 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Chitosan shows particularly high biocompatibility and fairly low cytotoxicity. However, chitosan is insoluble at physiological pH. Moreover, it lacks charge, so shows poor transfection. In order to develop a new type of gene vector with high transfection efficiency and low cytotoxicity, amphiphilic chitosan was synthesized and linked with low-molecular weight polyethylenimine (PEI).
Methods
We first synthesized amphiphilic chitosan – N-octyl-N-quatenary chitosan (OTMCS), then prepared degradable PEI derivates by cross-linking low-molecular weight PEI with amphiphilic chitosan to produce a new polymeric gene vector (OTMCS–PEI). The new gene vector was characterized by various physicochemical methods. We also determined its cytotoxicity and gene transfecton efficiency in vitro and in vivo.
Results
The vector showed controlled degradation. It was very stable and showed excellent buffering capacity. The particle sizes of the OTMCS–PEI/DNA complexes were around 150–200 nm with proper zeta potentials from 10 mV to 30 mV. The polymer could protect plasmid DNA from being digested by DNase I at a concentration of 2.25 U DNase I/μg DNA. Furthermore, they were resistant to dissociation induced by 50% fetal bovine serum and 1100 μg/mL sodium heparin. OTMCS–PEI revealed lower cytotoxicity, even at higher doses. Compared with PEI 25 KDa, the OTMCS–PEI/DNA complexes also showed higher transfection efficiency in vitro and in vivo.
Conclusion
OTMCS–PEI was a potential candidate as a safe and efficient gene vector for gene therapy.
Introduction
Based on genetic engineering and molecular genetics, gene therapy is a new method for the cure of cancer.Citation1 It has become the most promising field because it directly aims at the biological foundation of the occurrence and development of cancers. The nature of gene therapy is to feed therapeutic genes into target cells with gene vectors, and the key is to find a reliable and effective gene delivery system.Citation2 A perfect gene vector has to meet the following conditions: (1) be able to condense DNA effectively; (2) be stable in body fluid; (3) be able to target the specific cells; and (4) be able to cross membranes and release efficiently.Citation3
Presently, viral vectors and nonviral vectors are two kinds of common gene vectors. Viral vectors are frequently used in the field of medical research because of their relatively high transfection efficiency, but their clinical utility has been seriously limited by their fatal disadvantages, such as immunogenicity, oncogenic effects, and poor loading capacity.Citation4 By contrast, nonviral gene vectors provide a significant supplement to viral vectors. Nonviral gene vectors are nonimmunogenic, noninfectious, easier to prepare, and capable of carrying large amounts of genetic materials.Citation5–Citation7 Nonviral gene vectors have attracted more and more attention.Citation8
Among nonviral vectors, the cationic polymers have been widely explored in gene delivery research. Polyethylenimine (PEI) and its derivatives are the most extensively investigated because of their “proton sponge” effect.Citation9,Citation10 PEI, with abundant positive charges, condenses DNA by electrostatic interaction to compact complexes, which enables breaking through the various barriers to the nucleus of target cells.Citation11 DNA is condensed as nanoparticles so that it is not easily degraded by nuclease, or gathered as precipitate, which results in high transfection efficiency.Citation12,Citation13 However, it has been reported that the molecular weight of PEI has a strong influence on its transfection efficiency and cytotoxicity. PEI with high molecular weight performs not only with high transfection efficiency, but with high cytotoxicity. To the contrary, PEI with low-molecular weight performs with low cytotoxicity but also with poor transfection efficiency. In addition, there is an inconsistency between its stability in body fluid and its cellular uptake.Citation14 It has been proved that PEI derivatives obtained by cross-linking low-molecular weight PEI with degradable materials display higher transfection efficiency and lower cytotoxicity.Citation15
Chitosan, obtained by alkaline N-deacetylation of chitin, is one kind of the most widely used natural cationic polysaccharides.Citation16 Chitosan shows particularly high biocompatibility and fairly low cytotoxicity,Citation17 therefore in fact, can be used as a gene vector.Citation18,Citation19 Nevertheless, chitosan is insoluble at physiological pH, and lacks charge. These major drawbacks limit its use for gene delivery.Citation20
Based on the above analysis, we first synthesized amphiphilic chitosan, N-octyl-N-quaternary chitosan (OTMCS), then cross-linked the amphiphilic chitosan with low-molecular weight PEI. In this way we acquired a new type of degradable amphiphilic chitosan-PEI derivative with high transfection efficiency and low cytotoxicity.
The new gene vector was characterized in terms of its chemical structure and biophysical parameters. We also investigated the cytotoxicity and gene transfection efficiency of this vector in vitro and in vivo. The objective of this work was to reduce the cytotoxicity of PEI and improve its transfection efficiency, thereby enhancing the therapeutic effect of gene therapy on cancers.
Materials and methods
Materials
Branched PEI (MW 2000), heparin and 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St Louis, MO). DNase I was purchased from the Worthington Company (Worthington Company, Lakewood, NJ). RPMI 1640 culture medium and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad CA). Luciferase assay system for in vitro transfection assay and pGL3-Control vector with SC-40 promoter and enhancer encoding firefly (Photinus pyralis) luciferase were obtained from Promega Corp (Madison, WI). The plasmid encoding the enhanced green fluorescent protein (pEGFP-N2) was kindly provided by the Institute of Life Science and Technology of Tongji University in China. The plasmids were amplified using Escherichia coli DH5α and prepared using the Tiangen End-free Plasmid Mega Kit (DP117; Qiagen GmbH, Hilden, Germany). The purity of the purified and concentrated DNA was determined by measuring its UV absorbance value at 260 nm and 280 nm respectively.
Chitosan (MW 2000, degree of deacetylation > 90%), benzene, dichloromethane, triphosgene, N-hydroxysuccinimide, triethylamine, ethyl acetate, and absolute ethyl alcohol were purchased from Sinopharm Chemical Reagent Co, Ltd (Shanghai, China). Dialysis bag (MWCO 7000) was purchased from Spectrum Chemical & Instruments (Plainfield, IL).
Preparation of amphiphilic chitosan (OTMCS)Citation21
Chitosan (12 g) and octaldehyde were added into methanol with stirring at 30°C for 12 hours. Then KBH4 (6 g) dissolved in 60 mL of water was slowly added to the solution. After a further 24 hours of continuous stirring, the reaction solution was filtered, and the product was precipitated with methanol. The precipitate was washed repeatedly with water and methanol. The product, N-octyl chitosan, was dried under a vacuum at 50°C overnight.
N-octyl chitosan (0.96 g), N-methylpyrrolidone (15 mL), KI (2.4 g), NaOH (15%, 5 mL), and CH3I (5.2 mL) were added, in turn, into a three-necked bottle. This mixture was reacted at 60°C for 1 hour. Then the reaction solution was cooled and centrifuged for 30 minutes, and the precipitate gathered and dissolved in water. At last the solution was dialyzed (MWCO 7000) against distilled water and lyophilized. The product OTMCS was obtained.
Synthesis of OTMCS–PEI
Activation of OTMCS
Before the experiments, all the reagents were dehydrated by coevaporation. First, 3.86 g OTMCS and the same mole of triphosgene were dissolved in benzene/dichloromethane (3:1, 40 mL) with magnetic stirring for one night. Next, the same mole of N-hydroxysuccinimide as chitosan and plenty of triethylamine was added dropwise into benzene/dichloromethane (2:1, 30 mL) with stirring for 4 hours after evaporating the primary solvent. After rotary evaporating the solvent again, 50 mL of ethyl acetate was added and centrifuged (8000 rpm, 15 minutes). At last, the supernatant was evaporated and the residue was collected.
Linking OTMCS with PEI 2 KDa
Activated OTMCS (0.386 g, 2.14 mmol) was dissolved into 50 mL of dichloromethane. Dehydrated PEI 2 KDa (42.8 g, 21.4 mmol) was dissolved into 40 mL of alcohol. These two solutions were contemporarily added into 20 mL of dichloromethane, and stirred for one night. After evaporating solvent, the residue was dissolved in water, dialyzed, and then lyophilized. The polymer OTMCS–PEI was obtained.
The buffering capacity of the OTMCS–PEI polymerCitation22
The OTMCS–PEI polymer solution was prepared in a 50 mL flask (0.2 mg/mL, 30 mL) and pure water was used as a control. After adjusting the initial pH to 10.0 with 0.1 M NaOH, 25 μL increments of 0.1 M HCl were titrated into the solution while measuring the pH response with a pH electrode. The recorded pH varied from 10.0 to 3.0.
Characterizations of OTMCS–PEI (NMR, GPC)
First, 10 mg of OTMCS–PEI was dissolved in 0.6 mL of deuterium oxide (D2O) in an NMR tube, and the 1H NMR spectrum was recorded using a Varian 300 MHz spectrometer (Varian Medical Systems Inc, Palo Alto, CA) at room temperature.
The molecular weight and its distribution of the polymer was measured by gel permeation chromatography with multiangle laser light scattering (GPC-MALLS) (LC-20AD; Shimadzu Corp, Kyoto, Japan) (690 nm laser wavelength) using a TSK-GEL G5000PWXL column from TOSOH (Tokyo, Japan) (temperature 40°C) operated at a flow rate of 0.4 mL/min. Ammonium acetate (0.2 M) was used as a mobile phase.
Degradation of OTMCS–PEI
Degradation of OTMCS–PEI was estimated by the measurement of molecular weight. First, 0.5 g of the polymer was dissolved in 10 mL of phosphate-buffered solution (PBS) (0.1M, pH = 7.4), and then incubated at 37°C, with shaking at 100 rpm. After incubation for 0, 10, 20, 30, 40, 50, and 60 hours, respectively, solutions of the polymers were lyophilized, and the molecular weights of the lyophilized samples were measured by GPC-MALLS with a 690 nm laser wavelength.
Preparation of OTMCS–PEI/DNA complexes
The ratio of an amino group to a phosphate group is hereafter defined as the charge ratio. We controlled charge ratios of OTMCS–PEI/DNA by regulating the weight ratio of OTMCS–PEI and DNA. DNA solution and polymer solution were mixed to form self-assembly complexes with desired weight/weight (w/w) ratios. The complexes were allowed to stand at 4°C for 30 minutes before they were used in the next experiments.
Measurement of particle size, zeta potential and morphologic observation
The complexes were prepared at designed weight ratios and incubated at 4°C for 30 minutes before their particle sizes and zeta potentials were measured by an electrophoretic light-scattering spectrophotometer (Zetasizer Nano ZS90, MAN0317 Issue 5.0; Malvern Instruments Ltd, Malvern, UK). All the experiments were conducted in triplicate.
OTMCS–PEI/DNA complexes were prepared according to 2.7. The concentration of DNA was 50 μg/mL and the w/w ratio of the polymer to DNA was 10. A drop of the complex solution was placed on a copper grid. Transmission electron microscope (JEM 2100F; JEOL Ltd, Tokyo, Japan) was used to observe the morphological characteristics of the micelle after the sample was dried.
Agarose gel retardation assay
Before electrophoresis, 2 μL of 6 × loading buffer was added into 10 μL of prepared complex solution (250 ng of plasmid DNA). Electrophoresis was carried out on 1% agarose gel in Tris-Acetate-EDTA (TAE) buffer, at 120 V for about 40 minutes. Then the gel was dyed with ethidium bromide for 15 minutes, and illuminated on an ultraviolet illuminator to show the DNA migration patterns.
Resistance to DNase I digestion
In 0.5 mL Eppendorf tubes, the designed amount of DNase I solutions were added to 10 μL of prepared complex solutions (250 ng of plasmid DNA), and incubated at 37°C for 15 minutes. Then, 2 μL of 250 mM EDTA was added to each tube, and incubated at room temperature for 10 minutes to inactivate DNase I. At last, 10 μL of 10 mg/mL sodium heparin solution was added, and incubated at room temperature for 2 hours to dissociate the complex. After that, the resistance capacity of OTMCS–PEI/DNA to DNase I was evaluated by electrophoresis.
Resistance to serum and sodium heparin
In one tube, 10 μL of the prepared complex solution (250 ng of plasmid DNA) was added. Different volumes of FBS solution or 2 μL of different concentrations of sodium heparin solution were then added, and the mixed solution incubated at 37°C for 30 minutes. After that, the resistance capacities of OTMCS–PEI/DNA to serum and sodium heparin were evaluated by electrophoresis.
Cytotoxicity assay
The cytotoxicity of the polymer OTMCS–PEI was measured using the MTT assay. First of all, a 0.22 μm filtration membrane was used to sterilize the OTMCS–PEI polymer. HeLa cell line was purchased from ATCC (American Type Culture Collection, Manassas, VA). To begin, 5 × 103 cells were seeded in 200 μL of growth medium per well in 96-well plates, and incubated to 80% confluence. Then the culture medium was replaced with 200 μL of serum-free medium containing various concentrations of OTMCS–PEI, PEI 25 KDa, and PEI 2 KDa, and incubated for 72 hours. Next, the medium was replaced with 20 μL of MTT (5 mg/mL) and 180 μL of growth medium, and left to incubate for another 4 hours. After, the MTT solutions were replaced, 150 μL of dimethyl sulfoxide was added and kept in agitation for 10 minutes. The absorbance value at 570 nm was determined by an ELISA plate reader (Model 680; Bio-Rad Laboratories, Hercules, CA). Cell viability (%) was calculated according to these data and the following equation:Citation23
where Atest is the absorbance value of OTMCS–PEI or PEI treated cells and Acontrol is the absorbance value of the untreated cells.
Gene transfection efficiency
In vitro gene transfection
The transfection efficiency of OTMCS–PEI/DNA complexes in HeLa cells was examined using the plasmid pEGFP-N2 and pGL3-Control. Complexes were sterilized via a 0.22 μm filtration membrane. Cells were seeded in 500 μL of growth medium per well in 24-well plates and incubated to 80% confluence. Before transfection, the medium was replaced with 100 μL of complex solution at various weight ratios and 400 μL of serum-free RPMI 1640 medium. Cells were incubated for 4 hours at 37°C in a 5% CO2 atmosphere before the medium was replaced with 500 μL of medium containing 10% FBS and incubated for another 48 hours. After that, the pEGFP-N2 expression was observed with an inverted fluorescent microscope (AE-31; Motic Corporation, Wetzlar, Germany). Then the cells were trypsinized, centrifuged, and resuspended in PBS to determine the transfection efficiency by flow cytometry (BD FaCSAria; Becton Dickinson, Franklin Lakes, NJ). The data analysis was conducted using the CellQuest software (Becton Dickinson).
The luciferase assay was conducted according to the manufacturer’s specifications. The medium was replaced with 100 μL of cell culture lysis reagent (CCLR) and shaken for 30 minutes. After mixing with substrate, luciferase activity was examined by a luminometer (Turner Designs Luminometer Model TD-20/20; Promega Corp) as soon as possible. A bicinchoninic acid (BCA) protein assay kit (PP1001; Bioteke Corporation, Beijing, China) was used to measure protein contents. Transfection efficiency for the pGL3-Control was calculated by the relative light units (RLUs) against the corresponding protein contents.
In vivo gene transfectionCitation24,Citation25
Male BALB/C mice, 4–5 weeks and weighing 18–22 g, were purchased from National Rodent Laboratory Animal Resources, Shanghai Branch (Shanghai, China), and maintained under specific pathogen-free condition for in vivo transfection study. All animal procedures were approved by the Committee for Animal Research of Shanghai Ocean University, China (SCXK (HU) 2007-0003) and carried out according to the Guide for the Care and Use of Laboratory Animals.
The animals were divided into three groups (five rats per group). B16 cells were injected subcutaneously into the mice to establish the tumor models. When the subcutaneous transplanted tumors increased to 10 mm in diameter, the mice were injected with 250 μL of the sterilized complex solution containing 30 μg of the pGL3-Control reporter gene through the tail veins. Details are listed as follows: Group 1, OTMCS– PEI/DNA complexes, w/w 10; Group 2, OTMCS–PEI/DNA complexes, w/w 30; Group 3, PEI 25 KDa/DNA complexes, N/P 5. At 24 hours postinjection, mice were sacrificed by cervical dislocation, and the major tissues and the subcutaneous transplanted tumors were removed. The tissues and tumors were collected and homogenized in a cell lysis buffer. The cell lysate was centrifuged for 10 minutes at 10,000 xg (9800 rpm). Luciferase activity in the extracts was examined with a luciferase assay kit in a signal-well luminometer. The relative light units were detected against protein concentration in the cell tissue extracts, which was measured with the Bioteke PP1001 protein assay kit.
Results and discussion
Synthesis and characterization of OTMCS–PEI
shows the synthetic scheme of OTMCS–PEI. shows the structure of chitosan, and shows the 1H NMR spectra of OTMCS in D2O. Compared with the structure of chitosan, -CH2- proton peaks appear at 1.213 ppm and -CH3 proton peaks appear at 0.802 ppm, which could demonstrate that OTMCS was synthesized successfully. shows the 1H NMR spectra of PEI in D2O. shows the 1H NMR spectra of OTMCS–PEI in D2O. Compared with the spectra of PEI and OTMCS, the proton peaks of OTMCS–PEI moved to the lower magnet field due to the production of the groups with electronic screening effect. The performances of characteristic peaks have changed. -CH2- and -CH3 in OTMCS were merged and moved to higher magnet field. -H2CNH2- and -NCH2CH2 NH- in PEI were also changed. These results indicate that OTMCS-PEI was successfully prepared.
Figure 1 Synthetic scheme of OTMCS–PEI. First the N-octyl-N-quatenary chitosan (OTMCS) was synthesized. Then the OTMCS was crosslinked with PEI by N-hydroxysuccinimide.
Abbreviation: OTMCS–PEI, amphiphilic chitosan cross-linked with low-molecular weight polyethylenimine.
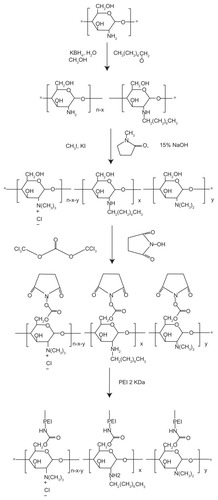
Figure 2 Representative 1H NMR spectra of (A) chitosan, (B) OTMCS, (C) PEI, and (D) OTMCS–PEI, in D2O at room temperature.
Abbreviations: OTMCS, amphiphilic chitosan; PEI, low-molecular weight polyethylenimine; OTMCS–PEI, amphiphilic chitosan cross-linked with low-molecular weight polyethylenimine; D2O, deuterium oxide.

From the integral ratio of the signal at 3.136 ppm, which corresponds to the proton of glucosamine from the OTMCS to the signal at 2.719–2.824 ppm of the -CH2CH2NH- from the PEI, the level of OTMCS substitution was calculated to be 81% to 90% ().
The buffering capacity of the OTMCS–PEI polymer
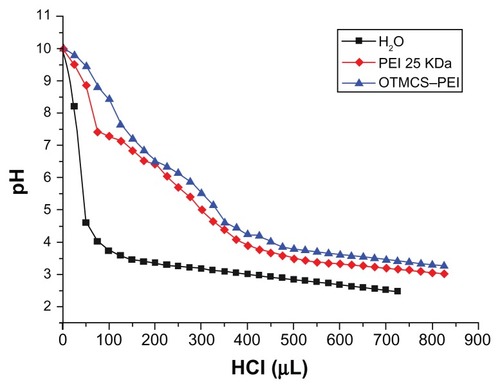
The buffering capacity of the gene vector is vital to the molecules escaping the endosomes of cells, because the molecules entering cells will experience a drop of pH from neutral to about 5. The proton sponge effect of the polymer ensures buffering in the endosomes, resulting in the degradation of the lysosomes so that genes can be protected. shows that the OTMCS–PEI polymer had relatively high buffering capacity in pH ranging from 4 to 7, compared with pure water. This result demonstrated that the polymer was sufficiently suitable for gene transfection.
Figure 3 Determination of the buffering capacity of PEI 25 KDa and the OTMCS–PEI polymer by acid-base titration.
Note: Solution containing the polymer (0.2 mg/mL) was adjusted to pH 10.0, and then titrated with HCl from 10.0 to 3.0.
Abbreviation: OTMCS–PEI, amphiphilic chitosan cross-linked with low-molecular weight polyethylenimine.
Degradation studies
Degradation of the gene vector is important for gene transfection. Degradable polymers can be degraded and secreted to the extracellular environment to show lower cytotoxicity on cells. Owing to its ester bond hydrolysis reaction at physiological conditions, OTMCS–PEI is a degradable vector (as compared with the nondegradable PEI 25 KDa) and was seen to degrade into low-molecular weight PEI and OTMCS, both of which were eliminated from cells easily, thereby reducing cytotoxicity. As is shown in , OTMCS–PEI degraded slowly and the degradation was completed after 60 hours.
Figure 4 Degradation of OTMCS–PEI.
Notes: The polymer was dissolved in 0.1 M PBS (pH = 7.4) and incubated at 37°C and 100 rpm. Determination of molecular weight (MW) was measured by gel permeation chromatography with multiangle laser light scattering (GPC-MALLS) (n = 3).
Abbreviations: OTMCS–PEI, amphiphilic chitosan cross-linked with low-molecular weight polyethylenimine; PBS, phosphate-buffered solution.
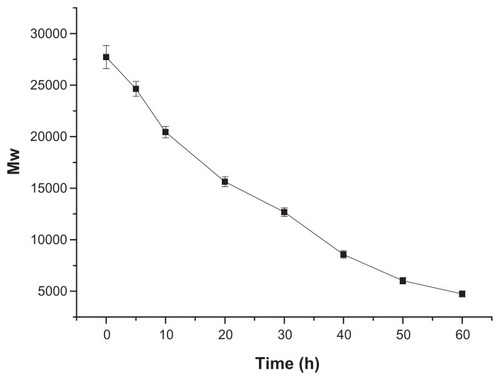
In order to obtain meaningful information from this degradation figure, the degradation figure was fitted to three kinds of kinetic models. The degradation figure of the OTMCS–PEI polymer was well described by a zero-order model. According to the model, the half-life of OTMCS–PEI was found to be 30 hours.
Particle size, zeta potential measurements and morphological characteristics
The particle sizes and zeta potentials of the OTMCS–PEI/DNA complexes were examined at different w/w ratios. From in the range of w/w ratios studied, no precipitation was observed (). It was seen that the OTMCS–PEI polymer could concentrate pDNA into nanoparticles. Depending on the composition of the complexes, their average particle sizes ranged from 150 nm to 200 nm, which were suitable for an efficient gene delivery in vivo. With the increase of OTMCS–PEI/DNA weight ratios, the particle size decreased. This result showed that a polymer with more charges could condense DNA more effectively.
Figure 5 (A) Particle sizes (nm) of the OTMCS–PEI/DNA complex at w/w ratios of 5, 10, 20, and 40. (B) Zeta potentials (mV) of the OTMCS–PEI/DNA complex at w/w ratios of 5, 10, 20, and 40. The data were expressed as mean values (±standard deviations, n = 3). (C) Transmission electron micrograph of OTMCS–PEI/DNA nanoparticles.
Abbreviation: OTMCS–PEI, amphiphilic chitosan cross-linked with low-molecular weight polyethylenimine.

The OTMCS–PEI/DNA complexes are able to bind successfully to cell membranes with negative charges because the complexes contain positive charges. It is important for a gene vector to rapidly enter into cells via endocytosis, however too strong cationic charges will lead to high cytotoxicity. Zeta potential allows the measurement of overall surface charge of the nanoparticles, and shows that the zeta potentials of all the complexes increased with the increase of their weight ratios. At low w/w ratios, zeta potential increased rapidly, to about 27 mV from a slight positive value. After w/w 20, the increase became slow. The increase of zeta potentials resulted from the increase of OTMCS–PEI with positive charges.
In , the OTMCS–PEI/DNA complexes are shown as spherical and the core/shell structure is obvious. The particle size was about 100 nm. This result demonstrated the micelle was formed successfully.
Condensation status of plasmid DNA by OTMCS–PEI polymer
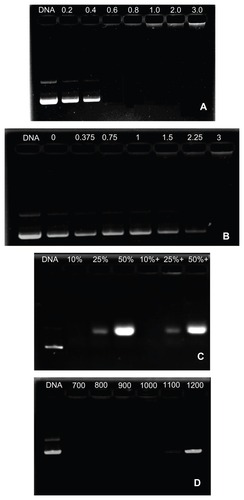
Agarose gel retardation was applied to determine the DNA condensation capacity of OTMCS–PEI at various ratios. As the proportion of OTMCS–PEI increased, the movement of the plasmid DNA was retarded. When the DNA was completely condensed by OTMCS–PEI, it stopped migrating to the anode. shows that OTMCS–PEI was able to condense DNA effectively and retard it completely at w/w ratio of 0.6. When the w/w ratios of the polymer and DNA exceeded 0.6, the complexes contained positive charges and stopped migrating to the anode.
Figure 6 (A) Agarose gel electrophoresis of the OTMCS–PEI/DNA complex at various w/w ratios. Protection of OTMCS–PEI on plasmid DNA. (B) Protection of plasmid DNA from degradation by DNase I at different concentrations of 0, 0.375, 0.75, 1, 1.5, 2.25, and 3 U DNase I/μg DNA. (C) Protection of plasmid DNA from dissociation by serum at varying concentrations of 10%, 25%, and 50%. The lanes 10%, 25%, and 50% without “+” refer to the presence of naked DNA with 10%, 25%, and 50% serum; the lanes 10%, 25%, and 50% with “+” refer to the presence of OTMCS–PEI/DNA complex at w/w ratio 20 besides 10%, 25%, and 50% serum. (D) Protection of plasmid DNA from dissociation by sodium heparin at varying concentrations of 700, 800, 900, 1000, 1100, and 1200 μg/mL.
Abbreviation: OTMCS–PEI, amphiphilic chitosan cross-linked with low-molecular weight polyethylenimine.
Protection of OTMCS–PEI on plasmid DNA
When the complex solution was incubated with DNase I, the polymer protected DNA from digestion by DNase I. At last, intact DNA was released completely by the action of sodium heparin. shows that with the amount of DNase I increasing, most DNA was still protected from digestion until the concentration of DNase I reached 3 U DNase I/μg DNA, which was higher than with other gene vectors. Under the same experimental conditions, DNA was digested by DNase I at the concentration of 0.08 U DNase I/μg DNA.Citation26
Naked DNA is also easily digested by blood components. Serum and sodium heparin were used to evaluate the stability of OTMCS–PEI/DNA complexes in vivo. As shown in , except for the DNA in serum itself, OTMCS–PEI/DNA complexes were not digested until the concentration of serum reached 50%, from which we inferred that OTMCS–PEI might protect DNA from dissociation by blood serum.
Sodium heparin was used to simulate molecules with negative charges in the blood, and dissociated the gene vector with its negative charges. At a w/w of 20, the OTMCS–PEI/DNA complexes were partially dissociated at a concentration of 1200 μg/mL (). They were completely dissociated once the concentration exceeded 1300 μg/mL. The capacity of OTMCS–PEI/DNA complexes to resist sodium heparin was very strong.
Cytotoxicity assay
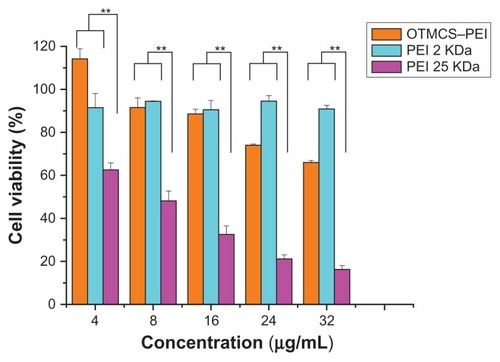
As high-molecular weight PEI showed relatively high cytotoxicity, we attempted to introduce a natural compound to form degradable PEI derivates. In this paper, the cytotoxicity of OTMCS–PEI was evaluated in HeLa cells using the MTT assay and compared with PEI 25 KDa. shows that PEI 25 KDa caused great cytotoxicity on cells at the concentration of 8 μg/mL, where more than 50% of the cells died. Cell viability fell to lower than 20% at the concentration of 48 μg/mL. By contrast, OTMCS–PEI showed much higher cell viability than PEI 25 KDa at any concentration (P < 0.01). At the highest concentration of 48 μg/mL, more than 60% of the cells survived under the influence of the OTMCS–PEI polymer. This indicated that OTMCS–PEI was quite suitable as a gene vector. Amino group density is lower in OTMCS–PEI than in PEI 25 KDa, which results in lower cytotoxicity to the cells. Also the polymer showed favorable degradability. OTMCS is a natural compound. These factors contribute to the lower cytotoxicity.
Figure 7 Cytotoxicity of OTMCS–PEI at various concentrations in HeLa cell lines using the MTT assay.
Notes: The data were expressed as mean values (±standard deviations, n = 3); **P < 0.01.
Abbreviations: OTMCS–PEI, amphiphilic chitosan cross-linked with low-molecular weight polyethylenimine; MTT, 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide.
Transfection efficiency
In vitro transfection efficiency
The transfection activity of the newly synthesized OTMCS–PEI polymer was investigated in HeLa cells using pEGFP-N2 and pGL3-Control reporter genes. The OTMCS–PEI/DNA complexes were prepared at various weight ratios ranging from 5 to 30. PEI 25 KDa and PEI 2 KDa polyplexes were prepared at the same weight ratios as controls.
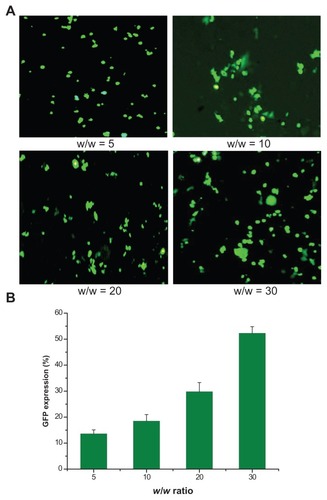
As shown in , bright green fluorescent segments demonstrated that the pEGFP reporter gene was effectively transfected into HeLa cells by the synthesized OTMCS–PEI polymer. With the increase of w/w ratios, more green fluorescence positive cells were observed. In , the percentage of green-fluorescent-protein-expressed cells was counted by a flow cytometer.Citation27 As w/w ratios increased, the percentage of green-fluorescent-protein-expressed cells increased. This was in accordance with the fluorescent images.
Figure 8 GFP gene transfection in HeLa cells by OTMCS–PEI at w/w ratios of 5, 10, 20, and 30. (A) Percentage of GFP gene transfection in HeLa cells by flow cytometry analysis.* (B) Representative fluorescence images for s transfection in HeLa cells using OTMCS–PEI at various w/w ratios.
Note: *The mean ± standard deviation, n = 6.
Abbreviations: GFP, green fluorescent protein; OTMCS–PEI, amphiphilic chitosan cross-linked with low-molecular weight polyethylenimin.
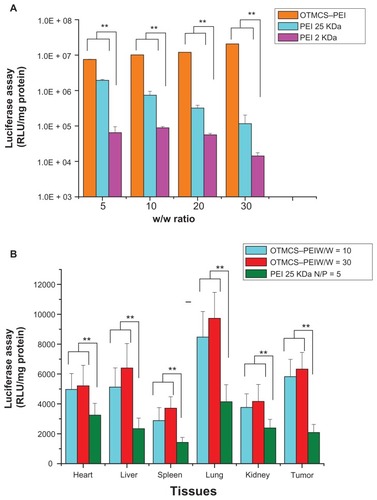
shows the results of the gene transfection efficiency of OTMCS–PEI/pGL3-Control complexes in HeLa cells in comparison with other controlled complexes. PEI 25 KDa showed the best transfection efficiency at w/w of 10. As the w/w ratios increased, its gene transfection decreased, which mainly resulted from high cytotoxicity. OTMCS–PEI showed much higher gene transfer ability compared with PEI 25 KDa at the designated w/w ratios. It exhibited the maximal transfection efficiency at the weight ratio of 30. The highest luciferase expression level obtained was more than 320 times higher compared with PEI 2 KDa, even about 10 times higher than PEI 25 KDa at optimal conditions.
Figure 9 (A) Transfection efficiency of OTMCS–PEI/DNA complex in HeLa cell line at w/w ratios of 5, 10, 20, and 30.* (B) Transfection efficiency of pGL3-Control as a reporter gene in mice.
Notes: BALB/c athymic mice were inoculated with B16 cells. Luciferase gene expression was determined after administration of OTMCS–PEI/DNA complex (w/w = 10) OTMCS–PEI/DNA complex (w/w = 30), and PEI 25 KDa/DNA complex (N/P = 5);† *each data point represents the mean ± standard deviation (n = 6); **P < 0.01; †results were expressed in RLU/mg protein.
Abbreviations: OTMCS–PEI, amphiphilic chitosan cross-linked with low-molecular weight polyethylenimine; RLU, relative light units.
In vivo transfection efficiency
In order to evaluate the DNA delivery efficiency of OTMCS–PEI, pGL3-Control gene transfection in vivo was performed using a tail-vein injection of pDNA complexed with OTMCS–PEI. It was found that the transfection efficiency of OTMCS–PEI was obviously higher than PEI 25 KDa in every tissue (). This was in line with the experimental results in vitro. The reporter gene expression of OTMCS–PEI/DNA complexes was highest in lung, followed by the subcutaneous transplanted tumor and liver. In contrast, the gene expression of PEI 25 KDa/DNA in tumor was lower than in lung and heart. In the case of the pulmonary accumulation of the complexes, on the one hand, because of abundant capillaries in the lung, the complexes with larger sizes were easily entrapped mechanically. At the same time, the complexes with surface positive charges were easily taken up by the lung due to electrostatic interaction.Citation28–Citation31 Therefore, in our future work, we intend to modify a tumor-targeted group to improve cell selection.
The transfection efficiency assay in vitro and in vivo showed that the micelle-like structure based on amphiphilic chitosan and low-molecular weight (LMW) PEI enhanced its stability in body fluid and transfection efficiency, compared with PEI 25 KDa.
Conclusion
We developed a new degradable gene vector OTMCS–PEI by cross-linking low-molecular weight PEI with amphiphilic chitosan. Through various physicochemical methods, we confirmed that OTMCS–PEI had great ability to form complexes with DNA, and suitable physicochemical properties for gene delivery. The vector showed controlled degradation. The degradation profile was suitable to a zero-order model. The half-life was about 30 hours. In the meantime, the OTMCS–PEI could deliver DNA to the nucleus, and then degraded into micromolecules. The polymer showed favorable buffering capacity. The particle sizes of OTMCS–PEI/DNA complexes were around 150–200 nm and the zeta potentials ranged from 10 mV to 30 mV, which was proper for a gene vector. The OTMCS–PEI polymer was able to condense DNA completely at the weight ratio of 0.6. The polymer could protect plasmid DNA from being digested by DNase I at a concentration of 2.25 U DNase I/μg DNA. At the same time, they were able to resist dissociation induced by 50% FBS and 1100 μg/mL sodium heparin. Additionally, the new gene vector showed much lower cytotoxicity and higher gene transfection efficiency in the HeLa cell lines, compared with PEI 25 KDa. The transfection efficiency of the polymer in vivo was also higher than that of PEI 25 KDa. Except for the accumulation in lung, more DNA transported by OTMCS–PEI polymer acted on transplanted tumors. In general, this new polymer could be a potential candidate in gene delivery, with high transfection efficiency and low cytotoxicity. However, it is worth improving its tumor targeting. Moreover, both the delivery of therapeutic gene transferred by this gene vector and the therapeutic efficiency need to be studied. We are currently conducting more comprehensive studies with the aim of answering these questions, to be presented in another manuscript.
Acknowledgments
This study was supported by the National Natural Science Foundation, China (81001024), the Leading Academic Discipline Project of the Shanghai Municipal Education Commission (J50704), the Scientific Research Innovation Project of the Shanghai Municipal Education Commission (13YZ097), and the Engineering Research Center Foundation of the Shanghai Municipal Science and Technology Commission (11DZ2280300).
Disclosure
The authors report no conflicts of interest in this work.
References
- MorilleMPassiraniCVonarbourgAClavreulABenoitJPProgress in developing cationic vectors for non-viral systemic gene therapy against cancerBiomaterials20082924–253477349618499247
- DanielsenSStrandSde Lange DaviesCStokkeBTGlycosaminoglycan destabilization of DNA-chitosan polyplexes for gene delivery depends on chitosan chain length and GAG propertiesBiochim Biophys Acta200517211–3445415652178
- VermaIMSomiaNGene therapy – promises, problems and prospectsNature199738966482392429305836
- PengMLiuWYangGInvestigation of the degradation mechanism of cross-linked polyethyleneimine by NMR spectroscopyPolym Degrad Stab2008932476482
- GuoQShiSWangXSynthesis of a novel biodegradable poly(ester amine) (PEAs) copolymer based on low-molecular-weight polyethyleneimine for gene deliveryInt J Pharm20093791828919539737
- GosselinMAGuoWLeeRJEfficient gene transfer using reversibly cross-linked low molecular weight polyethylenimineBioconjug Chem200112698999411716690
- RemautKSandersNNGeestBGBraeckmansKDemeesterJDe SmedtSCNucleic acid delivery: where material sciences and bio-sciences meetMater Sci Eng R2007583–5117161
- SeowWYYangYYFunctional polycarbonates and their self-assemblies as promising non-viral vectorsJ Control Release20091391404719470398
- WeissSISieverlingNNiclasenMUronic acids functionalized polyehtyleneimine (PEI)-polyethyleneglycol (PEG)-graft-copolymers as novel synthetic gene carriersBiomaterials200627102302231216337267
- DengRYueYJinFRevisit the complexation of PEI and DNA – how to make low cytotoxic and highly efficient PEI gene transfection non-viral vectors with a controllable chain length and structure?J Control Release20091401404619625007
- LungwitzUBreunigMBlunkTGöpferichAPolyethylenimine-based non-viral gene delivery systemsEur J Pharm Biopharm200560224726615939236
- LinEHKeramidasMRomeCLifelong reporter gene imaging in the lungs of mice following polyethyleneimine-mediated sleeping-beauty transposon deliveryBiomaterials20113271978198521168204
- NeuMFischerDKisselTRecent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivativesJ Gene Med200578992100915920783
- HuangHYuHTangGWangQLiJLow molecular weight polyethylenimine cross-linked by 2-hydroxypropyl-gamma-cyclodextrin coupled to peptide targeting HER2 as a gene delivery vectorBiomaterials20103171830183819942284
- KimTHCookSEAroteRBA degradable hyperbranched poly(ester amine) based on poloxamer diacrylate and polyethylenimine as a gene carrierMacromol Biosci20077561161917457939
- RomørenKThuBJEvensenØImmersion delivery of plasmid DNA. II. A study of the potentials of a chitosan based delivery system in rainbow trout (Oncorhynchus mykiss) fryJ Control Release2002851–321522512480326
- ManuelAMTaboadaPSabínJKrajewskaBVarelaLMRodríguezJRDNA–chitosan complexation: a dynamic light scattering studyColloids Surf A Physicochem Eng Asp20093391–3145152
- TuranKNagataKChitosan-DNA nanoparticles: The effect of cell type and hydrolysis of chitosan on in vitro DNA transfectionPharm Dev Technol200611450351217101521
- GaoYZhangZWChenLLGuWWLiYPChitosan N-betainates/DNA self-assembly nanoparticles for gene delivery: in vitro uptake and transfection efficiencyInt J Pharm200937115616219135139
- LaiWFLinMCNucleic acid delivery with chitosan and its derivativesJ Control Release2009134315816819100795
- ZhangCPingQZhangHShenJPreparation of N-alkyl-O-sulfate chitosan derivatives and micellar solubilization of taxolCarbohydr Polym2003542137141
- MaKHuMXQiYPAMAM-Triamcinolone acetonide conjugate as a nucleus-targeting gene carrier for enhanced transfer activityBiomaterials200930306109611819656564
- LiuKWangXFanWDegradable polyethylenimine derivate coupled to a bifunctional peptide R13 as a new gene-delivery vectorInt J Nanomedicine201271149116222412301
- GuoSTHuangYZhangWTernary complexes of amphiphilic polycaprolactone-graft-poly (N,N-dimethylaminoethyl methacrylate), DNA and polyglutamic acid-graft-poly (ethylene glycol) for gene deliveryBiomaterials201132184283429221450341
- ItoTYoshiharaCHamadaKKoyamaYDNA/polyethyleneimine/hyaluronic acid small complex particles and tumor suppression in miceBiomaterials201031102912291820047759
- HaoJShaXTangYEnhanced transfection of polyplexes based on pluronic-polypropylenimine dendrimer for gene transferArch Pharm Res20093271045105419641886
- WuXDingBGaoJSecond-generation aptamer-conjugated PSMA-targeted delivery system for prostate cancer therapyInt J Nanomedicine201161747175621980237
- KellawayIWFarrSJLiposomes as drug delivery systems to the lungAdv Drug Deliv Rev199051–2149161
- KawakamiSItoYCharoensitPYamashitaFHashidaMEvaluation of proinflammatory cytokine production induced by linear and branched polyethylenimine/plasmid DNA complexes in miceJ Pharmacol Exp Ther200631731382139016522808
- IntraJSalemAKCharacterization of the transgene expression generated by branched and linear polyethylenimine-plasmid DNA nanoparticles in vitro and after intraperitoneal injection in vivoJ Control Release2008130212913818538436
- KimEMJeongHJParkIKAsialoglycoprotein receptor targeted gene delivery using galactosylated polyethylenimine-graftpoly(ethylene glycol): in vitro and in vivo studiesJ Control Release20051082–355756716253376