 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
β-Carotene has been established as a known free radical scavenger with chain-breaking antioxidant properties. It has been documented for the treatment of epileptic convulsions at a 200 mg/kg body weight dose. The reported pathogenesis for epileptic convulsions is oxidative stress. Hence, experimental epileptic convulsions via oxidative stress was induced in albino mice epileptic models (maximal electroshock seizure and pentylenetetrazole [PTZ]). A dose concentration equivalent to 2 mg/kg was efficaciously administered in the form of brain-targeted polysorbate-80-coated poly(d,l-lactide-co-glycolide) nanoparticles. The nanoparticles were prepared by solvent evaporation technique and further characterized for their physical parameters, in-vitro release kinetics, and in-vivo brain release via various standard methods. Normal β-carotene nanoparticles (BCNP) and polysorbate-80-coated β-carotene nanoparticles (P-80-BCNP) of 169.8 ± 4.8 nm and 176.3 ± 3.2 nm in size, respectively, were formulated and characterized. Their zeta potential and polydispersity index were subsequently evaluated after 5 months of storage to confirm stability. In vivo activity results showed that a 2 mg unformulated β-carotene dose was ineffective as an anticonvulsant. However, salutary response was reported from BCNP at the same dose, as the hind limb duration decreased significantly in maximal electroshock seizure to 9.30 ± 0.86 seconds, which further decreased with polysorbate-80 coating to 2.10 ± 1.16 seconds as compared to normal control (15.8 ± 1.49 seconds) and placebo control (16.50 ± 1.43 seconds). In the PTZ model, the duration of general tonic–clonic seizures reduced significantly to 2.90 ± 0.98 seconds by the use of BCNP and was further reduced on P-80-BCNP to 1.20 ± 0.20 seconds as compared to PTZ control and PTZ-placebo control (8.09 ± 0.26 seconds). General tonic–clonic seizures latency was increased significantly to 191.0 ± 9.80 seconds in BCNP and was further increased in P-80-BCNP to 231.0 ± 16.30 seconds, as compared to PTZ (120.10 ± 4.50 seconds) and placebo control (120.30 ± 7.4 seconds). The results of this study demonstrate a plausible novel anticonvulsant activity of β-carotene at a low dose of 2 mg/kg, with brain-targeted nanodelivery, thus increasing its bioavailability and stability.
Introduction
Epilepsy debilitates more than 50 million people worldwide.Citation1 The majority of the affected population suffers from generalized tonic–clonic seizures, while others are affected with multiple forms of the disorder. One of the main pathogenesis of epilepsy is oxidative stress wherein the brain utilizes high amounts of oxygen as compared to other body organs. This consequently results in cellular disruptions, damage, and eventual cell death.Citation2 The consequences of oxidative stress are due to the oxidation of functional biomolecules such as proteins, lipids, and nucleotides in various cells.Citation3 Protein oxidation leads to the deactivation of several enzyme systems and their associated functional changes, while lipid peroxidation causes membrane structure alterations resulting in changes in membrane fluidity, permeation, and biological functionality.Citation4,Citation5
An increased calcium ion concentration in neuronal tissue leads to a cascade of biochemical responses which trigger neuronal cell death after status epilepticus.Citation6 High levels of intracellular calcium ions induce reactive oxygen species (ROS) in a biosystem mainly through xanthine oxidase and nicotinamide adenine dinucleotide phosphate oxidase mediated biochemical reactions.Citation7–Citation9 The increased level of superoxide radicals result in an equally elevated hydrogen peroxide level via a superoxide dismutase mediated reaction. Highly reactive hydroxyl (OH) free radicals generated by the reaction of peroxynitrite with superoxide radicals,Citation10 or through Fenton reactions,Citation11 readily oxidize the structural proteins of the cell membrane resulting in the disruption of cellular membrane fluidity, permeation, and overall biological functionality including that of the neuronal cells, DNA, and lipids.Citation9,Citation12 Physiological levels of ROS are maintained by means of scavenging through enzymatic (eg, superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, and peroxiredoxins) and nonenzymatic reactions of antioxidants.Citation3,Citation14–Citation16
Many biological experiments conducted by use of kainic acid, iron-salt, and electroshock induced seizures, as well as the kindling model by pentylenetetrazole (PTZ) have confirmed the relation of ROS and other species with convulsions.Citation17–Citation21 PTZ, an epilepsy inducing agent, is a selective blocker of GABAA receptor-chloride ionophore complex, which induces convulsions by triggering the glutamatergic transmitter system. This activation is due to an increased intracellular calcium ion influx, which results in an increased production of superoxide radicals (O2°–) ().Citation22,Citation23 The well-standardized and validated PTZ-induced model was selected for the present study.Citation24
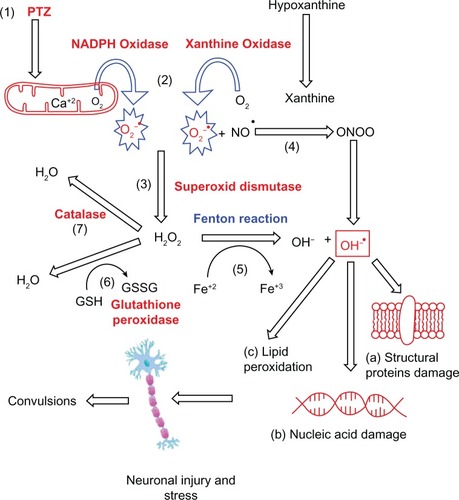
Figure 1 Mechanism of free radical generation.
Notes: (1) Pentylenetetrazole kindling activates the glutamatergic transmitter system which increases the intracellular calcium ion concentration and induces the formation of O2°− (2) Xanthine oxidase and nicotinamide adenine dinucleotide phosphate oxidase generates the superoxide radical, O2°− and has been widely applied as a O2°− generating system. (3) Increased hydrogen peroxide production through the catalyzed dismutation of O2°− by superoxide dismutase. (4) Production of ONOO− from NO and superoxide radical, which further produces OH°− free radical. (5) Production of OH°− free radical through Fenton reaction, readily oxidizes structural proteins of the Neuronal cells thus disrupting their fluidity, permeation and biological functioning (a) DNA (b) and lipids (c). (6) The enzyme glutathione peroxidase utilizes reduced glutathione to eliminate hydrogen peroxide as H2O. (7) Hydrogen peroxide is broken down into water and oxygen by the enzyme catalase.
Abbreviations: NADPH, nicotinamide adenine dinucleotide phosphate; O2°−superoxide radical; ONOO−, peroxynitrite; NO, nitric oxide; OH°−, highly reactive hydroxyl; DNA, Deoxyribonucleic acid.
β-Carotene, a known source of vitamin A, has exceptional antioxidant and free radical scavenging potential.Citation25,Citation26 Its antiepileptogenic activity has been established in PTZ-induced epilepsy models.Citation27 Earlier work provided evidence that β-carotene-enriched feeding of adult rats for a 1-week trial delayed the onset of induced seizures.Citation28 β-Carotene is converted into retinol in the biosystem, and due to its antioxidant activity, it plays an important role in preventing neurodegenerative disorders.Citation29 The high hydrophobicity of β-carotene makes it insoluble in an aqueous medium and thus restricts its absorption through the diet. Previous studies have focused on improving the dispersibility of carotenoids in water so as to increase its bioavailability.Citation30 Some researchers have developed water-dispersible formulations as carriers to entrap carotenoids and direct them to in vitro and in vivo systems.Citation31–Citation34 The polymer-based delivery system consisting of nanoscale carriers provides an alternative mode of carotenoid delivery. These formulations have shown increased physiochemical stability of the encapsulated β-carotene.Citation35,Citation36 The polyester poly(d,l-lactide-co-glycolide) (PLGA) nanoparticles were selected due to their biocompatibility, biodegradation properties, US Food and Drug Administration approval for biological use, successful central nervous system delivery, and its facile biodecomposition without being immunogenic or prone to producing inflammation at the site of action.Citation37,Citation38
Our objective was to focus on reducing the central nervous system’s oxidative stress with the use of known and natural antioxidants like β-carotene. The antioxidants were delivered to specific sites of disorder across the blood–brain barrier in neuronal tissues, which were then believed to control epilepsy through a free radical scavenging mechanism. However, drug targeting to neuronal regions require its delivery to be strictly localized, including its pharmacological active manifestation to the particular brain site. Polysorbate-80-coated β-carotene nanoparticles (P-80-BCNP) appeared to follow a similar required course of delivery in mimicking the adsorption of low-density lipoprotein, apolipoprotein-E, and apolipoprotein-A1 after their injection into the systemic blood circulation followed by receptor-mediated endocytosis of the nanoparticles by the brain barrier’s capillary endothelial cells.Citation39,Citation40 This hypothesis by Kreuter and Kim was supported by the fact that covalent coupling of apolipoprotein-E and apolipoprotein-A1 to human serum albumin nanoparticles lead to similar endocytosis.Citation41–Citation43 In the present study, polysorbate-80 was used as the coating surfactant for intended β-carotene-loaded nanoparticles for its brain-specific delivery. Hence, P-80-BCNP were prepared and anticonvulsant activity was evaluated with the intent to: (1) improve bioavailability at the site of action; (2) increase the stability of β-carotene in nanoparticle formulations; and (3) target required quantities of β-carotene to the brain site in reduced doses. To date, there has been no known documentation on the nanomodulated delivery of β-carotene to the brain with anticonvulsant activity.
Materials and methods
Procurement of raw materials
β-Carotene, PLA Mw 31,000–50,000, PLGA Mw 4,000–15,000, and hydrophobic polytetrafluoroethylene filters (pore diameter 0.5 mm) were purchased from Sigma-Aldrich (Milwaukee, WI). High-pressure liquid chromatography (HPLC)-grade acetone and Tween-80 (polysorbate-80) were purchased from SD Fine Chemicals (Mumbai, India). All materials were used as obtained.
Preparation of nanoparticles
Polymeric nanoparticles containing β-carotene at variable concentrations of polymers were prepared using a solvent displacement technique at room temperature (25°C). Briefly, in a typical preparation procedure providing the greatest entrapment of the drug, 10.0 mg of β-carotene was dissolved in 30.0 mL of acetone, followed by dissolution of 2.00 mg of α-tocopherol (as stabilizer), and 100.0 mg PLGA with 45.0 mg of PVA. The resulting solution was then gradually, in a drop-by-drop manner, added to a 30.0 mL aqueous phase containing 1% Tween-80 (as a dispersing agent) with continuous stirring at 10,000 rpm for 20 minutes. Following the addition, 70.0 mL of water was then added, and the organic solvent was allowed to diffuse into the aqueous phase. Acetone was eliminated by using a rotary vacuum evaporator under controlled temperature (<40°C) and vacuum. The resultant volume was further concentrated to one fifth of the original volume under the same conditions to give approximately 30.0 mL of final volume, which was subsequently freeze-dried and stored in a sealed dark amber vial at −4.0°C ± 2.0°C to produce BCNP. The lyophilized nanoparticles (BCNP) were reconstituted in double distilled water and were further coated by stirring in 1% polysorbate-80 (Tween-80) for 1 hour to produce P-80-BCNP.
Entrapment efficiency
β-carotene was extracted from nanoparticles by dissolving it in 2.0 mL ethanol and 3.0 mL n-hexane. The mixture was shaken, and the hexane phase was then recovered. The extraction was repeated twice and the recovered hexane phase was then further diluted with the same to 25 mL quantity sufficient. Absorbance was measured at a 450 nm wavelength using a Shimadzu UV-spectrophotometer (UV 1601; Shimadzu, Tokyo, Japan)Citation44 by means of a standard curve ranging from 1–10 μg/mL (r2 = 0.99). The percent entrapment efficiency was represented as follows:
W(initial) represents the drug taken in nanoparticle form for assay, and W(free drug) represents the free drug present in the supernatant.
Distribution and nanoparticle size
Particle size, zeta potential, and polydispersity index was determined by Zetasizer (Malvern Instruments, Worcestershire, UK). For long-term stability testing, particle size measurements were conducted over a 5-month storage period and frozen at −4°C ± 2°C.
Transmission electron microscopy (TEM)
Morphology of nanoparticles was analyzed using TEM (Morgagni 268D; SEI, Collegeville, PA). Briefly, a drop of sample was placed onto a 300-mesh copper grid and rested for 1 minute, followed by the addition of a drop of phosphotungstic acid and left again for 10 seconds. Residual phosphotungstic acid was removed by absorption, and the sample was analyzed at 60–80 KV at 1550× magnification.
X-ray diffractometer studies
An X-ray diffractometer (PW 1830; Phillips, Bangalore, Karnataka, India) was used. Samples were exposed to monochromatic Cu K- radiation (0.45 kV × 20 mA, λ =1.5406 Å) obtained by Ni filtration, and a system of D/R/S slides of 1°, 0.2 mm, and 1°, respectively. The diffraction pattern was determined in the area 10° <2θ <60°, using a stepwise method (0.2°/s).
Differential scanning calorimetric (DSC) studies
DSC experiments were carried out (TA Instruments, Brussels, Belgium) to determine the possible interactions between the drug and polymer. Briefly, between 5.0 to 10.0 mg of polyvinyl alcohol (PVA), PLGA, β-carotene, and lyophilized nanosuspension samples were added into aluminum pans, which were hermetically sealed. The heating rate was kept constant at 5°C/min, while nitrogen served as a purge gas to control the temperature of the system.
Animals
Male albino mice (25.0–30.0 gm weight) were received from Animal House after clearance from the institutional ethics committee (173/Committee for the Purpose of Control and Supervision on Experiments on Animals, Tamil Nadu, India). The animals were housed in colony cages under ambient temperature (25.0°C ± 2.0°C) with a 45%–55% relative humidity and under 10-hour light/14-hour dark cycles. The animals were allowed food and water ad libitum.
Dosing
Six groups of six animals each were taken for the maximal electroshock seizure (MES) study. Group 1 received 5% carboxymethylcellulose (CMC) as a normal control; Group 2 received β-carotene (200 mg/kg, 5% CMC); Group 3 received β-carotene (2 mg/kg, 5% CMC); Group 4 received placebo nanoparticles in 5% CMC as a placebo control; Group 5 received BCNP (2 mg/kg β-carotene, 5% CMC); and Group 6 received P-80-BCNP in 2 mg/kg β-carotene, 5% CMC. An apparatus with ear electrodes was used to deliver the stimuli.Citation45 The intensity of stimulus was 12 mA, 50 Hz for 0.2 seconds, wherein hind limb muscle extensor was the evaluation parameter.
In the PTZ kindling method, for the evaluation of general tonic–clonic seizure duration and latency, two sets of seven groups with six animals per group were taken. Group 1 was given 5% CMC as normal control; Group 2 received PTZ (60 mg/kg) as PTZ-control; Group 3 received PTZ (60 mg/kg) + β-carotene (2 mg/kg, 5% CMC); Group 4 received PTZ (60 mg/kg) + β-carotene (200 mg/kg, 5% CMC); Group 5 received PTZ (60 mg/kg) + placebo nanoparticles in 5% CMC as a placebo control; Group 6 received PTZ (60 mg/kg) + BCNP (2 mg/kg β-carotene, 5% CMC); and Group 7 animals received PTZ (60 mg/kg) + P-80-BCNP (2 mg/kg β-carotene, 5% CMC). All animals were observed for a period of 30 minutes after PTZ administration, and the duration and latency of general tonic–clonic seizure were evaluated as parameters.Citation46 Dose response studies ranged from a start of 200 mg/kg down to 2 mg/kg, and the dose fraction study was performed separately for the various antioxidants (Piperine, Capsaicin, Curcumin, β-carotene) (data are not shown). All the doses were administered via an intraperitoneal route.
In vitro β-carotene release
The release of β-carotene from nanoformulations were carried out in phosphate buffer saline (PBS, 154 mM, pH 7.4) containing 1% w/v Tween-80 at 37°C, 100 rpm in an orbital shaker (Barnstead Lab-Line, USA). Two milligram equivalent nanoparticle formulations were dispersed in 2.0 mL PBS and transferred into a dialysis bag (Sigma-Aldrich, cut off size 10 kDa), which was immersed into a 50.0 mL falcon tube containing 5.0 mL of PBS and ethyl alcohol (50.0% v/v). At predetermined intervals, all buffer solutions in the falcon tube were removed and replaced with a fresh solution. The β-carotene concentrations in the released samples were determined using a UV/VIS spectrophotometer using standard samples of β-carotene (0–10 μg/mL).
Release of β-carotene in brain
HPLC (Shimadzu, Japan) coupled with a UV/VIS detector (200 IC, USA), C18 column (4.6 mm × 250 mm, 5 μm, Theale Reading, Berks, UK), loaded with DataApex Clarity Lite (DataApex Ltd, Prague, Czech Republic) software was employed for all quantitative analyses. A mobile phase consisting of 1:1 (v/w) of acetonitrile in 0.01 M KH2PO4 adjusted to a pH 4.5 using orthophosphoric acid was used at a flow rate of 1.00 mL/min with detections assessed at 450 nm. β-carotene standards were freshly-prepared by diluting a stock solution in the above mentioned mobile phase to a concentration range of 2–100 ng/mL. The standards showed good linear correlation and accuracy with an average percentage curve value of less than 15%.
The brain of each sacrificed mouse was surgically isolated under anesthesia and homogenized with 0.2 mL of 1.15% KCl. The homogenate was thoroughly mixed with 0.3 mL of HPLC in the mobile phase, and was centrifuged at 10,000 rpm for 30 min (Spectrafuge, USA). The supernatant was then separated, and HPLC analysis was performed. All the data were obtained in triplicate (n = 3).
Statistical analysis
One-way analyses of variance (ANOVA) was performed on the data as mean ± SD to assess the size, polydispersity index, zeta potential, and in vitro and in vivo release (where, n = 3), and P < 0.05 was considered significant. However, the anticonvulsant data was expressed as mean ± SEM, and the significance of differences amongst the groups were determined using Tukey’s post hoc test, using a one-way ANOVA.
Results and discussion
All nanoparticle formulations were prepared by use of a solvent diffusion method,Citation47 which provided a high entrapment efficiency at 83.0% ± 3.8% for the drug. The placebo nanoparticles were an average size of 159.3 ± 6.1 nm; β-carotene-loaded nanoparticles were an average size of 169.8 ± 4.8 nm; while P-80-BCNP were an average size of 176.3 ± 3.2 nm. The nanoparticles were stored in reserve at −4.0°C ± 2.0°C. The average size following 5 months of storage was determined to be approximately 187.4 ± 3.8 nm for P-80-BCNP.
In general, particle aggregation is less likely to occur for charged particles (high ± zeta potential) due to electric repulsion. Lower ± zeta potential facilitates aggregation. Zeta potential of placebo nanoparticles was −20 ± 0.8 mV, which decreased to −18 ± 0.4 mV for β-carotene-loaded nanoparticles making nanoparticles denser (). The zeta potential of P-80-BCNP was further decreased to −16 ± 0.3 mV making it denser (). No appreciable changes were observed in the zeta potential after 5 months of storage at −4.0°C ± 2.0°C. The polydispersity index shows the distribution measure of nanoparticles. A polydispersity index greater than 0.5 indicates aggregation of particles; however, as the polydispersity index increased, it remained less than 0.5 after drug loading and polysorbate-80 coating, which confirms no appreciable aggregation. It consistently remained constant after 5 months of storage. As reported, P-80-BCNP crossed the blood–brain barrier through endocytosis via adsorption of apolipoprotein E on its surface. The nanoparticles mimic low-density lipoprotein particles, allowing for an interaction with low-density lipoprotein receptors at the brain microvessel endothelial cells for bypass.Citation39
Figure 2 Transmission electron microscopic images. (A) Placebo nanoparticles; (B) Polymeric nanoparticles containing BCNP at a dose equivalent to 2 mg/kg body weight; (C) P-80-BCNP dose equivalent to 2 mg/kg body weight; (D) P-80-BCNP nanoparticles after 5 months.
Abbreviations: BCNP, β-carotene nanoparticles; P-80-BCNP, polysorbate-80-coated β-carotene.
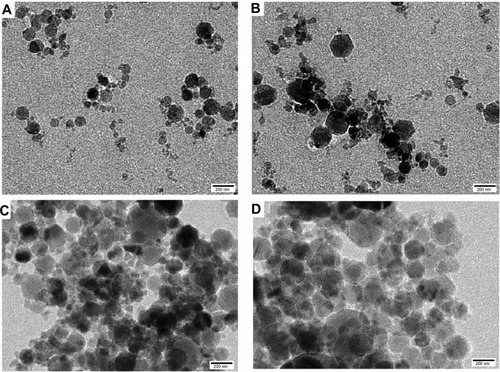
The TEM images of the placebo nanoparticles (), β-carotene-loaded nanoparticles (), P-80-BCNP (), and over 5 months stored nanoformulations () confirmed the shape, size, and storage stability of all the formulation versions used in this study. In addition, the morphological observations confirmed their spherical shape.
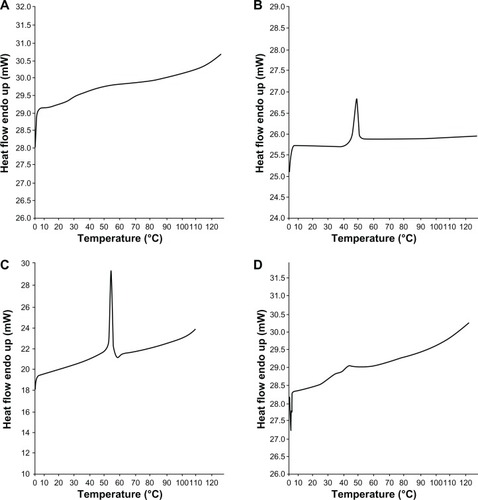
X-ray diffraction studies revealed the amorphous natures of PVA and PLGA polymeric constituents, while β-carotene was determined to be in a crystalline form (). The graphical comparison of nanoparticles with PVA and PLGA illustrate the idea of β-carotene dispersion inside the nanoparticles. In nanoparticle diffraction studies, no crystalline diffractions were observed, which led to the proposal of an amorphous nature of β-carotene constituent of polymeric nanoparticles. DSC is a thermal analytical technique that reveals the physical (crystalline/amorphous) nature and the possible interactions between different compounds of a mixture. The DSC studies revealed that both PVA and PLGA were amorphous at Tg 29°C () and 44°C (), respectively. Conversely. free β-carotene was crystalline in nature with an exothermic peak at Tg 181°C (). In the prepared formulation, very feeble and short peaks of PVA and PLGA polymers were found at Tg 27°C and Tg 39°C, respectively, without demonstrating any peak for β-carotene, thus confirming its amorphous state in prepared nanoparticles. The interaction of PVA and PLGA polymers with β-carotene was found to be negligible as their glass transition temperatures were found to be at 27°C and 39°C, respectively, indicating the level of the mentioned interactions ().
Figure 3 X-ray diffraction pattern. (A) β-carotene; (B) Polyvinyl alcohol; (C) poly(D,L-lactide-co-glycolide); (D) β-carotene nanoparticles.
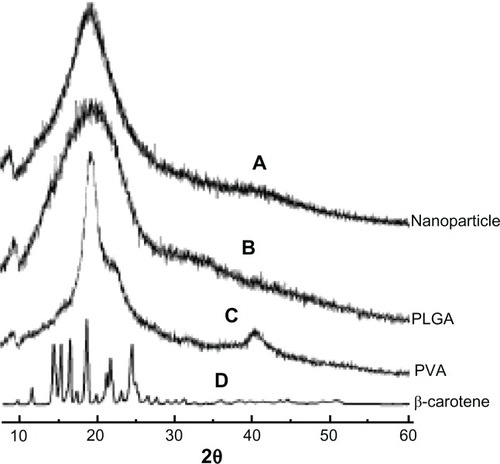
Figure 4 Differential scanning calorimetry studies. (A) Polyvinyl alcohol; (B) poly(D,L-lactide-co-glycolide); (C) β-carotene; (D) β-carotene nanoparticles.
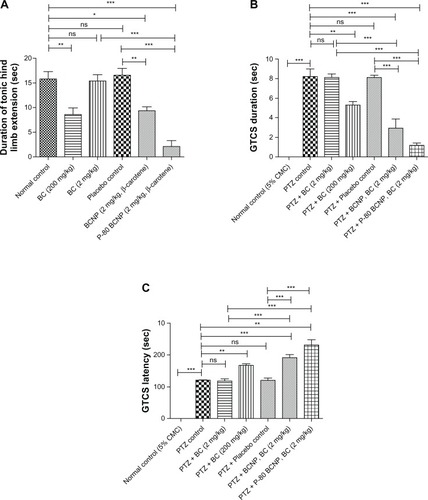
In the MES model, the duration of tonic hind limb extension (THLE duration) was used for biological study. In comparison to the normal control (15.8 ± 1.49 seconds), THLE duration of BC (200 mg/kg) at 8.60 ± 1.33 sec (P < 0.01); BCNP (BC, 2 mg/kg) at 9.30 ± 0.86 sec (P <0.05); and P-80-BCNP (BC, 2 mg/kg) at 2.10 ± 1.16 sec (P < 0.001) were found to be significant. In comparison to the placebo control group, THLE duration of BCNP (BC, 2 mg/kg, P < 0.01) and P-80-BCNP (BC, 2 mg/kg, P < 0.001) were also determined to be significant. The THLE duration of BC (2 mg/kg) at 15.36 ± 1.26 seconds and placebo at 16.50 ± 1.43 seconds were found to be nonsignificant as there was no appreciable change. THLE duration of BCNP (BC, 2 mg/kg, P <0.05) and P-80-BCNP (BC, 2.0 mg/kg, P < 0.001) was determined to be very significant as compared to BC (2.0 mg/kg). According to these findings, nonnanoformulated β-carotene at a dose level of 200 mg/kg was effective in reducing THLE duration, whereas the results of 2.0 mg/kg of the same group showed effects similar to normal control. This is indicative of the fact that 2.0 mg/kg is an ineffective dose. In the nanoformulated form, a dose level of 2.0 mg/kg showed an effective decrease in THLE duration, and the effect was further potentiated via polysorbate-80 coating, which established the successful targeting of 2.0 mg/kg nanoformulated β-carotene to the brain.
In the PTZ study, the GTCS duration in PTZ control (8.20 ± 0.80 seconds) was found to be significant (P < 0.001) in comparison to the normal control (0.0 ± 0.0 seconds). In comparison to the PTZ control, GTCS duration of PTZ + BC (200 mg/kg) at 5.28 ± 0.35 seconds (P < 0.01); PTZ + BCNP (BC, 2.0 mg/kg) at 2.90 ± 0.98 seconds (P < 0.001); and PTZ + P-80-BCNP (BC, 2.0 mg/kg) at 1.20 ± 0.20 seconds (P < 0.001) were found to be significant. In comparison to the PTZ-control group, GTCS duration of PTZ + BCNP (BC, 2.0 mg/kg) and PTZ +P-80-BCNP (BC 2.0 mg/kg) were also found to be significant (P < 0.001). The GTCS duration of PTZ + BC (2.0 mg/kg) at 8.12 ± 0.32 seconds and PTZ + placebo at 8.09 ± 0.26 seconds were found to be nonsignificant as there was no appreciable change. GTCS duration of PTZ + BCNP (BC, 2.0 mg/kg) and PTZ + P-80-BCNP (BC, 2.0 mg/kg) was found to be very significant (P < 0.001) as compared to PTZ + BC (2.0 mg/kg).
The GTCS latency of PTZ control was found to be highly significant (P < 0.001) at 120.10 ± 4.5 seconds compared to normal control. In comparison to the PTZ control, GTCS latency of PTZ ± BC (200 mg/kg) at 167.00 ± 5.2 seconds (P < 0.01); PTZ + BCNP (BC, 2.0 mg/kg) at 191.00 ± 9.80 seconds (P < 0.001); and PTZ + P-80-BCNP (BC, 2.0 mg/kg) at 231.00 ± 16.3 seconds (P < 0.01) were all found to be significant. In comparison to the PTZ control group, GTCS latency of PTZ + BCNP (BC, 2.0 mg/kg, P < 0.001) and PTZ + P-80-BCNP (BC 2.0 mg/kg, P < 0.001) were also found to be significant. The GTCS latency of PTZ + BC (2.0 mg/kg) at 118.0 ± 6.5 seconds and PTZ + placebo control at 120.30 ± 7.4 seconds were found to be nonsignificant compared to PTZ-control, which exhibited no appreciable change in GTCS latency. The GTCS latency of PTZ + BCNP (BC, 2.0 mg/kg) and PTZ + P-80-BCNP (BC, 2 mg/kg) were found to be very significant (P < 0.001) as compared to PTZ + BC (2.0 mg/kg).
As the decrease in GTCS duration and increase in GTCS latency were observed in comparison to both PTZ normal and placebo control, it was concluded that normal nonnano-formulated effective dose of β-carotene was 200 mg/kg. A dose of 2.0 mg/kg is effective only in a nanoformulation dosage form. A decrease in GTCS duration and an increase in GTCS latency of both BCNP and P-80-BCNP demonstrates an effectiveness of β-carotene at a 2.0 mg/kg concentration in nanoparticle form ().
Figure 5 Anticonvulsant activity. (A) Maximal electroshock seizure test; duration of hind limb muscle extension in normal control, placebo control, β-carotene 2 mg/kg, β-carotene 200 mg/kg, BCNP (2.0 mg/kg β-carotene), and P-80-BCNP (2.0 mg/kg β-carotene) are shown in the graph. Response of dosage forms were compared to placebo control. (B) General tonic–clonic seizure latency test. Latency duration was compared in normal control, PTZ Control, PTZ + BC (2.0 mg/kg), PTZ + BC (200 mg/kg), PTZ + placebo control, PTZ + BCNP (BC, 2.0 mg/kg), and PTZ + P-80-BCNP BC (2.0 mg/kg) are shown in the graph. (C) General tonic–clonic seizure duration in normal control, PTZ control, PTZ + BC (2.0 mg/kg), PTZ + BC (200 mg/kg), PTZ + placebo control, PTZ + BCNP (BC, 2.0 mg/kg), PTZ + P-80-BCNP, (BC, 2.0 mg/kg). The study was done on albino mice model of six animals in each group (n = 6). Comparisons among the groups are shown by arrows as indicated in the graph.
Notes: Results are expressed as mean ± SEM. Statistical analysis was performed using one-way ANOVAs with Tukey’s post hoc test. Significance compared with epileptic mice are *P < 0.05, **P < 0.001, ***P < 0.001.
Abbreviations: BCNP, β-carotene nanoparticles; PTZ, pentylenetetrazole; BC, β-carotene; P-80-BCNP, polysorbate-80-nanoparticles; ANOVA, analysis of variance; ns, nonsignificant.
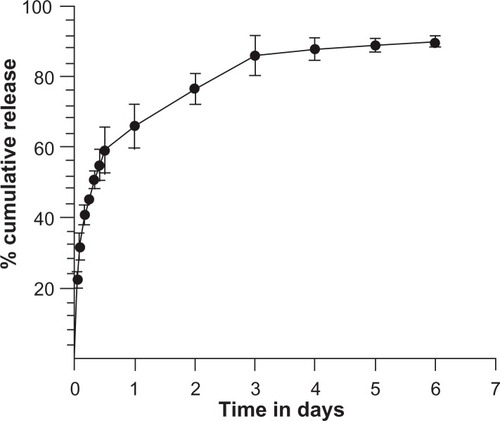
In vitro release kinetics
The release kinetics of β-carotene-loaded PLGA nanoparticles showed an initial burst release followed by a sustained release, which continued for seven days (). In vitro release studies are required to predict the release pattern of a drug from the encapsulating material as it affects a drug’s bioavailability.Citation48 Hence, knowing the quantity of residually encapsulated drug in in vitro release is crucial for drug delivery regime maintenance. The in vitro release profiles of β-carotene from PLGA nanoparticles are presented in , which shows a typical biphasic pattern. Initially, a burst release occurred during the first 12 hours with 56.0% ± 6.2% of β-carotene release from PLGA nanoparticles. Following this, a sustained drug release from BCNP was observed from 58.0% ± 7.7% to 88.6% ± 1.3% after 6 days. The sustained release profile of β-carotene from nanoparticles was consistent with the Higuchi diffusion equation (r2 = 0.95), which is defined as follows: Citation49
where Q is the cumulative amount of drug released per unit of surface area, C0 is the initial drug loading, D is the diffusion coefficient, t is the time after commencement of diffusion, and x is the constant of the equation.
Figure 6 In vitro release study of BCNP for one week.
Note: Determinations were done as mean ± SD from three samples (n = 3).
The drug release from the PLGA polymer displayed multiple release phases including initial burst release, lag phase, and zero order release.Citation50 The initial burst release is regulated by diffusion through the surface and pore-associated release of the drug. The lag phase and zero order releases are controlled by polymer erosion combined with the diffusion quotient.Citation51 The sustained release of the drug in a delivery system is an important property closely related with therapeutics-linked pharmacokinetics and contributes to the efficacy of the drug.Citation52
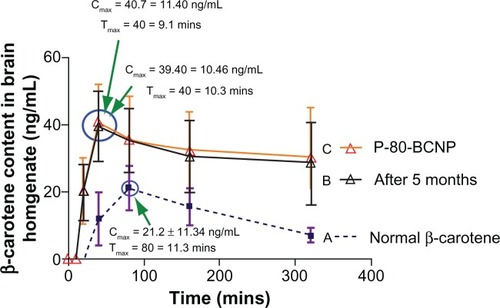
Brain release of β-carotene
The mean concentrations of free drug and nanoformulations in brain tissue homogenate after intraperitoneal administration are illustrated (). The relevant pharmacokinetic parameters including Cmax and Tmax are listed in the graph (). The concentrations (ng/mL) of β-carotene in brain tissue homogenate were plotted against time to generate the in vivo release study of P-80-BCNP. From the graph obtained by plotting concentration (ng/mL) against time, it was observed that the coated nanoformulation showed sustained release over a long period. Free drug β-carotene was noticed in very low brain concentration with a Cmax of 21.2 ± 11.80 ng/mL and a Tmax of 80 ± 11.3 minutes, which was followed by a rapid concentration decline in brain tissue homogenate over time. A relatively slow increase and sustained brain concentration of β-carotene was observed with the nanoformulation having Cmax of 40.7 ± 11.40 ng/mL with significantly delayed Tmax of 40 ± 9.1 minutes. Brain release pattern was not affected much by storing the nanoformulation for 5 months, as it showed a Cmax of 39.40 ± 10.46 ng/mL and a Tmax of 40 ± 10.3 minutes.
Figure 7 Brain release of β-carotene. (A) In BCNP formulation; (B) In P-80-BCNP formulation; (C) In P-80-BCNP formulation after 5 months storage.
Note: Data is shown in mean ± SD from three samples (n = 3).
Abbreviations: BCNP, β-carotene nanoparticles; P-80-BCNP, polysorbate-80 β-carotene nanoparticles.
Possible mechanism of β-carotene anticonvulsant action
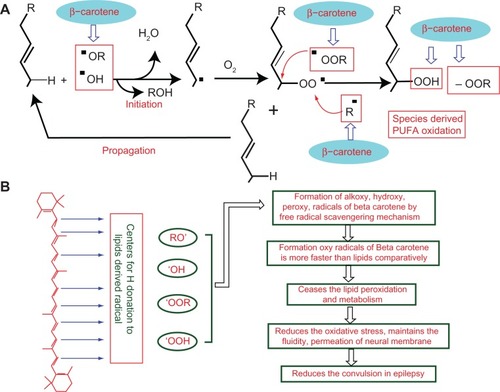
β-Carotene is a chain-breaking antioxidant, and a similar structural mechanism has been reported earlierCitation53,Citation54 (ie, it is able to impede oxidizing radicals directly, preventing the chain propagation step during lipid auto-oxidation). It reacts with alkoxy radicals, lipid peroxy radicals, and alkyl radicals derived from polyunsaturated fatty acids oxidation ().Citation55 The reaction between β-carotene and lipid free radicals occurs at the membrane water interphase, where β-carotene is believed to donate a hydrogen ion to lipid radicals with oxyl radical (BOH) generation.Citation55 In addition, it also acts as a chemical scavenger for oxygen radicals, especially for singlet oxygen (via irreversible oxidation), and acts as a physical deactivator of singlet oxygen by charge transfer mechanism ().Citation15,Citation54
Figure 8 Mechanism of free radical formation of PUFA and possible mechanism of β-carotene bio-action. (A) Mechanism of free radical formation of PUFA. Free radicals are formed via donation of hydrogen to alkoxy or hydroxy free radical. Free radical of PUFA further forms peroxides. Possible site of β-carotene action as free radical scavenger. (B) Possible mechanism of β-carotene bio-action.
Abbreviation: PUFA, polyunsaturated fatty acids.
Conclusion
β-Carotene containing polysorbate-80-coated polymeric nanoparticles were prepared by a solvent diffusion method and were well characterized for shape, average size, and zeta potential. The coated nanoformulation was stable for a longer period without significant deformities in size and shape, including their stable and unaltered zeta potential charge for approximately 5 months. The nanoformulation was delivered at a 2.0 mg/kg dose level through an intraperitoneal route, and sustained release accomplished the desired bioavailability demand which was confirmed as the slowdown dose requirement for induced convulsions in experimental animal models. Thus, polysorbate-80-coated nanoparticles potentiated the antioxidant biological action of β-carotene which is otherwise unfavored by conventional delivery systems. The dose, activity levels, and delivery mode of this formulation may herald a novel therapeutic approach for epilepsy in the near future.
Acknowledgements
The authors are grateful for laboratory and Animal House facilities at Hamdard University, New Delhi. Mohammad Yusuf is thankful to UGC, New Delhi for grant of a fellowship.
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health OrganizationFact Sheet No 165 Epilepsy: Epidemiology, Etiology and PrognosisGenevaWorld Health Organization2001
- PatelMMitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizuresFree Radic Bio Med200437121951196215544915
- LevineSAKiddPMAntioxidant adaptation: a unified disease theoryJ Orthomol Med19851411938
- StadtmanERProtein oxidation in aging and age-related diseasesAnn N Y Acad Sci2001928223811795513
- GajewskaEBernatPDługońskiJSkłodowskaMEffect of nickel on membrane integrity, lipid peroxidation and fatty acid composition in wheat seedlingsJournal of Agronomy and Crop Sciences20121984286294
- ChangRCHudsonPMWilsonBCLiuBAbelHHongJSHigh concentrations of extracellular potassium enhance bacterial endotoxin lipopolysaccharide-induced neurotoxicity in glial neuron mixed culturesNeuroscience200097475776410842021
- HyrcKHandranSDRothmanSMGoldbergMPIonized intracellular calcium concentration predicts excitotoxic neuronal death: observations with low-affinity fluorescent calcium indicatorsJ Neurosci9119971717666966779254679
- KuppusamyPZweierJLCharacterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generationJ Biol Chem615198926417988098842542334
- AbramovAYScorzielloADuchenMRThree distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenationJ Neurosci20072751129113817267568
- BrownGCBorutaiteVNitric oxide, mitochondria, and cell deathIUBMB Life2001523–518919511798032
- GrafEMahoneyJRBryantRGEatonJWIron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination siteJ Biol Chem19842596362036246323433
- LehningerALThe neuronal membraneProc Natl Acad Sci U S A1968604106910804299940
- ErakovicVZupanGVarljenJSimonicAPentylenetetrazol-induced seizures and kindling: changes in free fatty acids, superoxide dismutase, and glutathione peroxidase activityNeurochem Int200342217317812421597
- AshrafiMRShamsSNouriMA probable causative factor for an old problem: selenium and glutathione peroxidase appear to play important roles in epilepsy pathogenesisEpilepsia20074891750175517555528
- SharmaMKBuettnerGRInteraction of vitamin C and vitamin E during free radical stress in plasma: an ESR studyFree Radic Biol Med19931466496538392021
- WinyardPGMoodyCJJacobCOxidative activation of antioxidant defenceTrends Biochem Sci200530845346115996871
- Dal PizzolFKlamtFViannaMLipid peroxidation in hippocampus early and late after status epilepticus induced by pilocarpine or kainic acid in Wistar ratsNeurosci Lett2000291317918210984636
- KabutoHYokoiIOgawaNMelatonin inhibits iron-induced epileptic discharges in rats by suppressing peroxidationEpilepsia19983932372439578039
- RolaRSwiaderMCzuczwarSElectroconvulsions elevate the levels of lipid peroxidation products in micePol J Pharmacol200254552152412593541
- NieoczymDAlberaEKankoferMWlaźPMaximal electroshock induces changes in some markers of oxidative stress in miceJ Neural Transm20081151192517728996
- FrantsevaMVPerez VelazquezJLTsoraklidisGOxidative stress is involved in seizure-induced neurodegeneration in the kindling model of epilepsyNeuroscience200097343143510828526
- RaucaCWiswedelIZerbeRKeilhoffGKrugMThe role of superoxide dismutase and alpha-tocopherol in the development of seizures and kindling induced by pentylenetetrazol – influence of the radical scavenger alpha-phenyl-N-tert-butyl nitroneBrain Res200410091–220321215120598
- EkonomouAAngelatouFUpregulation of NMDA Receptors in hippocampus and cortex in the pentylenetetrazol-induced “kindling” model of epilepsyNeurochem Res199924121515152210591400
- Uma DeviPPillaiKKVohoraDModulation of pentylenetetrazole-induced seizures and oxidative stress parameters by sodium valproate in the absence and presence of N-acetylcysteineFundam Clin Pharmacol200620324725316671959
- KrinskyNIAntioxidant functions of carotenoidsFree Radic Biol Med1989766176352695406
- PalaceVPKhaperNQinQSingalPKAntioxidant potentials of vitamin A and carotenoids and their relevance to heart diseaseFree Radic Biol Med1999265–674676110218665
- SayyahMYousefi-PourMNarenjkarJAnti-epileptogenic effect of beta-carotene and vitamin A in pentylenetetrazole-kindling model of epilepsy in miceEpilepsy Res2005631111615716082
- BittermanNMelamedYBen-AmotzABeta-carotene and CNS oxygen toxicity in ratsJ Appl Physiol1994763107310768005847
- ObulesuMDowlathabadMRBramhachariPVCarotenoids and Alzheimer’s disease: an insight into therapeutic role of retinoids in animal modelsNeurochem Int201159553554121672580
- DemingDMErdmanJWJrMammalian carotenoid absorption and metabolismPure Appl Chem1999711222132223
- AuweterHHaberkornHHeckmannWSupramolecular structure of precipitated nanosize beta-carotene particlesAngew Chem Int Ed Engl199938152188219110425476
- HornDPreparation and characterization of microdisperse bioavailable carotenoid hydrosolsDie Angewandte Makromolekular Chemie19891661139153
- FessiHPuisieuxFDevissaguetJPAmmouryNBenitaSNanocapsule formation by interfacial polymer deposition following solvent displacementInt J Pharm1989551R1R4
- GrolierPAzais-BraescoVZelmireLFessiHIncorporation of carotenoids in aqueous systems: uptake by cultured rat hepatocytesBiochim Biophys Acta1992111111351381390859
- SzczepanowiczKHoelHJSzyk-WarszynskaLFormation of biocompatible nanocapsules with emulsion core and pegylated shell by polyelectrolyte multilayer adsorptionLangmuir20102615125921259720604580
- ZhuZMargulis-GoshenKMagdassiSTalmonYMacoskoCWPolyelectrolyte stabilized drug nanoparticles via flash nanoprecipitation: a model study with beta-caroteneJ Pharm Sci201099104295430620143406
- CostantinoLGandolfiFTosiGRivasiFVandelliMAForniFPeptide-derivatized biodegradable nanoparticles able to cross the blood-brain barrierJ Control Release20051081849616154222
- Dechy-CabaretOMartin-VacaBBourissouDControlled ring-opening polymerization of lactide and glycolideChem Rev2004104126147617615584698
- KreuterJInfluence of the surface properties on nanoparticle-mediated transport of drugs to the brainJ Nanosci Nanotechnol20044548448815503433
- KimHRAndrieuxKGilSTranslocation of poly(ethylene glycol-co-hexadecyl) cyanoacrylate nanoparticles into rat brain endothelial cells: role of apolipoproteins in receptor-mediated endocytosisBiomacromolecules20078379379917309294
- MichaelisKHoffmannMMDreisSCovalent linkage of apolipoprotein e to albumin nanoparticles strongly enhances drug transport into the brainJ Pharmacol Exp Ther200631731246125316554356
- PetriBBootzAKhalanskyAChemotherapy of brain tumour using doxorubicin bound to surfactant-coated poly(butyl cyanoacrylate) nanoparticles: revisiting the role of surfactantsJ Control Release20071171515817150277
- KreuterJHekmataraTDreisSVogelTGelperinaSLangerKCovalent attachment of apolipoprotein A-I and apolipoprotein B-100 to albumin nanoparticles enables drug transport into the brainJ Control Release20071181545817250920
- HungLCBasriMTejoBAAn improved method for the preparations of nanostructured lipid carriers containing heat-sensitive bioactivesColloids Surf B Biointerfaces201187118018621652183
- CashinCHJacksonHAn apparatus for testing anticonvulsant drugs by electroshock seizures in miceJ Pharm Pharmacol196214Suppl44T47T
- DhirAPentylenetetrazol (PTZ) Kindling Model of EpilepsyCurr Protoc Neurosci201210.1002/0471142301.ns0937s58 Epub January 1, 2012.
- HornDRiegerJOrganic nanoparticles in the aqueous phase-theory, experiment, and useAngew Chem Int Ed Engl200140234330436112404417
- MüllerRColloidal Carriers for Controlled Drug Delivery and Targeting: Modification, Characterization, and In Vivo DistributionBoca RatonCRC Press19914556
- HiguchiTMechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matricesJ Pharm Sci1963521145114914088963
- ZolnikBSBurgessDJEffect of acidic pH on PLGA microsphere degradation and releaseJ Control Release2007122333834417644208
- FaisantNSiepmannJBenoitJPPLGA-based microparticles: elucidation of mechanisms and a new, simple mathematical model quantifying drug releaseEur J Pharm Sci200215435536611988397
- ZolnikBSLearyPEBurgessDJElevated temperature accelerated release testing of PLGA microspheresJ Control Release2006112329330016644055
- SerbinovaEAPackerLAntioxidant properties of alpha-tocopherol and alpha-tocotrienolMethods Enzymol19942343543667808307
- BuettnerGRThe pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbateArch Biochem Biophys199330025355438434935
- Kamal EldinAAppelqvistLThe chemistry and antioxidant properties of tocopherols and tocotrienolsLipids19963176717018827691