?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Considering the observations that linoleic acid conjugated with paclitaxel (CLA-PTX) possesses antitumor activity against brain tumors, is able to cross the blood–brain barrier, but has poor water solubility, the purpose of this study was to prepare a novel CLA-PTX microemulsion and evaluate its activity against brain tumors in vitro and in vivo.
Methods
The in vitro cytotoxicity of a CLA-PTX microemulsion was investigated in C6 glioma cells. The in vivo antitumor activity of the CLA-PTX microemulsion was evaluated in tumor-bearing nude mice and rats. The pharmacokinetics of the CLA-PTX microemulsion were investigated in rats, and its safety was also evaluated in mice.
Results
The average droplet size of the CLA-PTX microemulsion was approximately 176.3 ± 0.8 nm and the polydispersity index was 0.294 ± 0.024. In vitro cytotoxicity results showed that the IC50 of the CLA-PTX microemulsion was 1.61 ± 0.83 μM for a C6 glioma cell line, which was similar to that of free paclitaxel and CLA-PTX solution (P > 0.05). The antitumor activity of the CLA-PTX microemulsion against brain tumors was confirmed in our in vivo C6 glioma tumor-bearing nude mice as well as in a rat model. In contrast, Taxol® had almost no significant antitumor effect in C6 glioma tumor-bearing rats, but could markedly inhibit growth of C6 tumors in C6 glioma tumor-bearing nude mice. The pharmacokinetic results indicated that CLA-PTX in solution has a much longer circulation time and produces higher drug plasma concentrations compared with the CLA-PTX microemulsion. The results of the acute toxicity study showed that the LD50 of CLA-PTX solution was 103.9 mg/kg. In contrast, the CLA-PTX microemulsion was well tolerated in mice when administered at doses up to 200 mg/kg.
Conclusion
CLA-PTX microemulsion is a novel formulation with significant antitumor efficacy in the treatment of brain tumors, and is safer than CLA-PTX solution.
Introduction
Conjugated linoleic acids are a structurally unique group of dietary long chain polyunsaturated fatty acids which have aroused considerable interest because of their significant antiproliferative effects on cancer cells in vitro and antitumor effects in animal models in vivo.Citation1–6 The synergistic antitumor activity of taxane agents incorporated with linoleic acid has been confirmed.Citation7,Citation8 Conjugation of linoleic acid with paclitaxel (CLA-PTX) has been previously reported by our laboratory.Citation9 The targeting effect of CLA-PTX on C6 glioma cells was demonstrated by in vitro cellular uptake experiments. The results showed that the extent of CLA-PTX uptake in C6 glioma cells was much greater than that of paclitaxel. Biodistribution results revealed that CLA-PTX, unlike paclitaxel, could distribute in brain tissue and remain there for a long time, indicating that CLA-PTX could cross the blood-brain barrier, reaching the brain and targeting brain tumors where it exerts a therapeutic effect. Unfortunately, the solubility of CLA-PTX was very low. In our previous research, the solvent used in the CLA-PTX formulation for intravenous administration was the same as that used for the paclitaxel injection (Taxol®, a mixture of Cremophor® EL [polyethoxylated castor oil, CrEL] and ethanol [1:1, v/v], Bristol Myers Squibb Co, Princeton, NJ).Citation9 Therefore, development of a novel formulation of CLA-PTX would be of great value.
It is well known that current strategies for increasing the solubility of poorly water-soluble drugs involve cosolvency, emulsification, microemulsification, drug complexation with cyclodextrins, and carrier mediation using liposomes or nanoparticles.Citation10–12 A microemulsion is an isotropic, thermodynamically stable, transparent (or translucent) system consisting of oil, water, and a surfactant, frequently in combination with a cosurfactant, and a droplet size usually in the range of 20–200 nm.Citation13–16 The major advantages of microemulsions are their high solubilization potential, thermodynamic stability, ability to improve the dissolution of lipophilic drugs, and surfactant-induced permeability enhancement.Citation17 The obvious benefits of microemulsions as drug delivery systems have led to the development of several systems for intravenous administration. Recently, a novel paclitaxel microemulsion was developed for intravenous administration in our laboratory.Citation18 In addition, considering that conjugated linoleic acid is a mixture of positional and geometric isomers of octadecadienoic acid, which is a component of some conjugated linoleic acid-rich soy oils, we hypothesized that the solubility of conjugated linoleic acid in oil would be high. We believed that the compatibility of CLA-PTX in oil, like conjugated linoleic acid, would be acceptable, and that development of a CLA-PTX microemulsion would be very feasible.
The present study describes the preparation of a CLA-PTX microemulsion and its characterization. The cytotoxicity of the CLA-PTX microemulsion in C6 glioma cells was assessed in vitro. The in vivo antitumor activity of the CLA-PTX microemulsion was evaluated in tumor-bearing nude mice and tumor-bearing rats, and its pharmacokinetics were investigated in rats. The acute toxicity of the CLA-PTX microemulsion was also evaluated in imprinting control region (ICR) mice.
Materials and methods
Chemicals and animals
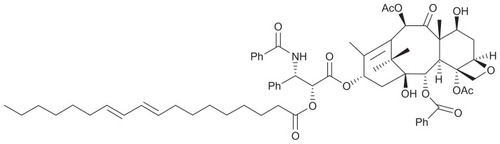
CLA-PTX was synthesized from conjugated linoleic acid and paclitaxel using the method described in a previous article published by our laboratory.Citation9 The structure and molecular weight of CLA-PTX are shown in . CrEL of higher purity was purchased from BASF Corporation (Shanghai, China). Egg phosphatidylcholine (Lipoid E 80) was obtained from Lipoid GmbH (Shanghai Toshisun Enterprise Co, Ltd, Shanghai, China). Paclitaxel was obtained from MeiLian Co, Ltd (Chongqing, China). Taxol® for injection was supplied by a local Beijing hospital, and contained 30 mg of paclitaxel in 5 mL of 50% CrEL (v/v) and 50% dehydrated ethanol (v/v). F10 cell culture medium, penicillin-streptomycin, fetal bovine serum, and donor equine serum were sourced from Gibco Invitrogen Co (Carlsbad, CA). All other reagents were of analytical or high-performance liquid chromatography grade.
Figure 1 Structure, molecular formula, and molecular weight of CLA-PTX.
Notes: Molecular formula of CLA-PTX C65H81NO15. Molecular weight of CLA-PTX 1116.
Abbreviation: CLA-PTX, linoleic acid conjugated with paclitaxel.
Sprague-Dawley rats weighing 200 ± 20 g, ICR mice weighing 20–22 g (aged 3–4 weeks), and female Balb/c nude mice weighing 18–22 g (aged 5–6 weeks) were obtained from the Experimental Animal Center of Peking University Health Science Center. All care and handling of the animals were performed in accordance with the requirements of the Institutional Authority for Laboratory Animal Care of Peking University.
Cell culture
Murine C6 glioma cells (Institute of Material Medical, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China) were used and maintained in Ham’s F10 medium supplemented with 2.5% fetal bovine serum, 15% donor equine serum, and 1% penicillin-streptomycin solution. The cells were incubated at 37°C in a humidified environment of 5% CO2.
Preparation of CLA-PTX microemulsion
The CLA-PTX microemulsion was prepared in our laboratory with some modification of the published method.Citation18 In brief, to prepare a concentrated CLA-PTX microemulsion, CLA-PTX was dissolved in dehydrated alcohol and then mixed with Lipoid E 80, CrEL, and soybean oil. The concentration of CLA-PTX in the microemulsion was 80 mg/mL. When this concentrated solution was mixed with a 5% glucose infusion, it underwent self-emulsification to form a CLA-PTX microemulsion (an oil/water microemulsion with a final CLA-PTX concentration of 2 mg/mL). For preparation of the CLA-PTX solution, CLA-PTX was dissolved in a mixture of CrEL and ethanol (1:1, v/v). The concentration of CLA-PTX in the CLA-PTX solution was 15 mg/mL.
Physicochemical characterization of CLA-PTX microemulsion
The volume of 5% glucose solution used for diluting the concentrated CLA-PTX microemulsion was 39 mL, and the final concentration of CLA-PTX in the microemulsion was 2 mg/mL. For droplet size analysis, this microemulsion was further diluted with 5% glucose solution to give a CLA-PTX concentration of about 0.8 mg/mL. The droplet size in the CLA-PTX microemulsion was measured using a Malvern Zetasizer (Nano-ZS, Malvern, Worcestershire, UK) at 25°C. Morphological examination of the CLA-PTX microemulsion was performed using a transmission electron microscope (TEM) at an acceleration voltage of 200 kV (JEM-200C, JEOL, Tokyo, Japan). Briefly, the TEM samples were prepared by dripping one drop of CAL-PTX microemulsion onto a 300-mesh Formvar-coated copper grid. The grids were negatively stained at room temperature with freshly prepared and sterile-filtered 2% (w/v) aqueous uranyl acetate solution. The grids were then washed twice and air-dried prior to imaging.
In vitro cytotoxicity
C6 glioma cells were seeded at a density of 5 × 103 cells/ well in 96-well transparent plates and incubated for 24 hours. The medium was then changed and various concentrations of the CLA-PTX microemulsion were used. At 48 hours, cell viability was determined using the sulforhodamine B assay. Briefly, the medium was removed, the cells were fixed with trichloroacetic acid, and then washed and stained with sulforhodamine B. Absorbance was measured at 540 nm using a 96-well plate reader (Bio-Rad 680, Bio-Rad, Hercules, CA). The survival percentages were calculated using the following formula:
where A540 nm is the absorbance value. Each assay was carried out in triplicate. Finally, dose-effect curves were constructed and IC50 values were calculated.
In vivo antitumor efficacy
The female Balb/c nude mice were inoculated subcutaneously in the right flank with 0.2 mL of a suspension of C6 glioma cells (1 × 106). When the tumor volume reached approximately 150 mm3 at about 7 days after inoculation, the mice were randomly divided into three groups, ie, a control group, a Taxol treatment group, and a CLA-PTX microemulsion treatment group. Each group consisted of six tumor-bearing mice. In this antitumor activity experiment, Taxol or the concentrated CLA-PTX microemulsion was diluted with 5% glucose infusion to give a concentration of 1.5 mg/mL or 2.0 mg/mL. The groups were treated with a 5% glucose infusion (control), Taxol (15 mg/kg intravenously once every 3 days for three doses), or CLA-PTX microemulsion (20 mg/kg [equimolar with 15 mg/kg of paclitaxel] intravenously once every 3 days for three doses). Formulations were given intravenously via the tail vein. Throughout the study, the mice were weighed, their tumors were measured with calipers every 2 days, and the tumor volume was calculated as [length × (width)2]/2. On day 21 post-tumor inoculation, the animals were sacrificed and tumors were harvested and weighed.
In a separate study, to establish a model of brain tumor-bearing rats, murine C6 glioma cells were inoculated into the brains of rats using a stereotaxic instrument (RWD Life Science Co, Ltd, Shenzhen, China). A sagittal incision was made through the skin to expose the cranium. Approximately 1 × 106 cells per 10 μL were stereotaxically implanted into the right forebrain of each rat using the following coordinates: 1.0 mm anterior and 3.0 mm lateral to bregma, at a depth of 5.0 mm from the brain surface.Citation19 Animals dying from complications associated with the anesthetic procedure (ie, death within 3 hours of surgery) were excluded from further analysis. The brain tumor-bearing rats were randomly divided into three groups, each containing ten animals. Animals in group 1, a blank control group, were given a 5% glucose infusion, animals in group 2 were treated with Taxol at a dose of 5 mg/kg, while animals in group 3 received the CLA-PTX microemulsion at a dose of 6.67 mg/kg (equimolar with 5 mg/kg of paclitaxel). Formulations were administered via the tail vein at days 7, 10, and 13 after tumor inoculation, and the animals were sacrificed on day 20 post-tumor inoculation. The tumors were harvested and weighed. Tumor weight inhibition (TWI) was calculated using the formula:
where WC and WD represent the tumor weight of the animals from the glucose infusion and treatment groups, respectively.Citation20
Pharmacokinetic studies
The CLA-PTX solution and concentrated CLA-PTX microemulsion were each diluted with 5% glucose infusion to give a final CLA-PTX concentration of 2 mg/mL. Ten male Sprague-Dawley rats were randomly assigned to two groups, containing five rats each. Group 1 received an intravenous injection of CLA-PTX solution at a dose of 6.67 mg/kg, while group 2 received an intravenous injection of CLA-PTX microemulsion at a dose of 6.67 mg/kg, respectively. After intravenous administration, blood samples of approximately 0.5 mL were collected into heparinized tubes from the orbit venous plexus of each rat at different times. Plasma samples were harvested by immediate centrifugation at 3000 g for 5 minutes and stored at −20°C until required for analysis by high-performance liquid chromatography.
Pharmacokinetic parameters were calculated from the CLA-PTX concentration-time data using noncompartmental methods and NONMEM version 7.2 (Icon Development Solutions, Ellicott City, MD). The C0.5 was the observed concentration at 0.5 hours. The area under the curve (AUC) was calculated using the linear trapezoidal method and was extrapolated to infinity (AUCinf) by dividing the last measured concentration by the terminal rate constant, λz, which was determined from the slope of the terminal phase of the plasma concentration-time curve. The terminal half-life (t1/2) was calculated as 0.693 divided by λz.
Acute toxicity
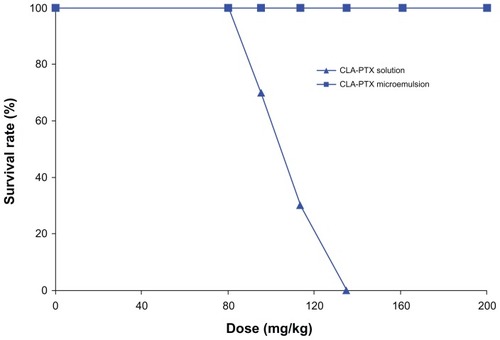
The method for evaluating the acute toxicity of the CLA-PTX microemulsion was modified from a previous report.Citation21 ICR mice were randomly divided into ten groups (five groups for treatment with the CLA-PTX solution and another five groups for treatment with the CLA-PTX microemulsion), containing ten mice each. All animals were fasted for 12 hours before intravenous administration of the study formulations. The doses used in the group receiving the CLA-PTX solution were 67, 80, 95.2, 113.4, and 135 mg/kg, respectively. Mice in the group receiving the CLA-PTX microemulsion were given doses of 80, 95.2, 113.4, 135, 160.7, and 200 mg/kg. The CLA-PTX formulations were dissolved in 5% glucose solution for injection and administered as individual doses via the intravenous route. All groups of mice were observed for one week, and the number of mice surviving was recorded. The LD50 was calculated using the Bliss method.
High-performance liquid chromatography analysis of CLA-PTX
CLA-PTX plasma samples were extracted using our previously reported method.Citation9 Briefly, 100 μL of plasma was mixed with 2.5 mL acetonitrile in a vortex mixer for 30 seconds. The mixture was centrifuged at 3000 g for 10 minutes. Next, 2.0 mL of supernatant was collected and dried under a gentle stream of nitrogen gas at 50°C in a water bath. The residue was dissolved in 100 μL of mobile phase and the supernatant (50 μL) was injected into the high-performance liquid chromatography system, which comprised a Waters 2487 dual λ absorbance detector and a 1525 pump (Waters, Milford, MA). An ODS 3 C-18 analytical column (5 μm, 250 × 4.6 mm, Phenomenex, Torrance, CA) was used and the wavelength was set at 227 nm. The flow rate was 1 mL per minute, with the mobile phase starting from 100% solvent A (acetonitrile:water = 60:40 v/v) for 10 minutes, then a linear gradient to 100% solvent B (acetonitrile) at 12 minutes, and sustained at 100% solvent B for 20 minutes. The retention time of CLA-PTX was approximately 15 minutes.
Statistical analysis
Data are presented as the mean ± standard deviation. One- way analysis of variance was used to determine significant differences among groups, after which post hoc tests with Bonferroni correction were used for comparisons between individual groups. Statistical significance was established at P < 0.05.
Results
Characterization of CLA-PTX microemulsion
The concentrated CLA-PTX microemulsion was transparent and slightly yellow in color. The concentrations of CLA-PTX, CrEL, and Lipoid E 80 in the concentrated microemulsion were 80, 95, and 190 mg/mL, respectively. In addition, the concentrated CLA-PTX microemulsion was stable for at least one month without any precipitation of CLA-PTX. The average droplet size in the concentrated CLA-PTX microemulsion after dilution with 5% glucose infusion was approximately 176.3 ± 0.8 nm, with a polydispersity index of 0.294 ± 0.024. The average zeta potential of the CLA-PTX microemulsion was −21.31 ± 4.61 mV, as shown in . A typical droplet size and distribution is shown in . TEM was used to examine the morphology of the CLA-PTX microemulsion () and showed that the CLA-PTX microemulsion droplets were spherical in shape. In addition, TEM imaging showed that the droplet size of the microemulsion was smaller than that in . This might be due to the effect of sample preparation (staining and drying) for the TEM analysis.
Figure 2 Typical droplet size and distribution of the CLA-PTX microemulsion (A) and transmission electron micrograph of CLA-PTX microemulsion (B).
Note: Bar 200 nm.
Abbreviation: CLA-PTX, linoleic acid conjugated with paclitaxel.
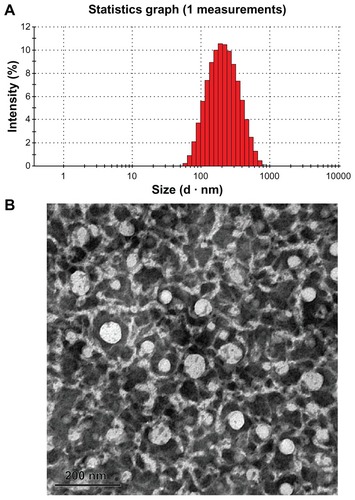
Table 1 Droplet size, polydispersity index, and zeta potential of CLA-PTX microemulsion (n = 3)
In vitro cytotoxicity
A C6 glioma cell line was used to investigate the cytotoxicity of the CLA-PTX microemulsion in comparison with free paclitaxel and CLA-PTX solution. The IC50 values are given in . It can be concluded from that the CLA-PTX microemulsion had a similar IC50 value to that of the CLA-PTX solution and free drug (P > 0.05).
Table 2 Cytotoxicity of various CLA-PTX formulations for C6 cells (n = 3)
In vivo antitumor efficacy
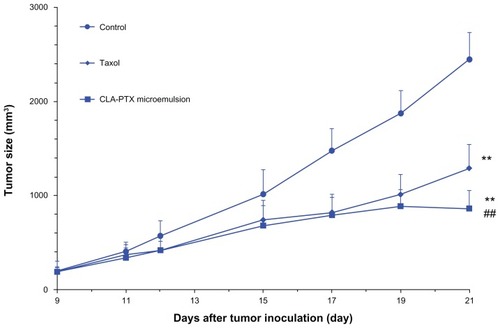
The in vivo antitumor activity of the CLA-PTX microemulsion was investigated in C6 tumor-bearing nude mice. As shown in , the CLA-PTX microemulsion markedly inhibited the growth of C6 tumors (P < 0.01). A similar result was observed in the group treated with Taxol (P < 0.01). The antitumor activity of the CLA-PTX microemulsion was significantly higher than that of Taxol (P < 0.01). The mean tumor sizes at day 21 after implantation in the Taxol and the CLA-PTX microemulsion groups were 1288 ± 257 and 857 ± 175 mm3, respectively, compared with 2449 ± 286 mm3 in the control group (P < 0.01). Corresponding tumor growth inhibition in the Taxol-treated group and in the CLA-PTX microemulsion-treated group was 47.4% and 65.0%, respectively.
Figure 3 In vivo antitumor activity of CLA-PTX microemulsion in C6 tumor-bearing nude mice.
Notes: Balb/C nude mice were inoculated subcutaneously with C6 cells and treated with a 5% glucose infusion, Taxol® (15 mg/kg, intravenously, every 3 days for three doses), or CLA-PTX microemulsion 20 mg/kg (equimolar with 15 mg/kg of paclitaxel), intravenously every 3 days for three doses, respectively. The control group was given the 5% glucose infusion. Formulations were given intravenously via the tail vein. Throughout the study, the mice were weighed and tumors were measured with calipers every two days. **P < 0.01 versus 5% glucose infusion as control; ##P < 0.01 versus Taxol.
Abbreviation: CLA-PTX, linoleic acid conjugated with paclitaxel.
The antitumor effect of the CLA-PTX microemulsion was also compared with that of Taxol by measuring the tumor weight in C6 glioma tumor-bearing rats after cell implantation. As shown in , the CLA-PTX microemulsion markedly inhibited growth of the C6 glioma tumors (P < 0.01). In contrast, Taxol had no significant inhibitory effect on the growth of C6 glioma tumors at a dose of 5 mg/kg (P > 0.05). The average tumor weight in the groups treated with 5% glucose infusion, Taxol 5 mg/kg, and CLA-PTX microemulsion (6.67 mg/kg) at day 20 after C6 glioma cell implantation was 386, 361, and 150 mg, respectively. As shown in , the TWI (%) value in the group treated with the CLA-PTX microemulsion versus that in the control group and the Taxol treatment group (5 mg/kg) was about 61.1% and 58.5%, respectively.
Figure 4 In vivo antitumor activity of CLA-PTX microemulsion in C6 brain tumor-bearing rats.
Notes: Tumor weight is shown at the time of sacrifice, 20 days post-inoculation. Formulations were administered intravenously via the tail vein at days 7, 10, and 13 after tumor inoculation. Animals were sacrificed on day 20 post-tumor inoculation (n = 10). Control group, 5% glucose infusion; Taxol® treatment group, at a dose of 5 mg/kg; CLA-PTX microemulsion treatment group, at a dose of 6.67 mg/kg (equimolar with 5 mg/kg of paclitaxel). **P < 0.01 versus 5% glucose infusion as control; ##P < 0.01 versus Taxol treatment group.
Abbreviation: CLA-PTX, linoleic acid conjugated with paclitaxel.
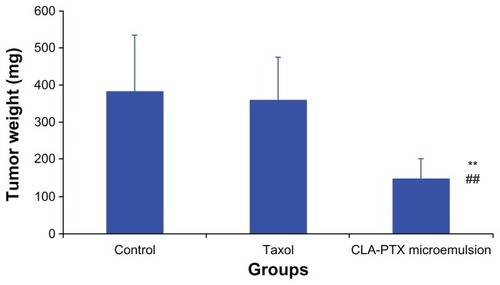
Table 3 Tumor weight inhibition (%) of CLA-PTX microemulsion against control and Taxol®
In vivo pharmacokinetics
The plasma concentration-time profiles of CLA-PTX after intravenous administration of the CLA-PTX formulations were characterized in rats. As shown in , the concentration of CLA-PTX in the group treated with the CLA-PTX solution was significantly higher than that in the group treated with the CLA-PTX microemulsion. The main pharmacokinetic parameters of CLA-PTX are summarized in , and show that the CLA-PTX solution had significantly higher values for C0.5, AUCinf, t1/2, and mean residence time (132.9 μM/mL, 2296 hours·μM/mL, 59.6 hours, and 36.6 hours, respectively) than did the CLA-PTX microemulsion (68.5 μM/mL, 389 hours·μM/mL, 24.4 hours, and 18.9 hours, respectively, P < 0.01), whereas the CLA-PTX microemulsion produced significantly higher values for Vss and Cl (0.62 L/kg and 16.8 mL/hour·kg, respectively) than did the CLA-PTX solution (0.22 L/kg and 2.26 mL/hour·kg, respectively, P < 0.01).
Figure 5 Plasma concentration-time profiles of CLA-PTX after intravenous administration of CLA-PTX solution or CLA-PTX microemulsion at 6.67 mg/kg CLA-PTX in Sprague-Dawley rats.
Notes: Mean ± standard deviation, n = 5.
Abbreviation: CLA-PTX, linoleic acid conjugated with paclitaxel.
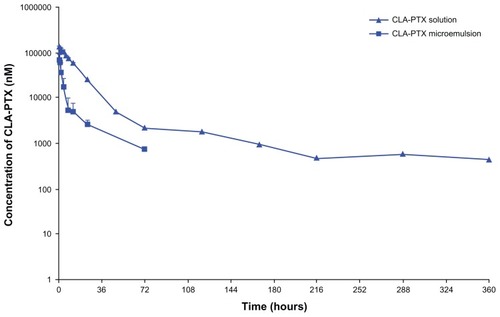
Table 4 Main pharmacokinetic parameters of CLA-PTX after intravenous administration of CLA-PTX solution or CLA-PTX microemulsion at 6.67 mg/kg of CLA-PTX in Sprague-Dawley rats (n = 5)
Acute toxicity
shows the acute toxicity of the intravenous CLA-PTX solution and CLA-PTX microemulsion in mice, as determined by the median lethal dose (LD50). No mortality or significant toxic symptoms were observed in mice treated with up to 200 mg/kg of the CAL-PTX microemulsion. Therefore, the LD50 of the CLA-PTX microemulsion is expected to be higher than 200 mg/kg. In contrast with the CLA-PTX solution, there were 3, 7, and 10 deaths within 4 hours of administration of the CLA-PTX solution at doses of 95.2, 113.4, and 135 mg/kg, respectively. The LD50 of the CLA-PTX solution administered by the intravenous route to mice was 103.9 mg/kg. The 95% confidence limits were found to lie within the range of 96.1–112.3 mg/kg.
Discussion
In the present study, we designed and prepared a novel CLA-PTX microemulsion. The droplet size and TEM results show that the concentrated CLA-PTX microemulsion would self-emulsify to form nanosized microemulsion droplets ( and ). The antitumor activity of the CLA-PTX microemulsion for the treatment of brain tumors was confirmed in our in vivo C6 glioma tumor-bearing nude mice as well as in a rat model ( and ). In contrast, Taxol had almost no significant antitumor effect on inhibition of tumor growth in C6 glioma tumor-bearing rats (), although Taxol markedly inhibited the growth of C6 tumors in the C6 glioma tumor-bearing nude mice (). One possible reason for this may be that the blood–brain barrier, which is a formidable barrier to many agents directed against malignant cells within the brain,Citation22 prevents paclitaxel reaching brain tissue. Our previous study showed that CLA-PTX, unlike paclitaxel, could distribute and maintain high levels in brain tissue, indicating its potential ability to cross the blood–brain barrier.Citation9 Therefore, we suggested that CLA-PTX in the microemulsion could cross the blood–brain barrier and have antitumor activity in the brain. According to our previous results for the antitumor activity of CLA-PTX solution in C6 glioma tumor-bearing rats (average tumor weight 126.9 ± 95.1 mg),Citation9 we suggest that the antitumor activity of the CLA-PTX microemulsion (average tumor weight 149.9 ± 52.0 mg) is similar to that of CLA-PTX solution in C6 glioma tumor-bearing rats (P > 0.05).
There are many reported strategies for drug targeting to brain tumors,Citation23 such as modified nanoparticles or solid lipid nanoparticles.Citation24–27 It has been also reported that intranasal administration of micro/nanoemulsions could have a brain-targeting effect.Citation28–37 In the present study, we confirmed that a CLA-PTX microemulsion administered intravenously could also produce antitumor activity in brain tissue.
The plasma concentration of CLA-PTX was determined after a single intravenous injection of CLA-PTX solution or CLA-PTX microemulsion. As shown in and , CLA-PTX in the solution showed a much longer circulation time and a higher drug plasma concentration than did CLA-PTX in the microemulsion formulation (P < 0.01). The C0.5, AUCinf, t1/2, and mean residence time of the drug in plasma for the group receiving the CLA-PTX solution were increased by 1.9-fold, 5.9-fold, 2.4-fold, and 1.9-fold compared with the group receiving the CLA-PTX microemulsion (P < 0.01). Although the higher plasma concentration and prolonged plasma circulation are responsible for the increased therapeutic efficacy of the CLA-PTX microemulsion, the present study shows that the antitumor activity of the CLA-PTX microemulsion was similar to that of the CLA-PTX solution in C6 glioma tumor-bearing rats. We suggest that this is due to the enhanced permeability and retention effect via a passive targeting mechanism, which is the principal physiology-based mechanism for accumulation of larger molecules and small particles in tumor tissue.Citation38 Therefore, these CLA-PTX droplets in the microemulsion would be removed from the circulation and accumulate in tumor tissues to produce an antitumor activity.
The solvent used in the CLA-PTX solution is a mixture of CrEL and dehydrated ethanol (1:1, v/v). The concentration of CLA-PTX in the CLA-PTX solution was 15 mg/mL, containing the CrEL at 527 mg/mL and dehydrated ethanol at 396 mg/mL. In contrast, the concentration of CLA-PTX in the concentrated CLA-PTX microemulsion was 80 mg/mL, containing only 95 mg/mL CrEL. The ratio of CLA-PTX to CrEL in the CAL-PTX microemulsion and CLA-PTX solution is 35.1 and 1.2, respectively, indicating that the CrEL in the CLA-PTX microemulsion is significantly reduced. It has been reported that treatment with CrEL alone produces asthenia, tachypnea, convulsions, and ultimately death in dogs and mice.Citation39,Citation40 In the present acute toxicity study, the LD50 of the CLA-PTX solution was calculated to be 103.9 mg/kg. According to the components of the CLA-PTX solution, when 103.9 mg/kg CLA-PTX was injected into mice, the dose of CrEL was 3584 mg/kg. In contrast, the calculated CrEL in the CLA-PTX microemulsion at the given dose of 200 mg/kg for mice was only 235 mg/kg. Therefore, it may be concluded that the acute toxicity of CLA-PTX solution is primarily due to the toxicity of CrEL. However, the cause of CrEL-related acute mortality was not investigated in this study. Considering the LD50 results, we suggest that the CLA-PTX microemulsion is safer than the CLA-PTX solution, and a high dose of CLA-PTX microemulsion can be used for future therapeutic studies due to the fact that the concentration of CrEL in this microemulsion is significantly reduced.
Conclusion
In summary, we have designed and prepared a novel CLA-PTX microemulsion. The droplet size and TEM results show that the concentrated CLA-PTX microemulsion self-emulsifies to form nanosized microemulsion droplets. The antitumor activity of the CLA-PTX microemulsion in the treatment of brain tumors was confirmed in our in vivo C6 glioma tumor-bearing nude mouse model as well as in a rat model. In contrast, Taxol had almost no significant antitumor effect in C6 glioma tumorbearing rats, but could markedly inhibit growth of tumors in C6 glioma tumor-bearing nude mice. Although the CLA-PTX in the CLA-PTX solution showed a much longer circulation time and higher drug plasma concentrations compared with the CLA-PTX microemulsion, the antitumor activity of the CLA-PTX microemulsion was similar to that of the CLA-PTX solution. Due to the reduction in CrEL, the CLA-PTX microemulsion is safer than CLA-PTX solution, and a high dose of CLA-PTX microemulsion could be used for future therapeutic studies.
Acknowledgments
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (81172992), the National Basic Research Program of China (973 Program 2009CB930300, 2013CB932501) and the Innovation Team of the Ministry of Education (BMU20110263).
Disclosure
The authors report no conflicts of interest in this work.
References
- De la TorreADebitonEDurandDConjugated linoleic acid isomers and their conjugated derivatives inhibit growth of human cancer cell linesAnticancer Res2005253943343916309181
- StachowskaEConjugated dienes of linoleic acid and tumorigenesisAnn Acad Med Stetin200854122125 Polish19839523
- BeppuFHosokawaMTanakaLKohnoHTanakaTMiyashitaKPotent inhibitory effect of trans9, trans11 isomer of conjugated linoleic acid on the growth of human colon cancer cellsJ Nutr Biochem20061783083616563722
- CunninghamDCHarrisonLYShultzTDProliferative responses of normal human mammary and MCF-7 breast cancer cells to linoleic acid, conjugated linoleic acid and eicosanoid synthesis inhibitors in cultureAnticancer Res1997171972039066651
- MaggioraMBolognaMCerùMPAn overview of the effect of linoleic and conjugated-linoleic acids on the growth of several human tumor cell linesInt J Cancer200411290991915316938
- TanmahasamutPLiuJHendryLBSidellNConjugated linoleic acid blocks estrogen signaling in human breast cancer cellsJ Nutr200413467468014988466
- MenéndezJAdel Mar BarbacidMMonteroSEffects of gamma-linolenic acid and oleic acid on paclitaxel cytotoxicity in human breast cancer cellsEur J Cancer20013740241311239764
- FiteAGouaMWahleKWSchofieldACHutcheonAWHeysSDPotentiation of the anti-tumour effect of docetaxel by conjugated linoleic acids (CLAs) in breast cancer cells in vitroProstaglandins Leukot Essent Fatty Acids200777879617900885
- KeXYZhaoBJZhaoXThe therapeutic efficacy of conjugated linoleic acid – paclitaxel on glioma in the ratBiomaterials2010315855586420430438
- SinghAWorkuZAVan den MooterGOral formulation strategies to improve solubility of poorly water-soluble drugsExpert Opin Drug Deliv201181361137821810062
- ShiYPorterWMerdanTLiLCRecent advances in intravenous delivery of poorly water-soluble compoundsExpert Opin Drug Deliv200961261128219941409
- FahrALiuXDrug delivery strategies for poorly water-soluble drugsExpert Opin Drug Deliv2007440341617683253
- BagweRPKanickyJRPallaBJPatanjaliPKShahDOImproved drug delivery using microemulsions: rationale, recent progress and new horizonsCrit Rev Ther Drug Carrier Syst2001187714011326744
- TalegaonkarSAzeemAAhmadFJKharRKPathanSAKhanZIMicroemulsions: a novel approach to enhanced drug deliveryRecent Pat Drug Deliv Formul2008223825719075911
- HeCXHeZGGaoJQMicroemulsions as drug delivery systems to improve the solubility and the bioavailability of poorly water-soluble drugsExpert Opin Drug Deliv2010744546020201713
- ShenQLiXYuanDJiaWEnhanced oral bioavailability of daidzein by self-microemulsifying drug delivery systemChem Pharm Bull (Tokyo)20105863964320460789
- YinYMCuiFDMuCFDocetaxel microemulsion for enhanced oral bioavailability: preparation and in vitro and in vivo evaluationJ Control Release2009140869419709639
- WangYWuKCZhaoBXA novel paclitaxel microemulsion containing a reduced amount of Cremophor EL: pharmacokinetics, biodistribution, and in vivo antitumor efficacy and safetyJ Biomed Biotechnol2011201185487221331356
- von EckardsteinKLPattSKratzelCKiwitJCReszkaRLocal chemotherapy of F98 rat glioblastoma with paclitaxel and carboplatin embedded in liquid crystalline cubic phasesJ Neurooncol20057220921515937642
- PetrangoliniGSupinoRPratesiGEffect of a novel vacuolar- H+-ATPase inhibitor on cell and tumor response to camptothecinsJ Pharmacol Exp Ther200631893994616714402
- LiuXSunJChenXPharmacokinetics, tissue distribution and anti-tumour efficacy of paclitaxel delivered by polyvinylpyrrolidone solid dispersionJ Pharm Pharmacol20126477578222571255
- PuduvalliVKSawayaRAntiangiogenesis – therapeutic strategies and clinical implications for brain tumorsJ Neurooncol20005018920011245279
- PardridgeWMDrug targeting to the brainPharm Res2007241733174417554607
- WagnerSZensiAWienSLUptake mechanism of ApoE-modified nanoparticles on brain capillary endothelial cells as a blood-brain barrier modelPLoS One20127e3256822396775
- XinHShaXJiangXZhangWChenLFangXAnti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticlesBiomaterials2012338167817622889488
- GaoHQianJCaoSPrecise glioma targeting of and penetration by aptamer and peptide dual-functioned nanoparticlesBiomaterials2012335115512322484043
- BondìMLDi GesùRCraparoEFLipid nanoparticles for drug targeting to the brainMethods Enzymol201250822925122449929
- Biddlestone-ThorpeLMarchiNGuoKNanomaterial-mediated CNS delivery of diagnostic and therapeutic agentsAdv Drug Deliv Rev20126460561322178615
- ZhangQJiangXJiangWLuWSuLShiZPreparation of nimodipine- loaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brainInt J Pharm2004275859615081140
- PiaoHMBalakrishnanPChoHJPreparation and evaluation of fexofenadine microemulsions for intranasal deliveryInt J Pharm201039530931620635476
- BondìMLCraparoEFGiammonaGDragoFBrain-targeted solid lipid nanoparticles containing riluzole: preparation, characterization and biodistributionNanomedicine (Lond)20105253220025461
- VyasTKBabbarAKSharmaRKSinghSMisraAIntranasal mucoadhesive microemulsions of clonazepam: preliminary studies on brain targetingJ Pharm Sci20069557058016419051
- KumarMMisraABabbarAKMishraAKMishraPPathakKIntranasal nanoemulsion based brain targeting drug delivery system of risperidoneInt J Pharm200835828529118455333
- GaoeHPangZPanSAnti-glioma effect and safety of docetaxel-loaded nanoemulsionArch Pharm Res20123533334122370788
- GantaSDeshpandeDKordeAAmijiMA review of multifunctional nanoemulsion systems to overcome oral and CNS drug delivery barriersMol Membr Biol20102726027320929336
- KumarMMisraAMishraAKMishraPPathakKMucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targetingJ Drug Target20081680681418988064
- DesaiAVyasTAmijiMCytotoxicity and apoptosis enhancement in brain tumor cells upon coadministration of paclitaxel and ceramide in nanoemulsion formulationsJ Pharm Sci2008972745275617854074
- VyasTKShahiwalaAAmijiMMImproved oral bioavailability and brain transport of Saquinavir upon administration in novel nanoemulsion formulationsInt J Pharm20083479310117651927
- TorchilinVTumor delivery of macromolecular drugs based on the EPR effectAdv Drug Deliv Rev20116313113520304019
- RowinskyEKCazenaveLADonehowerRCTaxol: a novel investigational antimicrotubule agentJ Natl Cancer Inst199082124712591973737
- ParkJHChiSCLeeWSToxicity studies of Cremophor-free paclitaxel solid dispersion formulated by a supercritical antisolvent processArch Pharm Res20093213914819183887