the regeneration of bone tissue. On the one hand, osteoblasts could be supported by nanoparticle-based growth factor delivery. On the other hand, osteoclast resorption could be modulated by nanoparticles locally releasing specific inhibitors.
For the enhancement of osteogenic differentiation, bone morphogenetic proteins (BMPs), members of the transforming growth factor (TGF)-β superfamily, proved to be very suitable. Although TGF-β itself was mainly encapsulated in nanoparticles to potentially improve cartilage regeneration,Citation59–Citation61 the clinically approved BMP-2 was incorporated in nanoparticles to support bone formation.Citation50–Citation53,Citation62 BiodegradableCitation51,Citation53 and nondegradable nanoparticlesCitation50,Citation62 displayed sustained BMP-2 release for up to 2 weeks. Analyses of the viability of osteoblastic cells in the presence of BMP-2-loaded nanoparticles revealed that, depending on the material, cell viability could be negatively affected, thus requiring further material development.Citation51,Citation62 It is essential that the nanoparticles do not influence the biological activity of released growth factors so that osteogenic differentiation can be successfully supported by locally, sustainably released BMP-2.Citation51,Citation62 In relation to this criterion, encapsulated BMP-2 implemented into a hydrogel promoted in vivo bone remodeling in a rat calvarial critical-size defect.Citation53 Another clinically approved member of the TGF-β protein family, BMP-7, was investigated regarding its potential for inducing ectopic, subcutaneous bone formation in a rat model. Like BMP-2, BMP-7 encapsulated in PLGA nanospheres and immobilized in a degradable scaffold supported bone formation.Citation56
In addition to growth factors, the extracellular matrix molecule osteopontin was incorporated in hydroxyapatite nanoparticles located in a degradable matrix and was analyzed for its osteoinductive potential in a canine endosseous gap implant model. However, other than new bone formation within the matrix, no positive effects were observed in the gap itself.Citation47
In contrast to naturally occurring proteins, medium supplements for osteogenic differentiation in vitro, such as the synthetic glucocorticoid dexamethasone, are known to feature osteoinductive properties.Citation63 Accordingly, dexamethasone-loaded dendrimer nanoparticles in a scaffold supported the osteogenic differentiation of rat MSCs in vitro, like free dexamethasone.Citation48 However, due to possible unwanted side effects of the drug itself in vivo, such as secondary osteoporosis,Citation64 a clinical application appears questionable.
In addition to the manipulation of osteoblast behavior, the aforementioned second strategy of drug-loaded nanoparticle application in bone regeneration to inhibit osteoclast resorption activity has also been pursued. In a study using surface-immobilized, dexamethasone-containing nanoparticles, their potential to decrease the number of osteoclast precursors was tested. The released dexamethasone did in fact lead to a local growth inhibition of such progenitors.Citation55 General excessive osteoclast activity, which eventually leads to bone loss or insufficient regeneration in the case of bone defects, is often treated with bisphosphonates, a class of osteoporosis-antagonizing drugs. However, orally administered bisphosphonates display poor bioavailability (only up to 3% of the ingested amount).Citation65 In contrast, controlled, sustained delivery in the case of local bone regeneration might be feasible by using bisphosphonate-loaded nanoparticles. As a model drug, alendronate was used in in vitro studies, and the inhibitory effect of the particle-associated bisphosphonate on osteoclasts (and their precursors) was confirmed for nondegradable gold nanoparticles as well as for biodegradable nanoparticles.Citation46,Citation54 Alendronate-modified nanoparticles were localized in the cytosol and, as expected, were observed to reduce the number of multinucleated, tartrate-resistant acid phosphatase-positive cells.Citation46 However, based on the inhomogeneity of the osteoclast cell culture due to the differing stages of osteoclast development at a given point in time, the effects of encapsulated, osteoclast-influencing substances are difficult to identify. Because of the high affinity of bisphosphonates to bone, and in particular to hydroxyapatite, bisphosphonate-loaded nanoparticles could be targeted to the bone surface, which might help to specifically address and treat, for example, skeletal tumors.Citation11,Citation12 Additionally, bisphosphonate-loaded nanocarriers were strongly retained in mineral-comprising implants, eg, hydroxyapatite-containing scaffolds, providing the opportunity to accurately adjust the delivery system.Citation49 Again, the chemical composition of nanoparticle constructs is of great importance to minimize potential material toxicity and to avoid cellular responses to the material itself rather than to the incorporated drug.Citation11,Citation62
Thus, drug delivery in bone has considerable potential. Regarding prospective progress in the area of biomaterials development, improved release kinetics of nanoparticles will contribute to an even more strictly controlled and sustained drug delivery. Moreover, the constantly increasing understanding of bone tissue in general and the regulation of osteogenic differentiation in particular will be the basis for the identification of further proteins of interest and will lead to promising alternatives besides, eg, BMP-2. In addition to the concepts discussed previously to enhance bone formation, eg, with osteoblast growth factors, or to reduce bone resorption with established drugs, such as bisphosphonates, further applications, including the nanoparticle-mediated delivery of analgesicsCitation42 or the application of bone cement comprising antibiotic-loaded nanoparticles,Citation43 will be pursued in the future.
Gene delivery
Although the therapeutic effect of many proteins is often obvious in terms of bone metabolism, the delivery of proteins of interest or growth factors still represents a great challenge due to aggregation, short lifetime in the bloodstream, and, moreover, very low efficiency due to short and abrupt release. In respect to this, the application of nanoparticles as gene carriers represents a wide and promising field, because the transfection approach potentially allows long-term expression and therefore a longer therapeutic effect.
Many proof-of-principle studies have already been performed to demonstrate that nanoparticles of an inorganic as well as an organic nature are able to deliver plasmids into bone cells. In such studies, a green fluorescent protein-encoding reporter plasmid combined with different carriers was normally used, such as calcium-doped organosilicate nanoparticles,Citation66 calcium phosphate nanoparticles,Citation67 arginine-functionalized hydroxyapatite nanorods,Citation68 or polymers.Citation69
The alternative approach, an application of nanoparticles as oligonucleotide (eg, small interfering ribonucleic acid [siRNA]) carriers, was also effective in other studies, using gold,Citation70 calcium phosphate,Citation71 and cationic polymer/lipid TransIT-TKO® nanoparticles.Citation72 A very promising system was developed by Zhang et alCitation15 and consisted of siRNA-loaded cationic liposomes attached to oligopeptides. Here, oligopeptide (AspSerSer6) provided efficient bone targeting to bone-formation surfaces, whereas siRNA that targeted casein kinase-2 interacting protein-1 promoted osteoblast activity, resulting in increased bone formation and enhanced bone microarchitecture.Citation15
The applied studies often used a transfection approach to enhance bone regeneration in the case of fractures, delivering plasmids encoding transcription factors, growth factors, and even hormones. Studies performed in this direction described a broad range of carriers, including viral particles,Citation73,Citation74 folate-chitosan nanoparticles,Citation75 PLGA nanospheresCitation76 and nanoparticles,Citation77 liposomes,Citation78 and even nonnanoparticulate, polymeric matrixes for the delivery of plasmid, encoding human parathyroid hormone.Citation79
The most well-known and widely investigated proteins for bone regeneration are the BMPs, which are known to possess the greatest in vivo bone stimulatory capacity and to stimulate the differentiation of MSCs along osteoblastic and chondrogenic lineages.Citation41 For example, Krebs et alCitation80 reported a gene delivery system composed of calcium phosphate nanoparticles carrying a BMP-2-encoding plasmid embedded in injectable alginate hydrogels. Hosseinkhani et alCitation81 used polyethylenimine/DNA nanoparticles encapsulated into poly(glycolic acid) scaffolds to transfect MSCs and promote ectopic bone formation in these scaffolds in vivo.
Among other growth factors, potential stimulators of bone regeneration are vascular endothelial growth factor (VEGF) and TGF-β. It was demonstrated that adeno-associated viral vectors carrying a VEGF plasmid were able to induce neovascularization as well as bone remodeling and resorption in autografts and allografts,Citation73 whereas retroviral VEGF transduction resulted in enhanced MSC recruitment and accelerated bone formation.Citation74 TGF-β was described as stimulating bone healing after injection of viral carriers functionalized with a TGF-β-encoded plasmid.Citation78 Deng et alCitation82 demonstrated effective gene delivery of plasmid TGF-β1 to rat MSCs when combined with ethylenediamine-modified polysaccharide from mulberry leaves.
Among transcription factors, the osteoblast-specific transcriptional activator Runx2 and LIM mineralization protein-1 (named after protein domains Lin-11, Isl-1, Mec-3) were considered in particular because of their ability to stimulate bone formation.Citation83
All these strategies normally include a spatial application, thus avoiding systemic toxicity and undesirable side effects. Nevertheless, in some cases the systemic application of nanoparticles (naturally in combination with desirable bone-targeting properties) is preferable, eg, in osteoporosis, arthritis, or bone tumors. Here, the combination of nanoparticles with an “osteoprotective” gene is an obvious strategy. For example, Fernandes et alCitation75 used folate-chitosan nanoparticles in combination with an interleukin-1 receptor antagonist (IL-1Ra) gene to decrease inflammation and reverse alterations in bone turnover in an arthritic rat model.
Such a protective effect could also be achieved via the introduction of tumor inhibitors, eg, IL-18.Citation84 Cytokine IL-18 is normally described as a T-cell and NK-cell activator and was previously shown to inhibit sarcoma growth via activation of the immune response and antiangiogenic activity in vivo. Nie et alCitation84 observed the inhibition of tumorigenesis in mice after the application of polymeric monomethoxy poly(ethylene glycol) (PEG)-PLGA-PEG nanoparticles complexed with IL-18 plasmid.
The alternative approach is to downregulate factors that enhance tumor formation. It is known that a certain expression profile in some tumors, eg, mammary carcinoma, is associated with metastases formation in the skeleton. With respect to this, Elazar et alCitation85 demonstrated that silencing of osteopontin and bone sialoprotein, which belong to the “abnormal” expression set described previously, with siRNA-functionalized PLGA nanoparticles led to the inhibition of metastatic bone lysis.
Thus, nanoparticle-based gene therapy offers great opportunities for fine modulation and treatment of bone diseases of different origin – from fractures to malignant tumors. Various possibilities for the delivery of enhancing as well as inhibitory genetic material have been explored over the past years, and several disadvantages of protein delivery, such as inadequate release kinetics, might be overcome by gene delivery approaches. Thereby, polymeric and inorganic nanoparticles represent a more promising field than viral vectors, due to their safety, degradability, and possible surface modifications, allowing nanoparticle targeting to cells of interest. The major advantages are potentially easy spatial application of particles (in terms of implants, cements, or direct bone injections), which minimizes systemic toxicity and potential side effects, and a prolonged therapeutic effect compared with pure proteins, thus making gene therapy a very promising strategy for the future.
Interactions of nanoparticles with bone cells
During bone regeneration, bone cells such as MSCs, which can differentiate into bone-forming osteoblasts, osteoblasts themselves, and bone-resorbing osteoclasts,Citation57,Citation58 tightly interact to ensure successful tissue reconstruction. Accordingly, it is important not to disturb such regenerative processes, eg, by eliciting undesired inflammatory responses induced by nanoparticles. Therefore, investigations on possible particle uptake as well as on the potential effects of nanoparticles on bone cell functions, such as limitation of MSC differentiation potential, bone mineralization by osteoblasts, or modulation of osteoclastic resorption activity, are required prior to any nanoparticle application in the field of bone research. Thus, the interactions of bone cells with diverse nanoparticles are discussed here.
Mesenchymal stem cells
MSCs are multipotent cells, which can be isolated, for example, from bone marrow. MSCs feature in vitro plastic adherence and can be characterized by the expression of a defined set of surface markers as well as by their differentiation potential into mesenchymal tissue lineages such as bone, cartilage, or adipose tissue.Citation86,Citation87 In contrast to osteoclasts, which are known to phagocytize particles due to their relationship to monocytes/macrophages,Citation88 MSCs do not necessarily take up particles effectively.
With respect to the cellular internalization of nanoparticles or foreign material in general, the uptake behavior depends on the cell type, particle chemistry, charge, and shape, as well as particle carrier systems and cellular microenvironment. Therefore, it is difficult to deduce universally valid rules for the prediction of particle uptake. In terms of charge, positively charged substances such as poly(L-lysine) generally promote the internalization of genetic material.Citation89 Equally, positive charges on the surface of polymeric nanoparticles were found to be beneficial for particle uptake by MSCs.Citation90,Citation91 Negatively charged polymeric nanoparticles such as carboxyl- or phosphonate-functionalized particles were also readily internalized by MSCs.Citation92,Citation93 These observations suggest specific interactions of both, positive and negative functional groups, with the cell surface. Most of the particles were then localized in the cytosol, often in membrane-enclosed clusters.Citation91,Citation93 For up to 3 weeks in long-term culture, these particles did not hamper the viability of undifferentiated and differentiated MSCs.Citation91,Citation93 With regard to differing particle chemistry, metallic silver nanoparticles, too, were taken up into endolysosomal structures in the cytosol.Citation94 Although certain silver nanoparticles did not compromise MSC viability,Citation94 others of approximately the same size caused serious DNA damage even at lower concentrations in a different study.Citation95 In addition to polymeric and metallic nanoparticles, quantum dots of just a few nanometers play an important role in MSC research, based on their excellent cell-labeling qualities.Citation25 Quantum dots, either unfunctionalized or provided with, eg, arginine-glycine-aspartic acid peptides, were spontaneously taken up by MSCs and could be detected inside the cells over a period of 3 weeks.Citation35,Citation96
With respect to the mechanisms for nanoparticle uptake, cells can rely on diverse endocytotic internalization pathways such as clathrin- or caveolae-mediated endocytosis and macropinocytosis.Citation97 Studies with specific inhibitors for the different uptake routes revealed a heterogeneous picture. Although polymeric and metallic particles were in part taken up via the clathrin-mediated pathway,Citation90,Citation92,Citation94 macropinocytosis was also involved to some extent.Citation94
Following the elucidation of nanoparticle uptake and the underlying mechanisms, the analysis of potential influences on MSC characteristics and function is essential. Again, the literature is quite heterogeneous on this topic, and nanoparticles of different chemical nature and shape will be considered here.
Calcium phosphate nanoparticles present an interesting alternative when attempting to imitate bone structure and function with regard to the in vivo crystallinity of calcium phosphates.Citation98 MSC proliferation was affected in a size-dependent manner by the exposure of these cells to calcium phosphate nanoparticles, with larger particles being more harmful.Citation99 The same research group further investigated a potential dependency on particle concentration and their form of appearance. It was observed that with increasing concentration as well as with amorphous particles in contrast to crystalline calcium phosphate particles, osteogenic cell differentiation and matrix mineralization decreased.Citation100,Citation101
Likewise, metallic particles, eg, commercially pure titanium nanoparticles, decreased adhesion and suppressed MSC differentiation.Citation102 In contrast, other metallic particles, such as gold nanotracers, did not affect MSC differentiation into the osteogenic and adipogenic lineages.Citation28
Similarly, polymeric phosphonate-functionalized particles, which exhibited excellent binding properties to metallic surfaces such as titanium dioxide,Citation103 were investigated with regard to their effect on MSC differentiation. Qualitative characteristic staining as well as comprehensive quantitative analyses of marker gene expression for the osteogenic, adipogenic, and chondrogenic lineages revealed no detrimental effect arising from the phosphonate-functionalized particles.Citation93
The MSC differentiation potential was investigated in the presence of various other particle types. Quantum dots, eg, consisting of CdSe/ZnS, restrained osteogenic differentiation in terms of alkaline phosphatase activity as well as osteopontin and osteocalcin expression.Citation35 In contrast, arginine-glycine-aspartic acid-conjugated quantum dots did not affect the differentiation potential, as assessed by characteristic staining for the osteogenic, adipogenic, and chondrogenic lineages,Citation96 and thus might be better suited with regard to biocompatibility with MSCs. Equally, mesoporous silica nanoparticles, either functionalized with different amounts of positively charged functional groups or with gadolinium, did not influence MSC differentiation, as confirmed by qualitative analyses after short-term exposure.Citation104,Citation105
In addition to the effects on the differentiation potential, further MSC functions can be modulated by nanoparticles. Ferucarbotran particles with superparamagnetic properties were efficiently taken up by MSCs.Citation106 Due to the intrinsic peroxidase-like activity of such magnetic nanoparticles,Citation107 ferucarbotran particles stimulated MSC growth through a reduction in intracellular hydrogen peroxide.Citation108
Thus, the heterogeneous picture of research on the interactions of nanoparticles with MSCs makes it difficult to draw general conclusions. However, it becomes clear that parameters such as chemistry, size, and shape in some cases greatly affect the particle uptake behavior of MSCs as well as their natural differentiation potential. Thus, it will be necessary to continue the thorough verification of nanoparticle innocuousness independent of their characteristics to ensure unaffected MSC differentiation.
Osteoblasts
Osteoblasts are bone-forming cells with the ability to mineralize and continuously remodel bone.Citation57 Additionally, osteoblasts make an essential contribution to the regulation of osteoclast activity and thus to bone homoeostasis by secreting the osteoclast-regulating factors macrophage colony-stimulating factor (M-CSF), receptor activator of nuclear factor κB ligand (RANKL), and its decoy receptor osteoprotegerin.Citation57,Citation58 Therefore, nanoparticles exerting effects on osteoblasts might, in addition, indirectly influence osteoclasts.
Similar to MSCs, osteoblasts were demonstrated to internalize a variety of nanoparticles, such as wear debris,Citation109 quantum dot/hydroxyapatite composites,Citation110 calcium phosphate nanoshells,Citation111 and polymeric particles.Citation112 Again, various factors influenced nanoparticle uptake and potential cellular effects.
With regard to the particle charge, the internalization of positively charged nanoparticles is generally facilitated. In line with this observation, positive surface groups appeared to enhance internalization by osteoblastic cells when comparing negatively and positively charged hydroxyapatite nanoparticles or mesoporous silica nanoparticles with increasing positive charge.Citation104,Citation113 However, studies with negatively charged phosphonate-functionalized nanoparticles indicated that these particles, too, were taken up by osteoblasts.Citation112
In addition to particle charge, shape plays an important role. Normally, spherical nanoparticles are readily internalized and do not impede cell functions. In accordance with this general observation, spherical zinc oxide nanoparticles were not cytotoxic for osteoblastic cells, in contrast to rod- shaped particles of the same material.Citation114 However, needle- shaped as well as spherical hydroxyapatite nanoparticles decreased osteoblast cell numbers in a different study.Citation115
With respect to nanoparticle size, the findings again diverged. On the one hand, hydroxyapatite and titania nanoparticles of up to 40 nm diameter decreased osteoblastic cell proliferation and viability, respectively.Citation99,Citation116 On the other hand, in another study, only hydroxyapatite nanoparticles with a diameter of 20 nm enhanced cell growth of osteoblast-like cells compared with larger particles with a diameter of 80 nm.Citation117
In addition to particle uptake by osteoblasts and potential effects on proliferation and viability, the consequences of nanoparticle presence on the differentiation and mineralization of osteoblastic cells were assessed in a variety of studies with a wide range of particles. The expression of alkaline phosphatase, a marker for osteoblast differentiation,Citation118 was found to be reduced by the addition of titanium and silver nanoparticles,Citation109,Citation119 whereas alkaline phosphatase activity and collagen type I synthesis, as well as calcium deposition and mineralization, were increased in the presence of calcium phosphate nanoshells, hydroxyapatite-coated iron oxide particles, strontium-doped hydroxyapatite nanocrystal surfaces, and nanostructured zinc oxide and titania in a different experimental series.Citation111,Citation120–Citation122 These collected findings imply the involvement of complex mechanisms in the internalization of differently composed nanoparticles and in the evocation of variable osteoblast responses.
As osteoblasts secrete factor s that regulate osteoclastogenesis,Citation58 the expression of RANKL, M-CSF, and osteoprotegerin was analyzed following incubation with various nanoparticles. A catabolic phenotype was induced by polyethylene particles through alteration of the RANKL/osteoprotegerin ratio.Citation123 Similar osteoclastogenic effects were triggered in osteoblastic cells by titania nanoparticles via increased gene expression of granulocyte colony-stimulating factor.Citation116 In contrast, phosphonate-functionalized nanoparticles did not affect the expression of osteoclast-regulating genes in primary human osteoblasts.Citation112
Further examined effects of nanoparticles on osteoblasts comprised DNA damage, confirmed for hydroxyapatite nanoparticles in osteoblastic cells,Citation115 and titania particles in various murine organs, including bone marrow.Citation124 Furthermore, a possible improvement in implant fixation by applying nanoparticle coatings was investigated. Contrary to expectations, calcium phosphate nanoparticle coatings did not enhance the osseointegration of implants in vivo.Citation125 In contrast, titanium implants coated with a composite of hydroxyapatite nanoparticles and poly(D,L-lactide) improved the osseointegration in terms of new bone formation and mechanical fixation in sheep compared with implants without hydroxyapatite nanoparticles.Citation126
In summary, the number of publications on current research regarding the interactions of nanoparticles with osteoblasts appears manageable. Still, findings with regard to nanoparticle influence on differentiation and mineralization of osteoblasts clearly diverge, depending on particle characteristics. As osteoblast behavior is closely connected to osteoclast formation and activity, it will be important to generally extend respective analyses to factors such as RANKL, which affect bone homeostasis in general, in order to ensure covering different fields of potential nanoparticle influence.
Osteoclasts
Osteoclasts, bone-resorbing cells, are derived from hematopoietic precursor cells and are closely related to macrophages.Citation127 Because macrophages are known to activate the immune response by phagocytizing pathogens,Citation128 it is no surprise that osteoclasts, too, are capable of phagocytizing foreign matter such as biomaterial particles.Citation88 Osteoclasts are part of a complex network coordinating bone homeostasis and regenerative processes, eg, in the case of bone defects. In this regard, the osseointegration of bone substitutes as well as a favored bone formation is highly desired. Osteoclasts, like MSCs and osteoblasts, play a decisive role in fracture healing.Citation129 If disadvantageously influenced, eg, by metallic wear particles of implants used for fracture stabilization,Citation130,Citation131 excessive osteoclast activation frequently causes aseptic loosening of implants.Citation132 Thus, locally applied (drug-loaded) nanoparticles might favorably modulate osteoclast resorption. However, due to the potential for affected osteoclast activity as a result of contact with foreign matter itself, it is a prerequisite to investigate the basic interactions of osteoclasts with nanoparticles.
In fact, diverse nanoparticles were readily taken up by undifferentiated monocytes and differentiated osteoclasts.Citation94,Citation112 However, differing subsets of monocytes, eg, those involved in chronic disease and in tissue repair, respectively, differentially incorporated nanoparticles,Citation133 implying a whole set of parameters influencing nanoparticle uptake in osteoclastic cells. With respect to the intracellular localization of nanoparticles, varying observations were made. Although metallic and polymeric nanoparticles were localized in the cytoplasm, partly surrounded by membrane-like structures,Citation94,Citation112 hydroxyapatite nanoparticles were detected within specialized cellular cavities with a connection to the extracellular environment, the so-called surface-connected compartments.Citation134 The uptake of various nanoparticles may lead to changes in osteoclast morphology, eg, a less pronounced ruffled border and modulation of cell proliferation,Citation120 as well as interference with osteoclast formation and resorption activity. An established characteristic to assess the formation of osteoclasts is the number of tartrate-resistant acid phosphatase-positive, multinucleated cells that are generated in vitro.Citation135 Based on the analysis of this feature, diverse metallic and silica nanoparticles negatively influenced osteoclast formation.Citation119,Citation136–Citation138 In contrast, other particles did not compromise the formation of multinucleated cellsCitation112,Citation139 or even promoted osteoclast maturation. Citation140 Similarly, the influence of nanoparticles on osteoclast resorption activity is heterogeneous. Although the resorption activity of undifferentiated monocytes and osteoclasts could be enhanced by metal ions or nanoparticles,Citation141,Citation142 other types of particles did not necessarily modulate resorption.Citation112 In addition to the effects that nanoparticles exert on osteoclast function, inflammatory responses can be induced by nanoparticles, eg, in implant wear, which, in turn, may affect osteoclasts.Citation140,Citation143 Osteoclast precursors themselves, too, were observed to react to titanium alloy and SPIO nanoparticles with increased cytokine production.Citation144,Citation145 However, the exposure of osteoclasts and their precursors to nanoparticles does not coercively lead to an upregulation of inflammatory factors in these cells.Citation66,Citation112 In addition to potential inflammation, further severe effects such as DNA damage or initiation of apoptosis can be elicited by nanoparticles in osteoclast precursors.Citation116,Citation146
In short, osteoclasts originate from the same precursors as other phagocytizing cells, such as macrophages, and readily take up nanoparticles. Arising clinical issues include aseptic implant loosening due to excessive osteoclast activity potentially caused by implant wear in the form of particles. However, only a few studies actually investigated osteoclast resorption activity, even though this osteoclast feature is crucial for eventual changes in bone mass. In general, nanoparticles stimulated osteoclasts. Thus, a careful selection of promising candidates for continuing research is essential.
Conclusion
Nanoparticles represent a promising tool for research purposes as well as for therapeutic approaches in bone. These could be finely modified, taking into account that the type of interaction between nanoparticle and cell varies depending on nanoparticle composition. Initial studies confirmed the innocuousness of several nanoparticles with respect to MSC differentiation potential and osteoclast function. Thus, different strategies for nanoparticle application in bone (eg, as cell-labeling agents and for drug or gene delivery) have great potential for monitoring and supporting tissue regeneration. In other areas, such as cancer treatment, nanoparticles already contribute to successful clinical approaches, and similar efforts to use nanoparticulate systems to promote bone regeneration will gradually lead to therapeutic success here as well.
Disclosure
The authors report no conflicts of interest in this work.
References
- NavarroMMichiardiACastanoOPlanellJABiomaterials in orthopaedicsJ R Soc Interface20085271137115818667387
- HuebschNMooneyDJInspiration and application in the evolution of biomaterialsNature2009462727242643219940912
- MieszawskaAJKaplanDLSmart biomaterials – regulating cell behavior through signaling moleculesBMC Biol201085920529238
- WebsterTJAhnESNanostructured biomaterials for tissue engineering boneAdv Biochem Eng Biotechnol200610327530817195467
- BiondiMUngaroFQuagliaFNettiPAControlled drug delivery in tissue engineeringAdv Drug Deliv Rev200860222924218031864
- BhirdeAXieJSwierczewskaMChenXNanoparticles for cell labelingNanoscale20113114215320938522
- PanyamJLabhasetwarVBiodegradable nanoparticles for drug and gene delivery to cells and tissueAdv Drug Deliv Rev200355332934712628320
- DutzSKetteringMHilgerIMullerRZeisbergerMMagnetic multicore nanoparticles for hyperthermia – influence of particle immobilization in tumour tissue on magnetic propertiesNanotechnology2011222626510221576784
- GradisharWJTjulandinSDavidsonNPhase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancerJ Clin Oncol200523317794780316172456
- MirtschingBCosgriffTHarkerGKeatonMChidiacTMinMA phase II study of weekly nanoparticle albumin-bound paclitaxel with or without trastuzumab in metastatic breast cancerClin Breast Cancer201111212112821569998
- CenniEAvnetSGranchiDThe effect of poly(D,L-lactide-co-glycolide)-alendronate conjugate nanoparticles on human osteoclast precursorsJ Biomater Sci Polym Ed7202011 [Epub ahead of print.]
- SalernoMCenniEFotiaCBone-targeted doxorubicin-loaded nanoparticles as a tool for the treatment of skeletal metastasesCurr Cancer Drug Targets201010764965920578992
- YewleJNPuleoDABachasLGEnhanced affinity bifunctional bisphosphonates for targeted delivery of therapeutic agents to boneBioconjug Chem201122122496250622073906
- WangDMillerSCShlyakhtenkoLSOsteotropic peptide that differentiates functional domains of the skeletonBioconjug Chem20071851375137817705416
- ZhangGGuoBWuHA delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapyNat Med201218230731422286306
- AllenTMCullisPRDrug delivery systems: entering the mainstreamScience200430356651818182215031496
- EvansCGene therapy for the regeneration of boneInjury201142659960421489526
- EdelmanRRWarachSMagnetic resonance imaging (1)N Engl J Med1993328107087168433731
- BruderSPFinkDJCaplanAIMesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapyJ Cell Biochem19945632832947876320
- BiancoPRiminucciMGronthosSRobeyPGBone marrow stromal stem cells: nature, biology, and potential applicationsStem Cells200119318019211359943
- Granero-MoltoFMyersTJWeisJAMesenchymal stem cells expressing insulin-like growth factor-I (MSCIGF) promote fracture healing and restore new bone formation in Irs1 knockout mice: analyses of MSCIGF autocrine and paracrine regenerative effectsStem Cells201129101537154821786367
- Granero-MoltoFWeisJAMigaMIRegenerative effects of transplanted mesenchymal stem cells in fracture healingStem Cells20092781887189819544445
- LeeSWPadmanabhanPRayPStem cell-mediated accelerated bone healing observed with in vivo molecular and small animal imaging technologies in a model of skeletal injuryJ Orthop Res200927329530218752273
- TsengSSLeeMAReddiAHNonunions and the potential of stem cells in fracture-healingJ Bone Joint Surg Am200890Suppl 1929818292363
- ByersRJHitchmanERQuantum dots brighten biological imagingProg Histochem Cytochem201145420123721196026
- Muller-BorerBJCollinsMCGunstPRCascioWEKypsonAPQuantum dot labeling of mesenchymal stem cellsJ Nanobiotechnology20075917988386
- HuangDMHungYKoBSHighly efficient cellular labeling of mesoporous nanoparticles in human mesenchymal stem cells: implication for stem cell trackingFaseb J200519142014201616230334
- RiclesLMNamSYSokolovKEmelianovSYSuggsLJFunction of mesenchymal stem cells following loading of gold nanotracersInt J Nanomedicine2011640741621499430
- Jasmin TorresALNunesHMOptimized labeling of bone marrow mesenchymal cells with superparamagnetic iron oxide nanoparticles and in vivo visualization by magnetic resonance imagingJ Nanobiotechnology20119421542946
- LuCWHungYHsiaoJKBifunctional magnetic silica nanoparticles for highly efficient human stem cell labelingNano Lett20077114915417212455
- Schmidtke-SchrezenmeierGUrbanMMusyanovychALabeling of mesenchymal stromal cells with iron oxide-poly(L-lactide) nanoparticles for magnetic resonance imaging: uptake, persistence, effects on cellular function and magnetic resonance imaging propertiesCytotherapy201113896297521492060
- WangYXHussainSMKrestinGPSuperparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imagingEur Radiol200111112319233111702180
- ArbabASYocumGTRadAMLabeling of cells with ferumoxides-protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cellsNMR Biomed200518855355916229060
- BalakumaranAPawelczykERenJSuperparamagnetic iron oxide nanoparticles labeling of bone marrow stromal (mesenchymal) cells does not affect their “stemness”PLoS One201057e1146220628641
- HsiehSCWangFFLinCSChenYJHungSCWangYJThe inhibition of osteogenesis with human bone marrow mesenchymal stem cells by CdSe/ZnS quantum dot labelsBiomaterials20062781656166416188313
- ChenYCHsiaoJKLiuHMThe inhibitory effect of superparamagnetic iron oxide nanoparticle (Ferucarbotran) on osteogenic differentiation and its signaling mechanism in human mesenchymal stem cellsToxicol Appl Pharmacol2010245227227920338187
- FarrellEWielopolskiPPavljasevicPEffects of iron oxide incorporation for long term cell tracking on MSC differentiation in vitro and in vivoBiochem Biophys Res Commun200836941076108118336785
- PawelczykEArbabASPanditSHuEFrankJAExpression of transferrin receptor and ferritin following ferumoxides-protamine sulfate labeling of cells: implications for cellular magnetic resonance imagingNMR Biomed200619558159216673357
- van SchooneveldMMCormodeDPKooleRA fluorescent, paramagnetic and PEGylated gold/silica nanoparticle for MRI, CT and fluorescence imagingContrast Media Mol Imaging2010523123620812290
- LaurencinCTAmbrosioAMBordenMDCooperJAJrTissue engineering: orthopedic applicationsAnnu Rev Biomed Eng19991194611701481
- KofronMDLiXLaurencinCTProtein- and gene-based tissue engineering in bone repairCurr Opin Biotechnol200415539940515464368
- KimYTCaldwellJMBellamkondaRVNanoparticle-mediated local delivery of methylprednisolone after spinal cord injuryBiomaterials200930132582259019185913
- ShenSCNgWKShiZChiaLNeohKGTanRBMesoporous silica nanoparticle-functionalized poly(methyl methacrylate)-based bone cement for effective antibiotics deliveryJ Mater Sci Mater Med201122102283229221786132
- ShahDAKwonSJBaleSSBanerjeeADordickJSKaneRSRegulation of stem cell signaling by nanoparticle-mediated intracellular protein deliveryBiomaterials201132123210321921296414
- ChiBParkSJParkMHLeeSYJeongBOligopeptide delivery carrier for osteoclast precursorsBioconjug Chem20102181473147820715852
- FanordFFairbairnKKimHGarcesABhethanabotlaVGuptaVKBisphosphonate-modified gold nanoparticles: a useful vehicle to study the treatment of osteonecrosis of the femoral headNanotechnology201122303510221149961
- JensenTBaasJDolathshahi-PirouzAOsteopontin functionalization of hydroxyapatite nanoparticles in a PDLLA matrix promotes bone formationJ Biomed Mater Res A20119919410121800419
- OliveiraJMSousaRAKotobukiNThe osteogenic differentiation of rat bone marrow stromal cells cultured with dexamethasone-loaded carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticlesBiomaterials200930580481319036432
- WangGMostafaNZIncaniVKucharskiCUludagHBisphosphonate-decorated lipid nanoparticles designed as drug carriers for bone diseasesJ Biomed Mater Res A2012100368469322213565
- XieGSunJZhongGLiuCWeiJHydroxyapatite nanoparticles as a controlled-release carrier of BMP-2: absorption and release kinetics in vitroJ Mater Sci Mater Med20102161875188020300953
- ChenLLiuLLiCTanYZhangGA new growth factor controlled drug release system to promote healing of bone fractures: nanospheres of recombinant human bone morphogenetic-2 and polylactic acidJ Nanosci Nanotechnol20111143107311421776677
- MercadoAEMaJHeXJabbariERelease characteristics and osteogenic activity of recombinant human bone morphogenetic protein-2 grafted to novel self-assembled poly(lactide-co-glycolide fumarate) nanoparticlesJ Control Release2009140214815619699244
- ChungYIAhnKMJeonSHLeeSYLeeJHTaeGEnhanced bone regeneration with BMP-2 loaded functional nanoparticle-hydrogel complexJ Control Release20071211–2919917604871
- Cohen-SelaEChornyMKoroukhovNDanenbergHDGolombGA new double emulsion solvent diffusion technique for encapsulating hydrophilic molecules in PLGA nanoparticlesJ Control Release20091332909518848962
- LoCTVan TasselPRSaltzmanWMBiodegradable poly(lactide-co-glycolide) nanoparticle assembly for continuous release of bioactive agents from medical devicesBiomaterials201031133631364220149428
- WeiWZeveDSuhJMBiphasic and dosage-dependent regulation of osteoclastogenesis by beta-cateninMol Cell Biol201131234706471921876000
- NeveACorradoACantatoreFPOsteoblast physiology in normal and pathological conditionsCell Tissue Res2011343228930221120535
- TeitelbaumSLBone resorption by osteoclastsScience200028954841504150810968780
- LimSMOhSHLeeHHYukSHImGILeeJHDual growth factor-releasing nanoparticle/hydrogel system for cartilage tissue engineeringJ Mater Sci Mater Med20102192593260020577785
- ParkJSParkKWooDGYangHNChungHMParkKHPLGA microsphere construct coated with TGF-beta 3 loaded nanoparticles for neocartilage formationBiomacromolecules2008982162216918630961
- ParkJSYangHNWooDGChungHMParkKHIn vitro and in vivo chondrogenesis of rabbit bone marrow-derived stromal cells in fibrin matrix mixed with growth factor loaded in nanoparticlesTissue Eng Part A20091582163217519413492
- ZhangSWangGLinXPolyethylenimine-coated albumin nanoparticles for BMP-2 deliveryBiotechnol Prog200824494595619194903
- ChengSLYangJWRifasLZhangSFAvioliLVDifferentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasoneEndocrinology199413412772868275945
- den UylDBultinkIELemsWFGlucocorticoid-induced osteoporosisClin Exp Rheumatol2011295 Suppl 68S939822018192
- DelmasPDTreatment of postmenopausal osteoporosisLancet200235993222018202612076571
- MüllerKSkepperJNPosfaiMEffect of ultrasmall superparamagnetic iron oxide nanoparticles (Ferumoxtran-10) on human monocyte-macrophages in vitroBiomaterials20072891629164217178155
- KlesingJChernousovaSEppleMFreeze-dried cationic calcium phosphate nanorods as versatile carriers of nucleic acids (DNA, siRNA)J Mater Chem201222199204
- Gonzalez-McQuireRGreenDWPartridgeKAOreffoROCMannSDavisSACoating of human mesenchymal cells in 3D culture with bioinorganic nanoparticles promotes osteoblastic differentiation and gene transfectionAdv Mater2007191722362240
- HarrisTJGreenJJFungPWLangerRAndersonDGBhatiaSNTissue-specific gene delivery via nanoparticle coatingBiomaterials2010315998100619850333
- JenCPChenYHFanCSA nonviral transfection approach in vitro: the design of a gold nanoparticle vector joint with microelectromechanical systemsLangmuir20042041369137415803721
- ZhangXKovtunAMendoza-PalomaresCSiRNA-loaded multi-shell nanoparticles incorporated into a multilayered film as a reservoir for gene silencingBiomaterials201031236013601820488536
- AndersenMØNygaardJVBurnsJSsiRNA nanoparticle functionalization of nanostructured scaffolds enables controlled multilineage differentiation of stem cellsMol Ther201018112018202720808289
- ItoHKoefoedMTiyapatanaputiPRemodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapyNat Med200511329129715711561
- PengHWrightVUsasASynergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4J Clin Invest2002110675175912235106
- FernandesJCWangHJreyssatyCBone-protective effects of nonviral gene therapy with folate-chitosan DNA nanoparticle containing interleukin-1 receptor antagonist gene in rats with adjuvant-induced arthritisMol Ther20081671243125118500247
- LabhasetwarVBonadioJGoldsteinSALevyRJGene transfection using biodegradable nanospheres: results in tissue culture and a rat osteotomy modelColloids Surf B Biointerfaces199916281290
- KimJHParkJSYangHNThe use of biodegradable PLGA nanoparticles to mediate SOX9 gene delivery in human mesenchymal stem cells (hMSCs) and induce chondrogenesisBiomaterials201132126827820875683
- KofronMDLaurencinCTOrthopaedic applications of gene therapyCurr Gene Ther200551376115638710
- BonadioJSmileyEPatilPGoldsteinSLocalized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regenerationNat Med19995775375910395319
- KrebsMDSalterEChenESutterKAAlsbergECalcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesisJ Biomed Mater Res A20109231131113819322877
- HosseinkhaniHHosseinkhaniMGabrielsonNPPackDWKhademhosseiniAKobayashiHDNA nanoparticles encapsulated in 3D tissue-engineered scaffolds enhance osteogenic differentiation of mesenchymal stem cellsJ Biomed Mater Res A2008851476017688252
- DengW-WCaoXWangMEfficient gene delivery to mesenchymal stem cells by an ethylenediamine-modified polysaccharide from mulberry leavesSmall20128344145122213679
- KofronMDLaurencinCTBone tissue engineering by gene deliveryAdv Drug Deliv Rev200658455557616790291
- NieYZhangZRHeBGuZInvestigation of PEG-PLGA-PEG nanoparticles-based multipolyplexes for IL-18 gene deliveryJ Biomater Appl201126889391621273262
- ElazarVAdwanHBäuerleTRohekarKGolombGBergerMRSustained delivery and efficacy of polymeric nanoparticles containing osteopontin and bone sialoprotein antisenses in rats with breast cancer bone metastasisInt J Cancer201012671749176019739076
- DominiciMLe BlancKMuellerIMinimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statementCytotherapy20068431531716923606
- PittengerMFMackayAMBeckSCMultilineage potential of adult human mesenchymal stem cellsScience1999284541114314710102814
- WangWFergusonDJQuinnJMSimpsonAHAthanasouNABiomaterial particle phagocytosis by bone-resorbing osteoclastsJ Bone Joint Surg Br19977958498569331049
- KwohDYCoffinCCLolloCPStabilization of poly-L-lysine/DNA polyplexes for in vivo gene delivery to the liverBiochim Biophys Acta19991444217119010023051
- JiangXDausendJHafnerMSpecific effects of surface amines on polystyrene nanoparticles in their interactions with mesenchymal stem cellsBiomacromolecules201011374875320166675
- LorenzMRHolzapfelVMusyanovychAUptake of functionalized, fluorescent-labeled polymeric particles in different cell lines and stem cellsBiomaterials200627142820282816430958
- JiangXMusyanovychARockerCLandfesterKMailanderVNienhausGUSpecific effects of surface carboxyl groups on anionic polystyrene particles in their interactions with mesenchymal stem cellsNanoscale2011352028203521409242
- TautzenbergerALorenzSKrejaLEffect of functionalised fluorescence-labelled nanoparticles on mesenchymal stem cell differentiationBiomaterials20103182064207120004969
- GreulichCDiendorfJGessmannJCell type-specific responses of peripheral blood mononuclear cells to silver nanoparticlesActa Biomater2011793505351421651999
- HackenbergSScherzedAKesslerMSilver nanoparticles: evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cellsToxicol Lett20112011273321145381
- ShahBSClarkPAMoioliEKStroscioMAMaoJJLabeling of mesenchymal stem cells by bioconjugated quantum dotsNano Lett20077103071307917887799
- HillaireauHCouvreurPNanocarriers’ entry into the cell: relevance to drug deliveryCell Mol Life Sci200966172873289619499185
- BuckwalterJAGlimcherMJCooperRRReckerRBone biologyJ Bone Joint Surg Am199577812561275
- CaiYLiuYYanWQRole of hydroxyapatite nanoparticle size in bone cell proliferationJ Mater Chem20071737803787
- HuQTanZLiuYEffect of crystallinity of calcium phosphate nanoparticles on adhesion, proliferation, and differentiation of bone marrow mesenchymal stem cellsJ Mater Chem20071746904698
- LiuYWangGCaiYIn vitro effects of nanophase hydroxyapatite particles on proliferation and osteogenic differentiation of bone marrow-derived mesenchymal stem cellsJ Biomed Mater Res A20089041083109118671263
- OkaforCCHaleem-SmithHLaquerierePMannerPATuanRSParticulate endocytosis mediates biological responses of human mesenchymal stem cells to titanium wear debrisJ Orthop Res200624346147316450379
- ZellerAMusyanovychAKapplMNanostructured coatings by adhesion of phosphonated polystyrene particles onto titanium surface for implant material applicationsACS Appl Mater Interfaces2010282421242820690639
- ChungTHWuSHYaoMThe effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cellsBiomaterials200728192959296617397919
- HsiaoJKTsaiCPChungTHMesoporous silica nanoparticles as a delivery system of gadolinium for effective human stem cell trackingSmall2008491445145218680095
- MailänderVLorenzMRHolzapfelVCarboxylated super-paramagnetic iron oxide particles label cells intracellularly without transfection agentsMol Imaging Biol200810313814618297365
- GaoLZhuangJNieLIntrinsic peroxidase-like activity of ferromagnetic nanoparticlesNat Nanotechnol20072957758318654371
- HuangDMHsiaoJKChenYCThe promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticlesBiomaterials200930223645365119359036
- LohmannCHSchwartzZKosterGPhagocytosis of wear debris by osteoblasts affects differentiation and local factor production in a manner dependent on particle compositionBiomaterials200021655156110701456
- HsiehMFLiJKLinCATracking of cellular uptake of hydrophilic CdSe/ZnS quantum dots/hydroxyapatite composites nanoparticles in MC3T3-E1 osteoblast cellsJ Nanosci Nanotechnol2009942758276219438032
- SchmidtSMMoranKATweed KentAMUptake of calcium phosphate nanoshells by osteoblasts and their effect on growth and differentiationJ Biomed Mater Res A200887241842818186060
- TautzenbergerAKrejaLZellerADirect and indirect effects of functionalised fluorescence-labelled nanoparticles on human osteoclast formation and activityBiomaterials20113261706171421093909
- ChenLMcCrateJMLeeJCLiHThe role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cellsNanotechnology2011221010570821289408
- NairSSasidharanADivya RaniVVMenonDManzoorKRainaSRole of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cellsJ Mater Sci Mater Med200920Suppl 1S23524118716714
- XuJLKhorKASuiJJZhangJHChenWNProtein expression profiles in osteoblasts in response to differentially shaped hydroxyapatite nanoparticlesBiomaterials200930295385539119631375
- ZhangYYuWJiangXLvKSunSZhangFAnalysis of the cytotoxicity of differentially sized titanium dioxide nanoparticles in murine MC3T3-E1 preosteoblastsJ Mater Sci Mater Med20112281933194521681655
- ShiZHuangXCaiYTangRYangDSize effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cellsActa Biomater20095133834518753024
- BellowsCGAubinJEHeerscheJNInitiation and progression of mineralization of bone nodules formed in vitro: the role of alkaline phosphatase and organic phosphateBone Miner199114127401868267
- AlbersCEHofstetterWSiebenrockKALandmannRKlenkeFMIn vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrationsNanotoxicology10212011 [Epub ahead of print.]
- CapucciniCTorricelliPBoaniniEGazzanoMGiardinoRBigiAInteraction of Sr-doped hydroxyapatite nanocrystals with osteoclast and osteoblast-like cellsJ Biomed Mater Res A200989359460018437694
- ColonGWardBCWebsterTJIncreased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2J Biomed Mater Res A200678359560416752397
- TranNWebsterTJIncreased osteoblast functions in the presence of hydroxyapatite-coated iron oxide nanoparticlesActa Biomater2011731298130620937416
- AtkinsGJWelldonKJHoldingCAHaynesDRHowieDWFindlayDMThe induction of a catabolic phenotype in human primary osteoblasts and osteocytes by polyethylene particlesBiomaterials200930223672368119349075
- SychevaLPZhurkovVSIurchenkoVVInvestigation of genotoxic and cytotoxic effects of micro- and nanosized titanium dioxide in six organs of mice in vivoMutat Res2011726181421871579
- SchoutenCMeijerGJvan den BeuckenJJIn vivo bone response and mechanical evaluation of electrosprayed CaP nanoparticle coatings using the iliac crest of goats as an implantation modelActa Biomater2010662227223619944782
- JensenTJakobsenTBaasJHydroxyapatite nanoparticles in poly-D,L-lactic acid coatings on porous titanium implants conducts bone formationJ Biomed Mater Res A201095366567220725972
- YavropoulouMPYovosJGOsteoclastogenesis – current knowledge and future perspectivesJ Musculoskelet Neuronal Interact20088320421618799853
- AderemAUnderhillDMMechanisms of phagocytosis in macrophagesAnnu Rev Immunol19991759362310358769
- BeilFTBarvencikFGebauerMEffects of estrogen on fracture healing in miceJ Trauma20106951259126520173660
- MacQuarrieRAFang ChenYColesCAndersonGIWear particle-induced osteoclast osteolysis: the role of particulates and mechanical strainJ Biomed Mater Res A2004691104112
- NealeSDHaynesDRHowieDWMurrayDWAthanasouNAThe effect of particle phagocytosis and metallic wear particles on osteoclast formation and bone resorption in vitroJ Arthroplasty200015565466210960005
- GreenfieldEMBiYRagabAAGoldbergVMVan De MotterRRThe role of osteoclast differentiation in aseptic looseningJ Orthop Res20022011811855378
- SettlesMEtzrodtMKosankeKDifferent capacity of monocyte subsets to phagocytose iron-oxide nanoparticlesPLoS One2011610e2519721984904
- MotskinMMullerKHGenoudCMonteithAGSkepperJNThe sequestration of hydroxyapatite nanoparticles by human monocyte-macrophages in a compartment that allows free diffusion with the extracellular environmentBiomaterials201132359470948221889202
- VäänänenHKLaitala-LeinonenTOsteoclast lineage and functionArch Biochem Biophys2008473213213818424258
- BeckGRJrHaSWCamalierCEBioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivoNanomedicine11152011 [Epub ahead of print.]
- SommerBFelixRSprecherCLeunigMGanzRHofstetterWWear particles and surface topographies are modulators of osteoclastogenesis in vitroJ Biomed Mater Res A2005721677615536650
- SulOJKimJCKyungTWGold nanoparticles inhibited the receptor activator of nuclear factor-kappab ligand (RANKL)-induced osteoclast formation by acting as an antioxidantBiosci Biotechnol Biochem201074112209221321071867
- NabeshiHYoshikawaTAkaseTEffect of amorphous silica nanoparticles on in vitro RANKL-induced osteoclast differentiation in murine macrophagesNanoscale Res Lett20116146421777482
- PalNQuahBSmithPNGladkisLLTimmersHLiRWNano-osteoimmunology as an important consideration in the design of future implantsActa Biomater2011772926293421530692
- CadoschDChanEGautschiOPMeagherJZellwegerRFilgueiraLTitanium IV ions induced human osteoclast differentiation and enhanced bone resorption in vitroJ Biomed Mater Res20099112936
- HirayamaTFujikawaYItonagaITorisuTEffect of particle size on macrophage-osteoclast differentiation in vitroJ Orthop Sci200161535811289587
- CobelliNScharfBCrisiGMHardinJSantambrogioLMediators of the inflammatory response to joint replacement devicesNat Rev Rheumatol201171060060821894210
- KaufmanAMAlabreCIRubashHEShanbhagASHuman macrophage response to UHMWPE, TiAlV, CoCr, and alumina particles: analysis of multiple cytokines using protein arraysJ Biomed Mater Res2008842464474
- LiuYChenZGuNWangJEffects of DMSA-coated Fe3O4 magnetic nanoparticles on global gene expression of mouse macrophage RAW264.7 cellsToxicol Lett2011205213013921641980
- SunJDingTp53 reaction to apoptosis induced by hydroxyapatite nanoparticles in rat macrophagesJ Biomed Mater Res A200988367367918335527