Abstract
Background
Bioconjugates of a polyamidoamine (PAMAM) G3 dendrimer and an aldehyde were synthesized as carriers for vitamins A and B6, and the bioavailability of these vitamins for skin nutrition was investigated.
Methods
Nuclear magnetic resonance (NMR) and ultraviolet-visible methods were used to characterize the structure of the bioconjugates and for monitoring release of pyridoxal (Pyr) and pyridoxal phosphate (PLP) from these bioconjugates in vitro. A skin model permeation of bioconjugates was also studied in a Franz chamber.
Results
A transdermal G3 PAMAM dendrimer was used to synthesize bioconjugates with trans-retinal (Ret), pyridoxal (Pyr), or PLP. These nanomolecules, containing up to four covalently linked Ret, Pyr, or PLP (G34Ret, G34Pyr, and G34PLP), were able to permeate the skin, as demonstrated in vitro using a model skin membrane. PLP and Pyr bound to a macromolecular vehicle were active cofactors for glutamic pyruvic transaminase, as shown by 1H NMR spectral monitoring of the progress of the L-alanine + α-ketoglutarate → glutamic acid + pyruvic acid reaction.
Conclusion
PAMAM-PLP, PAMAM-Pyr, and PAMAM-Ret bioconjugates are able to permeate the skin. PLP and Pyr are available as cofactors for glutamic pyruvic transaminase.
Introduction
Vitamins are important skin nutrients delivered topically in cosmetic creams. The amount that reaches the skin depends on the solubility of the vitamins and their transepidermal diffusion. Permeability of vitamins can be increased by use of a transdermal carrier. In recent decades, the most explored macromolecular vehicles have been polyamidoamine (PAMAM) dendrimers.Citation1 These monodispersed dendritic molecules have a strictly defined shape and size and are able to encapsulate drug molecules, resulting in formation of host–guest complexes.Citation2–Citation10 On the other hand, full-generation PAMAM dendrimers have surface amine groups (32 amine groups in the third-generation PAMAM dendrimer, G3), offering an easy route for formation of conjugates with drugs.Citation11–Citation21 Due to the relatively low toxicity of PAMAM dendrimers,Citation22–Citation25 they are frequently used as transdermal diffusion promoters.Citation26 In previous studies, we have used PAMAM dendrimers as carriers for 8-methoxypsoralen, the photosensitizer used in psoralen and ultraviolet light A therapy (PUVA),Citation4 riboflavin,Citation5 and vitamin C.Citation27 In all these cases, the PAMAM dendrimers formed host–guest complexes with 8-methoxypsoralen, vitamin B2 and vitamin C, thereby modifying the transdermal flux of the guest molecule. Another approach to influencing the rate of transdermal diffusion is based on covalent binding of a prodrug and using such bioconjugates as a platform for introducing the drug into skin tissue. In terms of potential dermatological application, bioconjugates of folate,Citation11 biotin,Citation12 riboflavin,Citation13 cholic acid,Citation14 and phosphorylcholineCitation15 have been synthesized and tested as membrane permeability enhancers, increasing the cellular uptake of the drug. Here we report on bioconjugates obtained from third-generation PAMAM dendrimers, retinal ([Ret] vitamin A), pyridoxal ([Pyr] vitamin B6), or pyridoxal phosphate ([PLP] metabolically active coenzyme of vitamin B6). The G3 dendrimer was chosen in accordance with our previously reported results for optimization of dendrimer size as a transdermal carrier for vitamins B2 and C. The important issue concerning application of bioconjugates carrying biologically active molecules is their bioavailability, which was tested here in vitro.
Materials and methods
The common solvents and reagents used for synthesis of PAMAM dendrimers or PAMAM bioconjugates, ie, ethylenediamine, methyl acrylate, 13-cis-retinal and all- trans-retinal, Pyr, and PLP were purchased from Sigma- Aldrich (St Louis, MO). Glutamic pyruvic transaminase from porcine heart (EC 2.6.1.2, activity 75 units/mg of protein; Sigma-Aldrich) was used for catalytic tests.
1H and 13C nuclear magnetic resonance (NMR) spectra were recorded using a Bruker 300 MHz instrument. Ultraviolet-visible spectra were recorded on an Hitachi U-1900spectrophotometer. Permeation of G34Ret and G34PLP conjugates was studied using a Franz diffusion assembly (model DC 600; Copley Scientific Ltd, Nottingham, UK) equipped with 6 cm3 acceptor compartments. A polyvinylidene difluoride (PVDF) model membrane (0.125 mm thickness) was used for the permeability studies. The bioconjugates were released from emulsion, which was prepared using cetearyl alcohol (1.5 g), Brij™ 72 (1.2 g), and Brij 58 (0.3 g) as emulsifiers, Vaseline® (5.0 g), stearin (0.5 g), glycerine (1.5 g) (all from Sigma-Aldrich), and water (40.0 g). Samples for the permeability studies were prepared by dissolving 90 mg of G34PLP or G34Ret in 1 g of emulsion. Samples weighing approximately 250 mg were mounted over a commercial PVDF membrane for assessment of permeability.
The receiving medium was 0.067 M phosphate buffer (pH 7.4) in ethanol 7:3 v/v. The progress of diffusion was monitored spectrophotometrically at 355 nm for G34Ret or 275 nm for G34PLP using extinction coefficients calculated for the conjugate solutions in receiving medium. The receiving solution was stirred magnetically at 100 rpm and 32°C. Next, 10 mL aliquots of receptor solution were taken at 0.5 hours or longer time intervals and the receiver compartment was filled with a fresh 6 mL of receptor solution. The experiments were conducted until at least 1.5% of the initial amount of vitamin was received in the receptor solution. The results are presented as the mean (±standard deviation, shown as bars) of six sets of measurements. The results were analyzed calculating the flux in μmol · hour−1 · cm−2. The active area of the membrane determined by size of the ring in the Franz cell was 0.176 cm2. The cumulative amounts of G34PLP and G34Ret received as a function of diffusion time were crucial to determine the diffusion properties of the bioconjugates. For comparison of diffusion efficiency, the slope of the linear part of the plot showing the cumulative percentage of conjugate versus time was used as the quantitative parameter. The slope was obtained using a linear regression procedure with Origin 8.6 packet software (Gambit COD, Krakow, Poland).
Synthesis of PAMAM dendrimers
A third-generation PAMAM dendrimer with an ethylenediamine core (G3) was synthesized according to a previously published methodCitation28 and purified using dialysis in a water- water system through a cellulose membrane (ZelluTrans/Roth 3.5 with a 2 kDa cutoff), as described elsewhere.Citation29 The purity of G3 was confirmed by 1H and 13C NMR spectra in deuterium oxide and in methanol-d4. Standard one-dimensional and two-dimensional correlation spectroscopy, nuclear Overhauser enhancement spectroscopy, heteronuclear single-quantum correlation spectroscopy, and heteronuclear multiple bond correlation measurements were performed to obtain the spectral assignments of G3 and its bioconjugates.
Synthesis of PAMAM G3-Ret bioconjugates
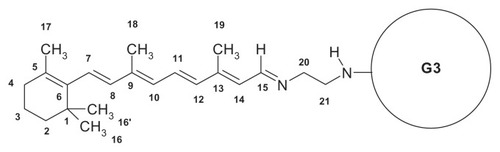
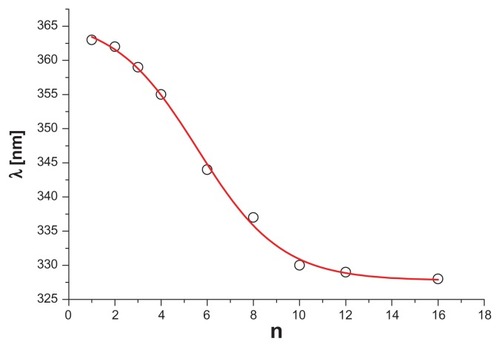
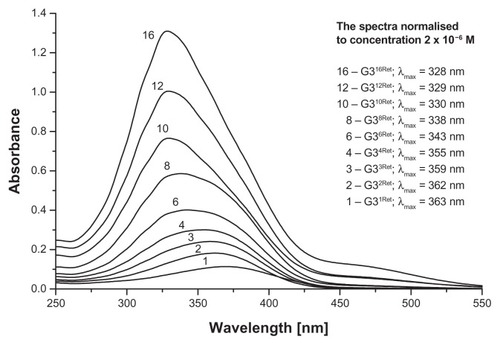
Synthesis of G34-cis−Ret and G34-trans−Ret was performed at a 300 mg scale of G3 (0.42 mmol) in methanol as follows: 1.138 g 13-cis- or 13-trans-retinal (1.68 mmol) in 20 mL of methanol was added dropwise to 30 mL of G3 solution with vigorous stirring in a nitrogen atmosphere in the dark. Then the mixture was then left for 30 minutes, followed by evaporation of the methanol under a stream of nitrogen. The progress of the reaction and final products (see for schematic formula of G34-trans−Ret) was characterized by 1H NMR spectroscopy. The bioconjugates were purified by extensive dialysis in a methanol-methanol system through a cellulose membrane (ZelluTrans/Roth 3.5 with a 2 kDa cutoff). The 1H NMR spectrum for G34-cis−Ret showed the presence of G34-trans−Ret. Therefore, pure G34-trans−Ret was used for further experiments. In a separate experiment, performed using 40 mg of G3, a stock solution of 13-trans-retinal was added in portions to obtain G3nRet bioconjugates (where n = 1, 2, 3, 4, 6, 8, 10, 12, and 16) as a methanolic solution, and ultraviolet-visible spectra were recorded in methanol ().
Figure 2 Relevant fragments of ultraviolet-visible spectra of G3nRet in methanol.
Note: The spectra are numbered according to n.
1H NMR (G34-trans−Ret in CD3OD, for atom numbering see ) showed 8.48 ppm (1H, d, H-15), 7.08 (1H, dd, H-11), 6.50 (1H, d, H-12), 6.38–3.23 (3H, three overlapped doublets, H-7, H-8, H-10), 6.21 (1H, d, H-14), 3.70 (2H, m, H-20), 3.51 (2H, m, H-21), 2.23 (3H, s, CH3–19), 2.11 (2H, H-4), 2.08 (3H, s, CH3–18), 1.78 (3H, s, CH3–17), 1.72 (2H, H-3), 1.57 (2H, H-2), and 1.27 (6H, s, CH3–16, 16′). Ultraviolet-visible (G34-trans−Ret in CH3OH): 355 nm (ɛ = 1.5 · 105 mol−1 · dm3 · cm−1).
Synthesis of PAMAM G3-Pyr and G3-PLP bioconjugates
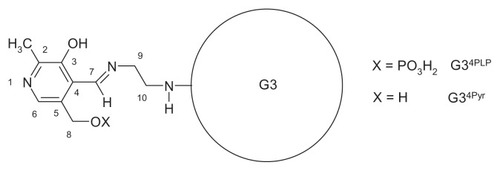
Condensation of Pyr with amine group-terminated G3 in methanol or PLP with G3 was performed in methanol and water, respectively, by stepwise addition of a 0.01 M solution of Pyr in methanol or PLP in water into an approximately 0.001 M solution of G3 in methanol or water, respectively (15 cm3 containing about 105 mg of G3). The stoichiometry of G3nPyr and G3nPLP bioconjugates (n = number of Pyr or PLP molecules covalently attached to terminal nitrogen atoms via an aldimine bond, ) was monitored by 1H NMR spectroscopy. Finally, the G34Pyr and G34PLP were purified by dialysis, as mentioned earlier, isolated, and characterized by 1H and 31P NMR spectra. In a separate smaller experiment, stepwise addition of PLP was continued across a broader range of PLP:G3 molar ratios in order to characterize the G3nPLP bioconjugates by ultraviolet-visible spectra ().
1H NMR (G34PLP in CD3OD; see for atom numbering): 8.89 ppm (1H, H-7), 7.57 (1H, H6), 4.79 (2H, H-8), 3.86 (2H, H-9), 3.55 (2H, H-10), 2.58 (3H, CH3–2). 31P NMR: 3.8 ppm. Ultraviolet- visible (G34PLP in CH3OH): 288 nm (ɛ = 2.5 · 104 mol−1 · dm3 · cm−1), 332 (ɛ = 9.4 · 103 mol−1 · dm3 · cm−1). 1H NMR (G34Pyr in CD3OD): 8.93 ppm (1H, H-7), 7.84 (1H, H6), 4.76 (2H, H-8), 3.84 (2H, H-9), 3.58 (2H, H-10) 2.58 (3H, CH3–2).
Catalytic tests
In vitro studies of transfer of an amine group from L-alanine to α-ketoglutarate were performed on an NMR sample scale. A solution of L-alanine and α-ketoglutarate (both at a concentration of 20 mM) and 2 mM Pyr or PLP were adjusted to pH 7.4 with sodium deuteroxide solution in D2O, and the 1H NMR spectrum was recorded. To this sample, 10 μL of stock solution of transaminase (5 mg/mL) was injected and NMR spectra were then recorded at 5-minute intervals. The same conditions were used to study the bioconjugates, except that Pyr and PLP were replaced with G34Pyr and G34PLP at 0.5 mM concentrations. The overlay spectra are presented in and in the Supplementary materials as Figures S1 and S2.
Results and discussion
The amine groups of the PAMAM dendrimers are readily convertible into Schiff bases via condensation with aldehydes. Citation29 We used a simple protocol for this reaction to obtain conjugates of G3 PAMAM dendrimer containing 32 terminal amine groups with biologically important vitamins, ie, Ret (13-cis and all-trans) and Pyr or PLP.
G3nRet bioconjugates
Stepwise addition of 13-cis-retinal or all-trans-retinal into G3 in methanol resulted in formation of G3nRet bioconjugates at variable substitution steps (n = 1–16), accompanied by marked changes in the ultraviolet-visible spectra (). Initially the absorption band attributed to aldimine n → π* transition was centered at 363 nm (for n = 1), which gradually shifted to a higher energy level (328 nm) upon increasing n to 16, ie, to the point where 50% of the amine groups in G3 were converted into Schiff base. Presumably the hypsochromic shift observed was due to interaction between the unsaturated hydrocarbon chains of all-trans-retinal within the bioconjugate. The sigmoidal plot of λmax versus n () showed the inflection point to be at about n = 6, corresponding to the statistical situation whereby every retinal substituent had at least one other retinal molecule attached to the proximal amine group of G3.
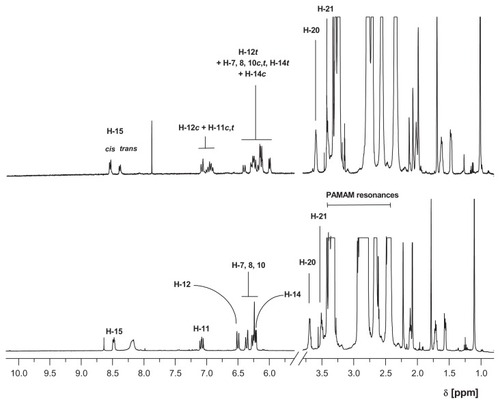
Both 13-cis-retinal and 13-trans-retinal were used to obtain G3Citation4cisRet and G3Citation4transRet bioconjugates. The 1H NMR spectra of these compounds were recorded in methanol-d4 (). The most characteristic resonance of the bioconjugates was that for the aldimine proton (H-15) which was diagnostic for Schiff base formation. The signal shifted from 10.15 ppm (13-cis-retinal) or 10.03 ppm (13-trans-retinal) to 8.59 ppm for G3Citation4cisRet or 8.48 ppm for G3Citation4transRet. The G3Citation4transRet bioconjugate was stable in solution when the sample was protected from air and light (, lower spectrum), while G3Citation4cisRet underwent spontaneous conversion within 12 hours under the same conditions into a mixture of G3Citation4xRet species (where x = cis or trans, , upper spectrum). The G3Citation4transRet bioconjugate actually corresponds to a mixture of species with predominantly n = 4. The high-intensity resonances within the 2.3–3.4 ppm region belong to the PAMAM dendrimer, while those below 2.25 ppm correspond to the methyl and methylene protons of the retinal substituent. The most characteristic resonances of PAMAM in G3Citation4transRet and the other bioconjugates obtained here are those of two methylene group protons, H-20 and H-21 (for atom numbering see ), seen at 3.70 and 3.51 ppm, respectively. The broad resonance at 8.2 ppm (lower spectrum) belongs to the internal N-H protons of the PAMAM dendrimer, which slowly undergoes substitution with the deuterium solvent and eventually disappears, and hence is not present in the upper spectrum for the bioconjugate mixture which was recorded after 12 hours of incubation of initial G3Citation4cisRet in methanol-d4.
Figure 6 1H NMR spectra of mixture of G3Citation4xRet species (x = cis or trans, upper spectrum) and G3Citation4transRet in methanol-d4 (lower spectrum).
Abbreviation: NMR, nuclear magnetic resonance; PAMAM, polyamidoamine.
The 13-cis-trans conversion in the G3nRet conjugates is not surprising in view of previous studies of retinal conformation in Schiff bases. Ab initio quantum chemical and free energy simulationCitation30 and dynamic simulationCitation31 show that lowest energy configuration is all-trans, which is about 2.1 kcal/mol lower than that of 13-cis. Moreover, the flexibility induced by the polyene chain of retinal in the bacteriorhodopsin-retinal Schiff base might be responsible for enabling the cis-trans transition and modifying the pKa of the retinal-protonated Schiff base in bacteriorhodopsin.Citation31
G3nPyr and G3nPLP bioconjugates
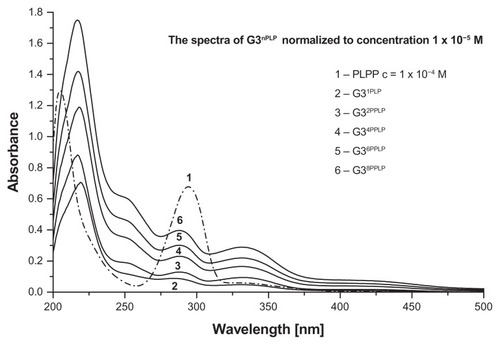
Stepwise addition of pyridoxal hydrochloride solution into G3 (both in methanol) resulted in formation of G3nPyr bioconjugates, which were characterized by 1H NMR spectra. The characteristic resonances of this bioconjugate are those of the aldimine proton (H-7) at 8.93 ppm, the aromatic proton (H-6) at 7.84 ppm, and the 5-CH2OH singlet at 4.76 ppm. The yellow compound is stable in aqueous and methanolic solutions. The solubility of the G34PLP analog in methanol is lower than that of G34Pyr, nevertheless the 1H NMR spectrum was recorded in methanol-d4 for comparative purposes. In this solvent, the chemical shift of the H-6 and H-7 protons was similar to those for G34Pyr (7.57 and 8.89 ppm, respectively), while the doublet of 5-CH2OPO3− protons (H-8) is shifted downfield to 4.79 ppm. Stepwise addition of PLP in water into an aqueous solution of G3 enabled recording of the ultraviolet-visible spectra for G3nPLP bioconjugates (), which differed considerably from that for PLP itself, in that the π → π* transition band centered at 295 nm for the PLP aldehyde split into three bands centered at 340 nm, 275 nm, and 255 nm (shoulder) of almost equal intensity upon formation of the Schiff base. The averaged extinction coefficient per one adjacent PLP remains constant (5.0 × 103 mol−1 dm3 cm−1) and is slightly lower than that for PLP itself (6.2 × 103 mol−1 dm3 cm−1), suggesting that no strong interaction between adjacent PLP substituents is present in G3nPLP until n = 16. However, the highly substituted G3nPLP became insoluble in water. Therefore, both types of Pyr bioconjugate containing four equivalents of vitamin, ie, G34Pyr and G34PLP, were used for subsequent biochemical and pharmacokinetic tests in vitro.
Release of Pyr and PLP from bioconjugates and transamination
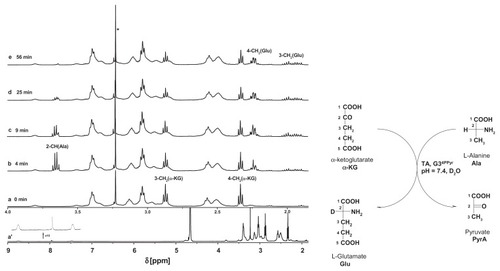
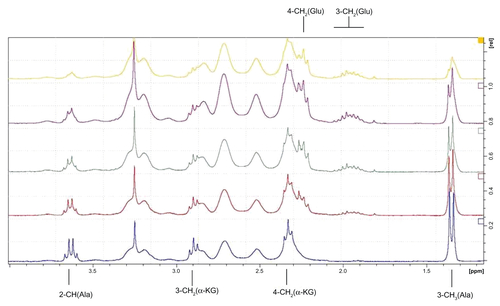
Schiff bases are labile in water. Every aldehyde condensation step with amines is reversible, including the formation of hemiaminals. The presence of hemiaminals and their back conversion into free aldehyde has been demonstrated using model systems,Citation29,Citation32 including Schiff bases obtained from PAMAM dendrimers and aldehydes. Because PLP is a crucial cofactor for transaminases,Citation33 we have tested G34PLP and also G34Pyr as potential cofactor donors. In order to test the bioavailability of PLP incorporated into a G3nPLP bioconjugate, we used glutamic pyruvic transaminase from porcine heart tissue (EC 2.6.1.2) and monitored amine transfer from the L-alanine donor to α-ketoglutarate under 1H NMR spectral control in 2H2O. In the control experiment, PLP was used as an enzyme cofactor. Amine transfer and formation of L-glutamate was triggered by addition of PLP (final concentration 0.002 M, ie, 2 μmol in 1 mL of an NMR sample) into the solution containing 0.02 M L-alanine and α-ketoglutarate and glutamic pyruvic transaminase (approximately 0.05 mg corresponding to three units) in a 1 cm3 sample at pH 7.6. Fifty percent conversion of α-ketoglutarate to L-glutamate was achieved in 45 minutes (see Figure S1 for 1H NMR spectra of the reaction mixture). An almost analogous spectral image was obtained when G34PLP was added as the cofactor source (). In this case, the transamination reaction was triggered by addition of L-alanine into a solution already containing α-ketoglutarate, glutamic pyruvic transaminase, and G34PLP (0.5 μmol, all other concentrations and numbers of μmol were the same as in the control experiment). The 1H NMR spectral assignments were done by two-dimensional correlation spectroscopy. For the starting solution, from which L-alanine was absent, the 3-CH2 and 4-CH2 triplet resonances are seen at 5.35 ppm and 3.50 ppm, respectively, while all other broad resonances belonging to the PAMAM protons and PLP residues are shown in the full-range spectrum at 8.73 (N = CH-7) and 7.50 (6-H). Four minutes after addition of L-alanine, the resonances of L-glutamate appear as multiplets from two magnetically nonequivalent 4-CH2 centered at 1.96 ppm and 3-CH2 centered at 2.24 ppm. No 2-CH (Ala) resonance was observed due to involvement of the deuterium oxide solvent. In the course of the reaction, the resonances from L-alanine disappear (2-CH) or broaden slightly due to formation of a Schiff base between L-alanine and PLP, which in fact is the species actively involved in transamination. Again, 50% conversion of α-ketoglutarate → L-glutamate was achieved within approximately 45 minutes, while the methyl group resonance of L-alanine was no longer detected in the 1H NMR spectrum of the reaction mixture. Thus, the puzzling issue remains concerning the fate of the amine groups not involved in transamination with α-ketoglutarate.
Figure 7 Relevant fragments of 1H NMR spectra of reaction mixtures, containing α-ketoglutarate (20 mM), glutamic-pyruvic transaminase (3 units), G34PLP (2 mM); the full spectrum is presented in (a) in the inset two resonances of PLP substituent at 8.8 ppm (H-7) and 7.5 ppm (H-6) are shown together with residual NH (PAMAM) resonance. (b) α-ketoglutarate and L-alanine (initial concentrations 20 mM), glutamic-pyruvic transaminase (3 units), and G34PLP (2 mM) after four minutes of reaction. (c–e) Components as in (b) after 9, 25, and 56 minutes, respectively.
Note: The assignments of resonances were done according to the numbering scheme (shown in the right margin).
Abbreviations: NMR, nuclear magnetic resonance; PAMAM, polyamidomine; PLP, pyridoxal phosphate.
The most surprising result was obtained in the last transamination experiment, in which G34Pyr was used as a potential cofactor source for transaminase. Transamination proceeded in the same way as that for G34PLP (Figure S2). No reaction was observed when Pyr was used instead of G34Pyr in the control experiment. The cofactor activity of G34Pyr in the amine transfer from L-alanine to α-ketoglutarate suggests that activation of Pyr as a cofactor for transaminase occurs via covalent bonding of Pyr to PAMAM G3.
Amine group transfer from pyridoxamine covalently incorporated into the core of PAMAM to phenylpyruvic and pyruvic acid,Citation34,Citation35 as well as racemization of alanine by Pyr covalently incorporated into the core of polyethyleneimine dendrimers has been reported.Citation36 In order to characterize the roles of the PAMAM carrier and the PAMAM-PLP and PAMAM-Pyr bioconjugates further, we performed additional control experiments. Amine transfer from PAMAM to α-ketoglutarate did not occur in the absence or presence of glutamic pyruvic transaminase when L-alanine was absent. Thus the PAMAM dendrimer was excluded as an amine group donor. G34PLP and G34Pyr in the absence of glutamic pyruvic transaminase did not catalyze amine transfer from L-alanine to α-ketoglutarate, demonstrating that the bioconjugates did not play a catalytic role. Finally, transamination did not occur when G3 was added to the system containing L-alanine, α-ketoglutarate, and glutamic pyruvic transaminase, indicating that G3 was not an activator of glutamic pyruvic transaminase.
Permeation through PVDF membrane
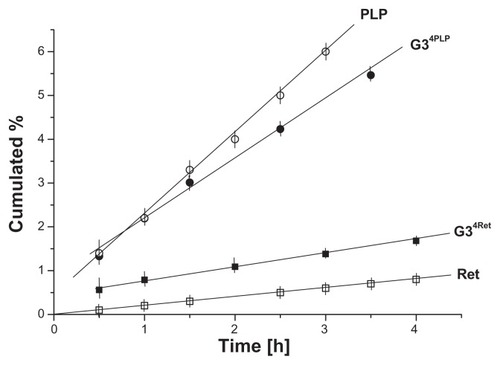
Permeation of G34Ret and G34PLP conjugates through PVDF was tested using a buffered water:ethanol receiving solution. The conjugates were soluble in this solvent to a concentration of 10−3 mol dm−3, so we could obtain the ultraviolet-visible spectra. We measured the extinction at λmax for G34Ret and G34PLP conjugates at 355 nm and 275 nm, respectively, and took the full spectra in order to confirm the identity of the conjugates by comparing them with the original spectra of these species. The flux, measured under reproducible conditions for both the test systems, changed in a typical manner, being high during the first half hour of the experiment and becoming stable (in a stationary condition) thereafter. The diffusion profiles were plotted as the cumulative amount of conjugate versus time or percentage of diffused species versus time. The conjugates showed different fluxes in stationary conditions, ie, the flux of G34PLP was 1.5 μmol h−1 cm−2, while that of G34Ret was as low as 0.4 μmol h−1 cm−2. The cumulative percentage plots for the diffused species versus time are shown in . The linear fit for these experimental profiles was applied in the region of stationary conditions, and the slopes of the fitted lines (expressed in % h−1) can be interpreted in terms of the diffusion ability of the conjugates. Thus, the slopes of the fitted line for G34Ret (0.44% ± 0.03% h−1) and for G34PLP (1.36% ± 0.06% h−1) demonstrate clearly an approximately two-fold lower permeation ability for the G34Ret conjugate than for the G34PLP. For comparison, control experiments were performed on release of trans-retinal and PLP from the same emulsion (). Comparing the diffusion rates for the G34Ret conjugate with those of Ret alone, one can see that the bioconjugate acts as a vitamin permeation promoter, while comparison of the diffusion profiles for G34PLP and PLP indicate that, in this case, the bioconjugate slows diffusion of PLP. Generally, by introducing enhancement factors (EF) as the ratio of the diffusion rate of bioconjugates (or host–guest complexes G3-X, where X = ascorbic acid, riboflavin, or 8-methoxypsoralen) to the diffusion rate of the free species X, the ability of the G3 PAMAM dendrimer to influence permeation can be estimated quantitatively. Thus, EF values of >1 demonstrate the ability of the G3 PAMAM dendrimer to promote transdermal diffusion, while EF values < 1 indicate that the dendrimer retards diffusion ().
Table 1 Diffusion ability of various species (G3 bioconjugates and host–guest complexes) studied by release from emulsions followed by permeation through polyvinylidene difluoride
Figure 8 Permeation profiles of G34Ret, G34PLP conjugates, and free vitamins through polyvinylidene difluoride.
Abbreviations: PLP, pyridoxal phosphate; Ret, retinal.
Permeation efficiency (diffusion rate of drug or prodrug, including bioconjugates) is influenced by two main factors, ie, size and flexibility of the species and its water solubility. We used a G3 PAMAM dendrimer as a macromolecular transdermal platform for drug diffusion. We have determined previously the rate of diffusion of G3 itself (G3 labeled with one fluorescein substituent [G3F]),Citation5 which gave us a reference value of 2.18% ± 0.23% h−1.
For bioconjugates bearing four substituents of Ret, Pyr, or PLP, the diffusion rate is lower, which can be attributed to the larger size of the bioconjugate than G3 itself (2.2 nm in diameter). Nevertheless, the bioconjugates do modify the diffusion of free Ret or PLP. In the case of Ret, which is insoluble in water (but soluble in oil phase emulsion) the bioconjugate diffuses faster than Ret due to better solubility of the G34Ret bioconjugate than Ret in water. In the case of PLP, the effect is opposite, in that PLP diffuses faster than the macromolecular G34PLP bioconjugate (both water-soluble) due to a steric effect.
For host–guest complexes with PAMAM as the host, the PAMAM carrier diminishes the rate of diffusion of water-soluble vitamin C by four times through porcine ear skin and by 1.5 times through PVDF.Citation27 On the other hand, G3 acts as a solubilizer for water-insoluble riboflavinCitation5 and 8-methoxypsoralene,Citation4 and promotes their diffusion. The enhancement factor was 4 through PVDF and 3 through porcine ear skin for riboflavinCitation5 and 3 for 8-methoxypsoralen as demonstrated in vitro using PVDF and porcine ear skin models, as well as in vivo studies using rat skin with confocal microscopy monitoring.Citation37
Conclusion
Vitamin B6 and vitamin A readily form conjugates with third-generation PAMAM dendrimers as Schiff bases. Condensation of 13-cis and 13-trans- retinal with G3 resulted in formation of conjugates with variable degrees of PAMAM substitution. The attached molecules of 13-cis-retinal undergo 13-cis → 13-trans conversion within the Schiff base. Polyene chains of 13-trans- retinal interact mutually within the bioconjugate. PLP is readily available as a cofactor for transaminase when released from a G3 bioconjugate, which was demonstrated in vitro by amine transfer from L-alanine to α-ketoglutarate, as confirmed by 1H NMR spectroscopy. The Pyr bioconjugated with G3 behaves the same as G34PLP and in contrary to free pyridoxal, which is not cofactor for transaminase. Thus, it undergoes activation upon binding to the PAMAM dendrimer. Bioconjugates of PLP and Ret with a G3 PAMAM dendrimer diffuse through a PVDF skin model membrane slightly slower than G3 labeled with single fluorescein. Finally, bioconjugates of G3 with vitamin A and vitamin B6 aldehydes can be used as transdermal drug delivery vehicles. In the case of bioconjugates with Pyr and PLP, the vitamins become available as transaminase cofactors, which is crucial when using these conjugates to feed skin.
Acknowledgment
The work was supported by a grant (N302 432839) from the Ministry of Higher Education and Research, Poland.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary figures
Figure S1 The 1H NMR spectra of reaction mixture containing pyridoxal phosphate (2 mM), L-alanine and α-ketoglutarate (both 20 mmM) and transaminase (3 units) in D2O, pH = 7.4.
Abbreviation: NMR, nuclear magnetic resonance.
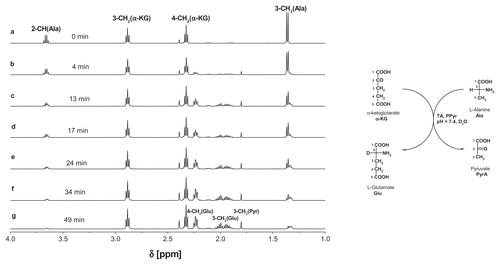
Figure S2 The relevant fragments of 1H NMR spectra of reaction mixture containing G34Pyr (2 mM), L-alanine and α-ketoglutarate (both 20 mmM) and transaminase (3 units) in D2O, pH = 7.4, recorded after (from bottom to top): 2, 6, 10, 20, and 45 minutes, respectively.
Abbreviation: NMR, nuclear magnetic resonance.
References
- TomaliaDANaylorAMGoddardWAIIIStarburst dendrimers: molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matterAngew Chem Int Ed Engl199029138175
- VenugantiVVKPerumalOPEffect of poly(amidoamine) (PAMAM) dendrimer on skin permeation of 5-fluorouracilInt J Pharm200836123023818582550
- ChengYLiMXuTPotential of poly(amidoamine) dendrimers as drug carriers of camptothecin based on encapsulation studiesEur J Med Chem2008431791179518215444
- BorowskaKLaskowskaBMagońAMyśliwiecBPydaMWołowiecSPAMAM dendrimers as solubilizers and hosts for 8-methoxypsoralene enabling transdermal diffusion of the guestInt J Pharm201039818518920655371
- FilipowiczAWołowiecSSolubility and in vitro transdermal diffusion of riboflavin assisted by PAMAM dendrimersInt J Pharm201140815215621272625
- ChengYQuHMaMPolyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinoline antimicrobials: an in vitro studyEur J Med Chem2007421032103817336426
- MaMChengYXuZEvaluation of polyamidoamine (PAMAM) dendrimers as drug carrier of anti-bacterial drugs using sulfamethoxazole (SMZ) as a model drugEur J Med Chem200742939817095123
- DevarakondaBOttoDPJudefeindAHillRAde VilliersMMEffect of pH on the solubility and release of furosemide from polyamidoamine (PAMAM) dendrimer complexesInt J Pharm200734514215317600643
- WangYGuoRCaoXShenMShiXEncapsulation of 2-methoxyestradiol within multifunctional poly(amidoamine) dendrimers for targeted cancer therapyBiomaterials2011323322332921315444
- ChengYLiYZhangJXuTGeneration-dependent encapsulation/electrostatic attachment of phenobarbital molecules by poly(amidoamine) dendrimers: evidence from 2D-NOESY investigationsEur J Med Chem2009442219222318635290
- ChandrasekarDSistlaRAhmadFJKharRKDiwanPVThe development of folate-PAMAM dendrimer conjugates and their pharmacokinetics and biodistribution in arthritic ratsBiomaterials20072850451216996126
- YangWChengYXuTWangXWenLPTargeting cancer cells with biotin-dendrimer conjugatesEur J Med Chem20094486286818550227
- ThomasTPChoiSKLiM-HKotylarABakerJRJrDesign of riboflavin- presenting PAMAM dendrimers as a new nanoplatform for cancer-targeted deliveryBioorg Med Chem Lett2010205191519420659800
- ZhangKYuAWangDSolvent controlled self-assembly of amphiphilic cholic acid-modified PAMAM dendrimersMater Lett201165293295
- JiaLXuJ-PWangHJiJPolyamidoamine dendrimers surface-engineered with biomimetic phosphorylcholine as potential drug delivery carriersColloids Surf B Biointerfaces201184495421237622
- WiwattanapatapeeRLomlimLSaramunaeKDendrimers conjugates for colonic delivery of 5-aminosalicylic acidJ Control Release2003881912586498
- KhandareJKolhePPillaiOKannanSLieh-LaiMKannanRMSynthesis, cellular transport, and activity of poalyamidoamine dendrimer- methylprednisolone conjugatesBioconjug Chem20051633033715769086
- MaKHuM-XQiYPAMAM-triamcinolone acetonide conjugate as a nucleus-targeting gene carrier for enhanced transfer activityBiomaterials2009306109611819656564
- KurtogluYENavathRSWangBKannanSRomeroRKannanRMPoly(amidoamine) dendrimer-drug conjugates with disulfide linkages for intracellular drug deliveryBiomaterials2009302112212119171376
- KurtogluYEMishraMKKannanSKannanRMDrug release characteristics of PAMAM dendrimer-drug conjugates with different linkersInt J Pharm201038418919419825406
- PatriAKKukowska-LatalloJFBakerJRJrTargeted drug delivery with dendrimers: comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complexAdv Drug Deliv Rev2005572203221416290254
- MukherjeeSPLyngFMGarciaADavorenMByrneHJMechanistic studies of in vitro cytotoxicity of poly(amidoamine) dendrimers in mammalian cellsToxicol Appl Pharmacol201024825926820736030
- KolhatkarRBKitchensKMSwaanPWGhandehariHSurface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeabilityBioconjug Chem2007182054206017960872
- ScutaruAMKrügerMWenzelMRichterJGustRInvestigations on the use of fluorescence dyes for labelling dendrimers: cytotoxicity, accumulation kinetics, and intracellular distributionBioconjug Chem2010212222222621049938
- JevprasesphantRPennyJJalalRAtwoodDMcKeonNBD’EmanueleAThe influence of surface modification on the cytotoxicity of PAMAM dendrimersInt J Pharm200325226326612550802
- VenugantiVVPerumalOPPoly(amidoamine) dendrimers as skin penetration enhancers: influence of charge, generation and concentrationJ Pharm Sci2009982345235618937369
- WołowiecSLaskowskiMLaskowskaBMagońAMyśliwiecBPydaMDermatological application of PAMAM – vitamin bioconjugates and host-guest complexes. Vitamin C case studyInnocentiAStoichiometry and Research – The Importance of Quantity in Biomedicine. Part 3Stoichiometry in Lipids and Polymer ArchitectureRijeka. CroatiaIntech2012
- TomaliaDAHuangBSwansonDRBrothersHMIIKlimashJWStructure control within poly(amidoamine) dendrimers: size, shape and regio–chemical mimicry of globular proteinsTetrahedron20035937993813
- SubikPWelcBWiszBWołowiecSStable hemiaminals attached to PAMAM dendrimersTetrahedron Lett20095065126514
- BaudryJCrouzySRouxBSmithJCQuantum chemical and free energy simulation analysis of retinal conformational energeticsJ Chem Inf Comput Sci19973710181024
- TajkhorshidEBaudryJSchultenKSuhaiSMolecular dynamics study of the nature and origin of retinal’s twisted structure in bacteriorhodopsinBiophys J20007868369310653781
- IwasawaTHooleyRJRebekJStabilization of labile carbonyl addition intermediates by a synthetic receptorScience200731749349617656719
- StryerLBiochemistry4th edNew York, NYWH Freeman and Co1995
- LiuLBreslowRDendrimeric pyridoxamine enzyme mimicsJ Am Chem Soc2003125121101211114518994
- BreslowRWeiSKeneskyCEnantioselective transaminations by dendrimeric enzyme mimicsTetrahedron2007636317632118596840
- LiuLBreslowRPolymeric and dendrimeric pyridoxal enzyme mimicsBioorg Med Chem2004123277328715158796
- BorowskaKWołowiecSRubajAGłowniakKSieniawskaERadejSEffect of polyamidoamine dendrimer G3 and G4 on skin permeation of 8-methoxypsoralene – in vivo studyInt J Pharm201242628028322310461