Abstract
Polyvinyl alcohol nanofibers incorporating the wide spectrum antibiotic gentamicin were prepared by Nanospider™ needleless technology. A polyvinyl alcohol layer, serving as a drug reservoir, was covered from both sides by polyurethane layers of various thicknesses. The multilayered structure of the nanofibers was observed using scanning electron microscopy, the porosity was characterized by mercury porosimetry, and nitrogen adsorption/desorption measurements were used to determine specific surface areas. The stability of the gentamicin released from the electrospun layers was proved by high-performance liquid chromatography (HPLC) and inhibition of bacterial growth. Drug release was investigated using in vitro experiments with HPLC/MS quantification, while the antimicrobial efficacy was evaluated on Gram-positive Staphylococcus aureus and Gram-negative Pseudomonas aeruginosa. Both experiments proved that the released gentamicin retained its activity and showed that the retention of the drug in the nanofibers was prolonged with the increasing thickness of the covering layers.
Introduction
The highly porous structure of nanofibers makes them attractive materials for various biomedical applications. Numerous reports have described the fabrication of electrospun polymer nanofibers applicable in tissue engineering for the regeneration of skin, bone, heart, cornea, the nervous system, and other tissues.Citation1–Citation3 The specific morphology of nanofibrous nonwoven materials makes them suitable for wound dressings.Citation3,Citation4 Their high porosity, consisting of relatively small pores, enables good permeability for oxygen and water but protects wounds from dehydration and bacterial penetration.Citation3,Citation5 Moreover, the large surface area and high pore volume of nanofibers promote the migration of keratinocytes on the wound surface and may play a role in accelerating the healing process. Nanofibers composed of natural polymers, such as chitin,Citation6 chitosanCitation7 or collagen,Citation8 or synthetic polymers or their blends, such as polyurethane (PUR),Citation9,Citation10 polyvinyl alcohol (PVA),Citation10 polyacrylonitrile,Citation10 or polyethersulfone,Citation11 have been specifically engineered as dressings for wound healing or as tissue engineered scaffolds for skin substitutes. In addition to physical protection of the wound site, nanofibrous materials have also been developed for the local delivery of therapeutic agents, such as antibiotics,Citation12 anesthetics,Citation13,Citation14 or growth factors.Citation7,Citation15
Various processing techniques have been used to produce nanofibers.Citation1,Citation16–Citation18 However, the electrospinning technique allows the production of continuous polymeric nanofibers and provides numerous opportunities to modify and control their morphological parameters, such as surface area, fiber diameter, the porosity of the nanofibrous layer (fiber density), basis weight,Citation19 or internal fiber structure.Citation18,Citation20,Citation21 Nanospider™ electrospinning technology is based on a needleless method in which polymeric jets are spontaneously formed from liquid surfacesCitation22,Citation23 and allows the production of fibers with diameters ranging from tens of nanometers to tens of micrometers. Nanospider™ (Elmarco, Liberec, Czech Republic) technology is very flexible, enabling the formation of nanofibrous materials from various polymers.Citation24 The process provides high production capacity, stability, and easy maintenance compared to other known industrial-scale technologies based on needles or capillary spinners.Citation25,Citation26 The incorporation of various additional substances, such as pharmacologically active compounds, in the nanofibers can be accomplished by simply adding the substances to the polymer solution. In our previous workCitation27 using Nanospider™ technology, we demonstrated that the immunosuppressive drug cyclosporine A can be incorporated directly into electrospun nanofibers without any loss of its pharmacological activity.
To achieve drug release from nanofibers, two basic delivery designs are known: matrices and reservoirs. In the matrix-type of carriers, the drug is homogeneously dispersed in the material of the nanofiber, and its release is based on solid-state diffusion or a desorption mechanism;Citation13,Citation28,Citation29 however, such drug-loaded systems tends to have a strong burst release within the first hours. In the second design, reservoir-type structures are formed by a drug-loaded core and a covering polymer shell.Citation21,Citation28,Citation30 The core-shell structure enables better control of the drug release profile by adjusting the shell’s properties, such as its microstructure, thickness, and degradability. On the other hand, some of the complications that persist during nanofiber preparation do not allow for easy large-scale production.
Several papers have described the preparation and testing of multi-layer polymer nanofibrous structures containing a middle layer that incorporates a pharmacologically active compound, covered by other nanofibrous layers of a different polymer.Citation31–Citation33 Chen et alCitation31 prepared biodegradable sandwich-structured nanofibers from poly(D,L)-lactide-co-glycolide and collagen with incorporated vancomycin, gentamicin, and lidocaine. They described the conditions of nanofibrous mat preparation by classical jet electrospinning and the in vitro release of the drugs, as well as their effect on the in vivo healing process in rat wounds. Kim et alCitation32 observed rhodamine and peptide release from multi-layered PCL/polyethylene oxide/PCL nanofibers. They found a decrease of the initial burst release with increasing thickness of the covering layer and a slower release of compounds from sandwiches with thicker covering layers. Nevertheless, all of these authors prepared nanofibers by classical electrospinning based on needle or capillary spinners.
In contrast, needleless electrospinning allows the sequential deposition of nanofibrous layers, and therefore, the preparation of compact sandwich structures in which the middle drug-loaded layer is covered by layers of a different polymer. These layers, with a large surface area and a microporous structure, improve the mechanical properties, promote wound healing, and also contribute to controlled drug release to the surrounding tissue. Combining more layers of the required composition, porosity, thickness, fiber diameter, and/or drug concentration, it is possible to obtain a nanofibrous material with properties suitable for specific applications.
The aim of this study was to employ needleless electrospinning for the preparation of multi-layer nanofibrous structures consisting of a drug-loaded middle layer and covering layers of various thicknesses, enabling the controlled release of the drug. The middle layer was made from crosslinked or noncrosslinked PVA nanofibers containing the wide spectrum antibiotic gentamicin, while the covering layers were made from PUR. The morphology of the prepared nanofibers was observed using scanning electron microscopy (SEM) and characterized by Brunauer, Emmet, and Teller (BET) nitrogen adsorption/desorption measurements and mercury porosimetry. Gentamicin release was quantified in vitro using high-performance liquid chromatography/mass spectrometry (HPLC/MS), and its bactericidal effect was confirmed on Staphylococcus aureus and Pseudomonas aeruginosa cultures. The effect of the preparation conditions on the morphology of the nanofibers and subsequent gentamicin release was evaluated.
Materials and methods
Polyvinyl alcohol (PVA) (type Z 220, viscosity of 4 wt% water solution at 20°C 11.5–15 mPa.s, saponification degree 90.5–92.5 mol%) was supplied by Nippon Gohsei (Osaka, Japan). PUR (Estane 5714 F1, viscosity of 15 wt% solution in THF 600–900 mPa.s) was obtained from Lubrizol Corp (Wickliffe, OH). Gentamicin was purchased from AppliChem GmbH (Darmstadt, Germany). N,N-Dimethylacetamide (Sigma-Aldrich, St Louis, MO) and toluene and phosphoric acid (Penta a.s., Prague, Czech Republic) were obtained in analytical reagent grade and used as received.
PVA and PUR nanofibers were prepared using Nanospider™ technology.Citation22 The process parameters were individually optimized for each polymer. PVA nanofibrous layers were made at a basis weight of 0.8 g/m2, while the covering PUR layers had basis weights of 1.5, 4.4, and 14.7 g/m2. Layers of each polymer intended for mercury porosimetry and nitrogen adsorption/desorption measurements were prepared separately in thicknesses (basis weights) similar to the sandwich structures.
PVA was dissolved in a water/phosphoric acid mixture at a polymer concentration of 11 wt%. The distance between the spinning electrode and the collector was 13 cm; the spinning electrode rotated at 2 rpm, and the high voltage supply was 45–55 kV/cm. The relative humidity was 25%–30% and the temperature 22°C. Gentamicin, at a concentration of 10 wt%, was added directly to the PVA electrospun mixture. The PVA layers were crosslinked thermally in a drying oven at 145°C for 15 minutes.
PUR was dissolved in an N,N-dimethylacetamide/toluene mixture at a ratio of 2:1 (w/w) and a polymer concentration of 10 wt%. The electrospinning conditions were: 4 rpm for the spinning electrode, a 15 cm distance between the electrodes, and a 5 kV/cm electric field strength. The relative humidity was kept lower than 30% due to the risk of solvent explosion.
The PUR/PVA/PUR multi-layered sandwich structures were prepared by multi-step deposition of each layer. All layers were prepared on the same Nanospider™ machine; only the process parameters were varied according to the polymer type as described above. Nonwoven polypropylene material (ATEX, Milan, Italy) was used as a support for the deposition of the first PUR layer. The different thickness of each layer (g/m2) results from the speed of the rotating collecting electrode. In the case of the PVA layer with a basis weight of 0.8 g/m2, the speed was 50 mm/min; for the PUR layers, the speeds were as follows: 1.5 g/m2 = 110 mm/min, 4.4 g/m2 = 30 mm/min, and 14.7 g/m2 = 18 mm/min with double electrospinning.
The structure of the prepared nanofibers was observed using a TS 5130 VEGA scanning electron microscope, TESCAN (Brno, Czech Republic). Multilayer samples were fractured in liquid nitrogen and sputtered with a gold layer. The fiber diameters and the thicknesses of the nanofibrous layers were determined as mean values of 30 measurements in various locations. Single layers of PVA and PUR nanofibers were prepared to determine porosity and surface area. Mercury porosimetry measurements were made using an Autopore IV 9500 porosimeter (Micromeritics, Norcross, GA), and specific surface areas were calculated from nitrogen absorption/desorption isotherms recorded on an ASAP 2020 apparatus (Micromeritics).
Gentamicin release from the nanofibrous mats was investigated under laboratory conditions and by in vitro bacteriological assay. Laboratory experiments were performed on agar dishes (Caso-Agar, Mercoplate®; Merck and Co, Whitehouse Station, NJ) in order to evaluate the time course of the drug release from the nanofibrous mats. Discs of 8 mm in diameter were cut from the nanofibrous mats and placed on agar previously wetted with 0.5 mL of distilled water, 1 day before the release experiment. After various time periods, the discs were removed, immersed into distilled water (1 mL each) and shaken for 2 days to rinse out the remaining gentamicin. The concentration of gentamicin in solution was quantified by an HPLC-MS Liquid Chromatograph Series 1200 with a Triple Quad LC/ MS 6460 tandem mass spectrometer (Agilent Technologies, Waldbroon, Germany). The amount of gentamicin released into the agar was calculated as the difference between the initial and residual amounts of gentamicin in the disc. The drug release from each nanofibrous sample was determined 15 times and the average values given. The HPLC-MS apparatus was also used to evaluate the stability of gentamicin during the electrospinning process.
The in vitro antimicrobial properties of electrospun layers containing gentamicin were evaluated from inhibitory zone measurements against the Gram-positive organism Staphylococcus aureus (ATCC 6538) and the Gram-negative organism Pseudomonas aeruginosa (ATCC 27853). The bacterial strains were obtained from the Czech Collection of Microorganisms, Brno, Czech Republic. A gelatin pellet infused with the bacterial strain was incubated in 9 mL of liquid media (Tryptic Soy Broth, Mecrotube®; Merck and Co) at 37°C for 24 hours, and then diluted 1000-fold in a phosphate buffer. An aliquot of the suspension (0.7 mL) was uniformly spread onto an agar plate (Caso-Agar, Mercoplate®; Merck and Co) and allowed to dry for several minutes. The nanofibrous mats (6-mm diameter discs) were placed on the agar plates and incubated at 37°C. The zones of inhibition were measured after 1, 2, and 6 days.
The release profiles of the gentamicin from the nanofibrous layered mats were evaluated using the inhibition of S. aureus after 1, 3, 6, 24, and 48 hours of treatment. Upon removal from the agar plate, the nanofibrous discs were placed on a new agar plate to further evaluate the inhibitory effect from the residual gentamicin in the sample. The plates were incubated overnight at 37°C, and the zones of inhibition were measured from photomicrographs using ImageJ software. To eliminate potential variability between the samples during the manufacturing process, the area of inhibition was calculated as (area of inhibition/area of inhibition after 48 hours) × 100, as the area of inhibition after 48 hours was found to be maximal; also, no residual inhibitory activity was detected in the samples after 48 hours of drug release. Measurements of the inhibitory zones were repeated four times for each nanofiber sample. Antimicrobial susceptibility testing discs (Oxoid, Cambridge, UK) containing 10, 30, or 120 μ of gentamicin were used as standards.
Results and discussion
Preparation and morphological characterization of nanofibrous mats
All nanofibrous layers were made using Nanospider™ technology. The multilayered nanofibrous mats were prepared in a multi-step process, changing the process parameters and evaluating the area weight after each step.
The structures of the prepared nanofibrous mats were observed using SEM. The images did not reveal any artifacts or heterogeneities, and uniform nanofibrous structures were found. From the SEM images, the fiber diameters were calculated as the mean value of 30 measurements at various locations. The mean fiber diameter obtained for PVA fibers was 185 ± 70 nm, while in the case of PUR fibers it was 420 ± 150 nm. The fiber diameters differed between PVA and PUR nanofibers; however, the diameters were similar for samples of the same polymer, regardless of the layer thickness. This is due to the method of preparation, by which the fiber thickness is particularly influenced by the viscosity of the solution, the strength of the electrostatic field, and the distance between the electrodes; the mat’s thickness is driven by the speed of rotation of the collecting electrode and the duration of fiber deposition, while the other parameters remain unchanged.
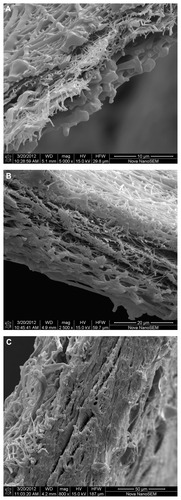
The structures of the three-layered nanofibrous PUR/ PVA/PUR mats evaluated by SEM are depicted in . It can be seen that fracturing the samples in liquid nitrogen successfully maintained the inner sandwich structure and the original thickness of each layer. On the other hand, some secondary changes in the nanofibers, especially local melting of the fibers, can be seen. The mean values of the layer thicknesses calculated from the SEM measurements are given in . The values obtained showed a proportional increase similar to the increasing basis weights. It should be mentioned that the actual thickness of the nanofibrous layers in the subsequent drug release experiments, as well as in their potential in vivo application, will certainly differ from the values obtained by SEM in a vacuum/dry state, due to the absorption of water under aqueous conditions.
Table 1 Morphological characteristics of the prepared nanofibers
Figure 1 SEM images of multilayer nanofibrous structures in which the middle crosslinked PVA layer (0.8 g/m2) containing 10 wt% gentamicin is covered with PUR layers of 1.5 (A), 4.4 (B), or 14.7 g/m2 (C) basis weight.
Abbreviations: PUR, polyurethane; PVA, polyvinyl alcohol; SEM, scanning electron microscopy.
Nitrogen adsorption/desorption BET measurements and mercury porosimetry were carried out with single PVA and PUR layers of the same basis weight as in the sandwich structures, and the results obtained are summarized in . BET measurements of specific surface areas revealed higher values in the case of the PVA fibers (12.8 m2/g) compared to the PUR ones (between 7.5 m2/g and 9.1 m2/g), which corresponds to the smaller diameter of the PVA fibers. Similar to the fiber diameters, comparing PUR layers of various thicknesses revealed that the surface areas were not influenced by the basis weight.
The porosity of the PVA nanofibers (85.8%) determined by mercury porosimetry was higher than the porosity of the PUR nanofibrous samples (70.7%–78.9%; see ). The porosity of the PUR layers slightly decreased with increasing basis weight, which was caused by the preparation procedure in which the fibers were deposited stepwise for a certain length of time. With the increasing deposition time needed to obtain thicker layers, a certain level of compression of a whole layer can occur, ie, the formed nanofibrous layer is more compact. The total pore volume of such layers consequently becomes lower. Mercury porosimetry, as well as BET measurements, proceeds on single layers, and therefore, the obtained values may be slightly different from those of real sandwich structures. Nevertheless, the measurements of single layers provided us with more detailed information about morphological parameters depending on polymer type or layer thickness. An important finding was that SEM images of the sandwich structures did not show any noticeable difference between the upper and lower PUR nanofibrous layers (see ), so the stepwise deposition of subsequent layers did not cause any significant compression of the lower layers.
Pore size distribution curves (not depicted) obtained from mercury porosimetry measurements did not show any pores less than tens of nanometers in size, suggesting that there is no porosity inside the PUR and PVA fibers and that the whole pore volume is given by the inter-fiber space. The relatively low values of specific surface areas (see ) obtained by BET measurements also correspond to this finding.
No morphological differences were observed between samples consisting of crosslinked or noncrosslinked PVA layers. It can be noted that the additional crosslinking of the PVA layers had no effect on any structural parameters of the resulting nanofibrous mats. Also, no statistically significant differences in morphological parameters were observed between nanofibers prepared with 10 wt% gentamicin and without the drug.
Drug release from nanofibrous mats
Gentamicin release experiments were carried out with multi-layer nanofibrous mats consisting of a crosslinked or noncrosslinked PVA layer of basis weight 0.8 g/m2 loaded with 10 wt% gentamicin and outer PUR layers with basis weights of 1.5, 4.4, or 14.7 g/m2. The concentration of the released gentamicin was assessed using HPLC-MS.
Before starting the gentamicin release experiments, it was crucial to prove the stability of the drug during the incorporation procedure. MS spectra of fresh gentamicin before incorporation were compared with the spectra of gentamicin released from sandwich nanofibers consisting of crosslinked PVA nanofibers and 14.7 g/m2 PUR covering layers. The mass spectra did not show any differences, suggesting that the structure of gentamicin remained unchanged.
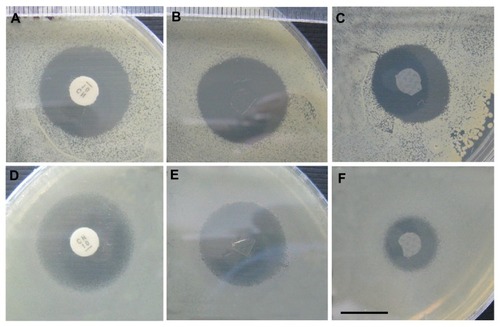
The antimicrobial effectiveness of gentamicin released from crosslinked and noncrosslinked PVA layers as well as from PUR/PVA/PUR sandwiches was evaluated from the inhibition zones of S. aureus and P. aeruginosa after 1, 2, and 6 days of culture. Clear inhibitory zones were observed with both strains around all of the gentamicin-loaded mats, while no inhibition was found around control nanofibers without the drug. The inhibitory zones on S. aureus cultures were comparable with those of gentamicin standard discs that released a similar amount of gentamicin (). Smaller inhibitory zones were found on P. aeruginosa cultures when compared to S. aureus; nevertheless, the inhibitory activity of gentamicin against P. aeruginosa was still preserved ().
Figure 2 The areas of inhibited growth of Staphylococcus aureus (A–C) and Pseudomonas aeruginosa (D–F) around gentamicin standard discs (A and D), a noncrosslinked PVA layer with 10 wt% gentamicin (B and E), and a crosslinked PVA layer with 10 wt% gentamicin (C and F).
Note: Scale: 1 cm.
Abbreviation: PVA, polyvinyl alcohol.
These tests proved that gentamicin survives “hard” electrospinning conditions, such as exposure to phosphoric acid, a high electrostatic field, and an elevated temperature during PVA crosslinking, in terms of maintaining its structure and antibacterial activity.
The gentamicin release experiments were first carried out by immersing samples of the nanofibrous mats (pieces of ca 0.1 g) in an excess of water (10 mL). shows the dependence of the released gentamicin (in percentage) on time. It can be seen that the release of almost 90% of the total drug amount occurred within 1 hour. An effect of the various thicknesses of the covering PUR layers was observed only for very short exposure times (10 and 30 minutes). For longer exposure times, regardless of the PUR layer thickness, the amount of the released drug was similar (90%–100%). No significant differences in the release profiles were found by comparing samples with a noncrosslinked or crosslinked PVA layer.
Figure 3 Release profiles of gentamicin into water from PUR/PVA/PUR nanofibrous sandwiches with PUR layers of 1.5, 4.4, or 14.7 g/m2 basis weight and a noncrosslinked (A) or crosslinked (B) PVA layer.
Abbreviations: PUR, polyurethane; PVA, polyvinyl alcohol.
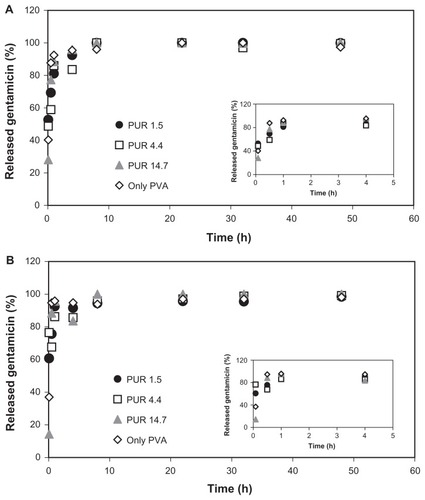
In the case of all tested samples, the fast release can be explained by several factors. During shaking, the flow of a liquid medium is greater and it can easily penetrate through a nanofibrous structure. The middle drug-loaded layer is easily accessible and the release of gentamicin as a water-soluble drug is thus accelerated. Moreover, the excess water and lower gentamicin concentration in solution contribute to a steeper concentration gradient, and, consequently, faster drug diffusion, respectively.
It is necessary to note that this experimental arrangement does not simulate the actual conditions of drug release in potential applications, particularly in wound healing. Therefore, it would be desirable to perform the experiments in such a way that would more closely approximate real drug release into surrounding living tissue. Unfortunately, this aspect has often been neglected by other authors, and most published reports rely on monitoring drug release into a large volume of liquid media. So, in the next step, release experiments were carried out by placing the sample discs on wet agar plates. The antibacterial assays (discussed below) were also done using agar plates. The discs were removed from the agar after various time periods, and the residual gentamicin was washed out into water and quantified by HPLC-MS analysis. The amount of gentamicin released to the agar was calculated from the differences between the initial and the residual amounts.
In the case of samples with gentamicin incorporated into sandwiches with a noncrosslinked PVA layer, the release was generally fast regardless of the thickness of the covering PUR layer. The faster release was influenced by the degradation of the PVA layer and the subsequent easier dissolution of the gentamicin.
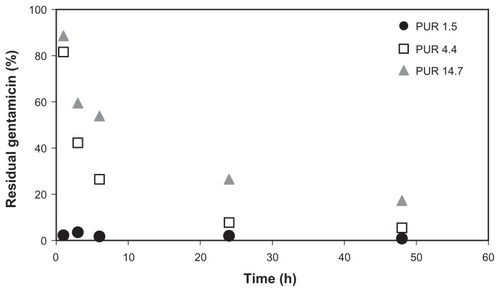
Significantly different results were obtained from sandwich samples with a crosslinked PVA layer. The release profiles are depicted in . In the case of the thinnest covering PUR nanofibrous layers (1.5 g/m2), the entire quantity of gentamicin was released within 1 hour of sample application onto the agar plate. There was no apparent difference between the amount of residual gentamicin in nanofibers applied to the agar plate for 1 hour and those applied for any longer time period. A similar gentamicin release profile was observed for crosslinked PVA nanofibers without PUR layers (not depicted). A significant difference was found in the case of samples covered by 4.4 g/m2 or 14.7 g/m2 PUR layers. Sandwiches with 4.4 g/m2 PUR layers retained about 10% of the gentamicin after 24 hours of application, while those with 14.7 g/m2 PUR layers retained 30% after 24 hours and still contained about 20% of gentamicin even after 48 hours.
Figure 4 Release profiles of gentamicin to agar from PUR/PVA/PUR nanofibrous sandwiches with crosslinked PVA layers and PUR layers of 1.5, 4.4, or 14.7 g/m2 basis weights, expressed as the percentage of residual gentamicin remaining in the nanofibrous discs.
Abbreviations: PUR, polyurethane; PVA, polyvinyl alcohol.
In accordance with the results discussed above, the antibacterial experiments were carried out only with sandwiches based on a crosslinked drug-loaded PVA layer. The gentamicin release profiles were evaluated from the area of inhibition of S. aureus after various time periods (). After 1 hour, the largest inhibitory area (85%) was found for 1.5 g/m2 PUR sandwiches, indicating the largest amount of released gentamicin, while the inhibitory zones were smaller around the thicker nanofibrous sandwiches with outer PUR layers of 4.4 g/m2 (59%) or 14.7 g/m2 (56%) (). After 6 hours of treatment, the inhibitory zones achieved their maximal size around all samples and did not increase further with time.
Figure 5 (A) Area of inhibition (% of the maximal inhibition area after 48 hours) of Staphylococcus aureus agar cultures around nanofibrous samples during gentamicin release. (B) Inhibitory zones from the residual gentamicin in the nanofibrous samples after various intervals of gentamicin release.
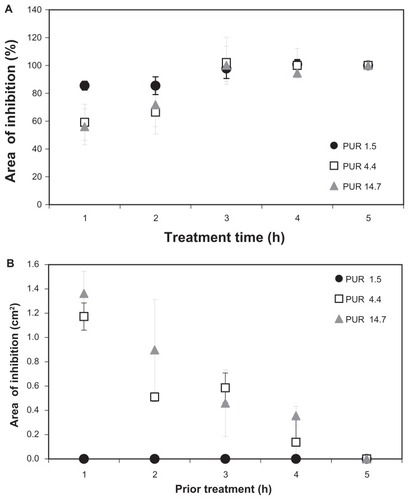
The residual gentamicin in the samples was evaluated by applying the removed discs onto fresh bacterial cultures. No inhibition zones were observed around the 1.5 g/m2 PUR layered mats, while the inhibition zones confirmed gentamicin retention in the 4.4 g/m2 and 14.7 g/m2 PUR layered mats even after 24 hours (). Bacterial inhibition, however, requires a certain limiting inhibitory concentration of gentamicin, and drug release is therefore not detectable in the more distant areas around the nanofibrous mats where the gentamicin concentration is below the level needed to exert an inhibitory effect.
It can be concluded that the 1.5 g/m2 PUR layers are too thin to influence the release of gentamicin and to prolong its retention in the nanofibrous mats. Prolonged drug release was found for thicker covering layers of 4.4 g/m2 or 14.7 g/m2 thicknesses, but even so, more than 70% of the gentamicin was released within 24 hours.
The prolongation of the gentamicin release was influenced by two factors: (1) since a covering PUR layer with a higher basis weight is thicker, the gentamicin molecules must travel a longer distance from the surface of the PVA nanofibers to the free volume; and (2) thicker nanofibrous mats are more compact with a lower porosity than thinner ones (see the column “Porosity” in ). Consequently, migrating molecules of the released drug must travel through a nanofibrous layer with smaller pores.
Both these factors are apparently involved in prolonging drug retention. Gibson et alCitation34 studied water vapor diffusion and gas convection through nanofibrous mats and found a linear dependence of flow resistance on the thickness of the nanofibrous mats.
This dependence was also mentioned by Kim et alCitation32 in the case of rhodamine and peptide release from multilayered PCL/polyethylene oxide/PCL nanofibers into a liquid environment. Unlike these authors, we have expressed the thickness of the nanofibrous layers in basis weight because, according to our experience, thicknesses expressed in micrometers can vary significantly due to the compression of nanofibrous mats during manipulation or in in vitro/in vivo experiments. Thickness expressed in micrometers is also dependent on the environment – whether the sample is in dry conditions or immersed in water or a saline solution. The values obtained by SEM measurements performed under vacuum/dry conditions may differ considerably from the real thickness of nanofiber mats in water/wet conditions during biological experiments or in medical applications. Obviously, these changes affect the thickness of nanofibers as well as their total porosity and the distribution of the pore sizes, particularly the mean and most frequent pore radius. The release of the incorporated compound is also significantly dependent on the conditions of the experiment – its release into a liquid environment is different when compared to its release into a solid or heterogeneous environment, such as biological tissues in a living organism.
We are aware that a PVA nanofibrous layer with a basis weight of 0.8 g/m2 and 10 wt% gentamicin was used to model gentamicin release from sandwiches of various thicknesses, but that this does not correspond to the actual therapeutic dosage of gentamicin commonly used for local application. The required therapeutic dose of gentamicin or other drugs can be easily obtained by adjusting the thickness (basis weight) of the middle drug-loaded layer or by adjusting the drug’s concentration in the electrospun mixture. Nevertheless, the thickness of the layers needs to be further optimized to control the drug release rate and to maintain the mechanical properties or other features of multi-layer nanofibrous mats.
In our previous studies,Citation35,Citation36 we demonstrated that nanofibers produced by needleless technology represent a convenient scaffold for cell cultivation and subsequent transfer to damaged tissue. The multilayered nanofibers studied in this work may offer a promising combination of such inherent properties of electrospun nanofibers with controlled drug release for developing suitable scaffolds for wound dressing applications. Moreover, the use of Nanospider™ technology represents a relatively simple and easily controllable way to fabricate such nanofibrous scaffolds in mass production, which is advantageous when compared to other methods.
Conclusion
In the present study, needleless electrospinning technology was successfully used for the preparation of PUR/PVA/PUR multi-layer nanofibrous mats with gentamicin incorporated into the middle PVA layer. This method enables effective large-scale production of nanofibrous sandwiches for various medical applications. Morphological characterization of the sandwiches revealed highly homogeneous nanofibrous structures with tight connection between the individual nanofiber layers. HPLC/MS analysis, as well as inhibition of bacterial growth, proved the stability of the gentamicin after its incorporation into the nanofibers during electrospinning. Increasing the thickness of the covering layers prolonged gentamicin retention. Nanofibrous multi-layer drug carriers seem to be promising materials for use in various medical applications.
Acknowledgments
This work was supported by grants: AS CR KAN200520804, SVV 2012265201, and MEYS MSM 0021620857. We thank James Dutt for his critical reading of our manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- VasitaRKattiDSNanofibers and their applications in tissue engineeringInt J Nanomedicine200611153017722259
- KubinovaSSykovaENanotechnologies in regenerative medicineMinim Invasive Ther Allied Technol2010193–414415620497067
- KhangDCarpenterJChunYWParetaRWebsterTJNanotechnology for regenerative medicineBiomed Microdevices201012457558719096767
- KumbarSGNairLSBhattacharyyaSLaurencinCTPolymeric nanofibers as novel carriers for the delivery of therapeutic moleculesJ Nanosci Nanotechnol200669–102591260717048469
- ChandrasekaranARVenugopalJSundarrajanSRamakrishnaSFabrication of a nanofibrous scaffold with improved bioactivity for culture of human dermal fibroblasts for skin regenerationBiomed Mater201161110
- NohHKLeeSWKimJMElectrospinning of chitin nanofibers: Degradation behavior and cellular response to normal human keratinocytes and fibroblastsBiomaterials200627213934394416574218
- ParkCJClarkSGLichtensteigerCAJamisonRDJohnsonAJWAccelerated wound closure of pressure ulcers in aged mice by chitosan scaffolds with and without bFGFActa Biomater2009561926193619342320
- RhoKSJeongLLeeGElectrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healingBiomaterials20062781452146116143390
- VerreckGChunIRosenblattJIncorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymerJ Control Release200392334936014568415
- LiuXLinTFangJAIn vivo wound healing and antibacterial performances of electrospun nanofibre membranesJ Biomed Mater Res Part A201094A2499508
- BabaeijandaghiFShabaniISeyedjafariEAccelerated epidermal regeneration and improved dermal reconstruction achieved by polyethersulfone nanofibersTissue Eng Part A201016113527353620624004
- KattiDSRobinsonKWKoFKLaurencinCTBioresorbable nanofiber-based systems for wound healing and drug delivery: Optimization of fabrication parametersJ Biomed Mater Res Part B200470B2286296
- ThakurRAFlorekCAKohnJMichniakBBElectrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressingInt J Phar200836418793
- PengHSZhouSBGuoTIn vitro degradation and release profiles for electrospun polymeric fibers containing paracetanolColloids Surf B Biointerfaces200866220621218691851
- ChoiJSLeongKWYooHSIn vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF)Biomaterials200829558759617997153
- HuangZZhangYKotakiMRamakrishnaSA review on polymer nanofibers by electrospinning and their applications in nanocompositesCompos Sci Technol20036322232253
- MartinsAReisRNevesNElectrospinning: processing technique for tissue engineering scaffoldingInt Mater Rev2008535257274
- BellanLCraigheadHApplications of controlled electrospinning systemsPolym Adv Technol201122304309
- ThompsonCChaseGYarinARenekerDEffects of parameters on nanofiber diameter determined from electrospinning modelPolymer2007482369136922
- LeeGHSongJCYoonKBControlled wall thickness and porosity of polymeric hollow nanofibers by coaxial electrospinningMacromol Res2010186571576
- WangCYanKLinYHsiehPBiodegradable core/shell fibers by coaxial electrospinning: Processing, fiber characterization, and its application in sustained drug releaseMacromolecules2010431563896397
- JirsakOSanetrnikFLukasDKotekLMartinovaLChaloupekJTechnical University of LiberecMethod of nanofibers production from polymer solution using electrostatic spinning and a device for carrying out the method United States patent US 2006029003112282006
- LukasDSarkarAPokornyPSelf-organization of jets in electrospinning from free liquid surface: A generalized approachJ Appl Phys2008103084309
- BarnesCPSellSABolandEDSimpsonDGBowlinGLNanofiber technology: Designing the next generation of tissue engineering scaffoldsAdv Drug Deliv Rev200759141413143317916396
- TaylorGElectrically driven jetsProc R Soc Lond A Math Phys Sci1969453475
- ParkJCFinetex Ene, IncElectric spinning apparatus for mass-production of nano-fiber United States patent US 79808383262008
- HolanVChudickovaMTrosanPCyclosporine A-loaded and stem cell-seeded electrospun nanofibers for cell-based therapy and local immunosuppressionJ Control Release2011156340641221802460
- SrikarRYarinALMegaridisCMBazilevskyAVKelleyEDesorption- limited mechanism of release from polymer nanofibersLangmuir200824396597418076196
- GandhiMSrikarRYarinALMegaridisCMGemeinhartRAMechanistic examination of protein release from polymer nanofibersMol Phar200962641647
- HuangZMHeCLYangAZEncapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinningJ Biomed Mater Res Part A200677A1169179
- ChenDWCLiaoJYLiuSJChanECNovel biodegradable sandwich-structured nanofibrous drug-eluting membranes for repair of infected wounds: an in vitro and in vivo studyInt J Nanomedicine2012776377122359454
- KimGYoonHParkYDrug release from various thicknesses of layered mats consisting of electrospun polycaprolactone and polyethylene oxide micro/nanofibersAppl Phys A Mater Sci Process2010100411971204
- Fathi-AzarbayjaniAChanSYSingle and multi-layered nanofibers for rapid and controlled drug deliveryChem Pharm Bull201058214314620118570
- GibsonPWSchreuder-GibsonHLRivinDElectrospun fiber mats: Transport propertiesAIChE J1999451190195
- DubskyMKubinovaSSircJNanofibers prepared by needleless electrospinning technology as scaffolds for wound healingJ Mater Sci Mater Med201223493194122331377
- ZajicovaAPokornaKLencovaATreatment of ocular surface injuries by limbal and mesenchymal stem cells growing on nanofiber scaffoldsCell Transplant201019101281129020573307