Abstract
This work aims to develop solid lipid nanoparticles (SLNs) loaded with retinoic acid (RA) to evaluate the influence of two lipophilic amines, stearylamine (SA) and benethamine (BA), and one hydrophilic, triethylamine (TA), on drug-encapsulation efficiency (EE) and cytotoxicity in cancer cell lines. The SLNs were characterized for EE, size, and zeta potential. The mean particle size decreased from 155 ± 1 nm (SLNs without amine) to 104 ± 4, 95 ± 1, and 96 ± 1 nm for SLNs prepared with SA, BA, and TA, respectively. SA-RA-loaded SLNs resulted in positively charged particles, whereas those with TA and BA were negatively charged. The EEs were significantly improved with the addition of the amines, and they increased from 36% ± 6% (without amine) to 97% ± 2%, 90% ± 2%, and 100% ± 1% for SA, TA, and BA, respectively. However, stability studies showed higher EE for BA-RA-loaded SLNs than TA-RA-loaded SLNs after 30 days. The formulations containing SA loaded or unloaded (blank SLNs) with RA were cytotoxic in normal and cancer cell lines. In contrast, the blank SLNs containing TA or BA did not show cytotoxicity in human breast adenocarcinoma cells (MCF-7), while RA-loaded SLNs with the respective amines were significantly more cytotoxic than free RA. Furthermore, the cytotoxicity of BA-RA-loaded SLNs was significantly higher than TA-RA-loaded SLNs. These findings are in agreement with the data obtained in the evaluation of subdiploid DNA content and cell-cycle analysis, which showed better anticancer activity for BA-RA-loaded SLNs than TA-RA-loaded SLNs and free RA. Taken together, these findings suggest that the BA-RA-loaded SLN formulation is a promising alternative for the intravenous administration of RA in the treatment of cancer.
Introduction
Vitamin A and its derivatives have the ability to reduce tumor growth and to induce apoptosis and differentiation in several types of cancer. In particular, all-trans retinoic acid (RA) has been studied for the treatment of cancer, including leukemia and breast cancer. The action of RA is attributed to its binding to the nuclear receptors, retinoic acid receptors, and retinoid X receptors, which regulate a variety of genes. RA can generally block the cell cycle in the G1 phase, causing cell proliferation inhibition and apoptosis.Citation1 The most effective clinical use of RA for human diseases was demonstrated in the treatment of acute promyelocytic leukemia.Citation2,Citation3
In clinical trials, RA has been given to cancer patients by oral administration. However, the bioavailability of oral RA has been considered low and quite variable.Citation4 Moreover, continuous treatment with oral RA has been associated with progressive reduction of plasma concentrations, potentially to levels below those required to carry out its effect, probably due to the induced cytochrome P-450-dependent metabolism of RA.Citation5 Whereas these factors limit the clinical use of oral RA, an intravenous formulation could circumvent this problem.
RA, a hydrophobic drug with an octanol/water partition coefficient log of 4.6,Citation6 has poor aqueous solubility, and this can be a great disadvantage for endovenous administration. Some efforts have been made to enable intravenous administration of RA using nanocarriers, and a number of previous publications demonstrated that nanoparticles loaded with RA have significant influence over cancer cell viability.Citation7 In addition, lipid nanocarriers such as liposomes, nanoemulsions, and solid lipid nanoparticles (SLNs) have also been used.Citation8–Citation10
Among the advantages associated with SLNs are, most notably, the fact that their production is easily transposed to an industrial scale, as they do not require the use of organic solvents. In addition, compared with the nanoemulsions, which are prepared with liquid lipids, SLNs have more potential for controlled release, due to their solid matrix.Citation11–Citation13 However, the RA encapsulation in SLNs is usually lowCitation14 unless a high surfactant/lipid ratio is used.Citation15,Citation16 This favors the drug location at the interface because of RA amphiphilicity,Citation17 thereby reducing the benefits obtained by the encapsulation in lipid matrix (increased stability, controlled release, and targeting effect).
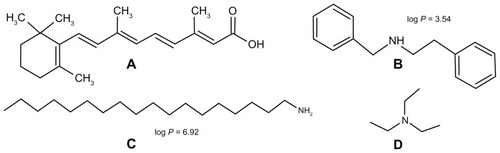
We previously reported that the in situ formation of an ion pairing between RA, a lipophilic acid (), and different amines provides an interesting alternative to increased RA encapsulation in SLNs. Among the tested amines, stearylamine (SA) (), a lipophilic amine, provided the highest encapsulation.Citation18,Citation19 These previous investigations were performed aiming for the development and evaluation of formulations for the topical treatment of acne. We hypothesized that SLNs loaded with RA, and different amines could be an interesting alternative for enabling intravenous administration of RA for cancer treatment. In the present study, we aimed to develop RA-loaded SLNs for parenteral administration, evaluating the influence of three amines: two lipophilic amines (SA and benethamine [BA]) () and a hydrophilic one (triethylamine [TA]) (). The in vitro anticancer activity of SLNs loaded with RA and SA, TA, or BA was investigated against different cancer cell lines.
Materials and methods
Materials
Retinoic acid (RA), Compritol® 888 ATO (glyceryl behenate, mixture of mono-, di-, and triacylglycerols of behenic acid [C22]) and Super Refined Tween 80™ (Polysobarte 80) were kindly provided by Basf (Ludwigshafen, Germany), Gattefossé (Lyon, France), and Croda Inc (Edison, NJ, USA), respectively. SA (octadecylamine), cholesterol, TA, and BA were purchased from Sigma-Aldrich (St Louis, MO, USA).
For in vitro studies, the Roswell Park Memorial Institute medium (RPMI) 1640 was obtained from Sigma-Aldrich; Dulbecco’s modified Eagle’s medium, fetal bovine serum, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and staurosporine were purchased from Gibco (Life Technologies, Carlsbad, CA, USA).
The following cancer cell lines were used: Jurkat (immortalized line of T lymphocyte) was provided by Gustavo Amarante-Mendes (São Paulo University, São Paulo, Brazil). HCT-116 (colorectal carcinoma) and MCF-7 (human breast adenocarcinoma) cells were purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA). All other chemicals were of analytical grade.
Preparation of SLNs
SLNs were prepared by the hot melting homogenization method using an emulsification-ultrasound.Citation19 The composition of the SLNs has previously been described, but with some modifications.Citation19 Considering the intended use of SLNs (intravenous administration), Tween 80™ was selected as surfactant. Briefly (batch 20 mL), the oily phase, composed of Compritol (400 mg), cholesterol (40 mg), Tween 80™ (300 mg), RA (10 mg), and the aqueous phase were heated separately to 85°C. Next, the aqueous phase was gently dropped onto the oily phase with constant agitation, at 8000 rpm in an Ultra Turrax T-25 homogenizer (Ika Labortechnik, Staufen, Germany). This emulsion was immediately submitted to the high-intensity probe sonication (20% amplitude) for 10 minutes, using a high-intensity ultrasonic processor (CPX 500 model; Cole-Palmer Instruments, East Bunker Court Vernon Hills, IL, USA). The influence of the in situ formation of an ion pairing between RA and amines was investigated. The SA or BA, and TA were selected as lipophilic and hydrophilic amines, respectively (RA/amine molar ratio was 1/2). The pH of the SLNs containing the amines was adjusted to 7.0 with a solution of 0.01 M HCl (Digimed DM 20, Santo Amaro, São Paulo, Brazil).
Particle size analysis
The mean particle diameter of SLNs in the dispersion was determined by unimodal analysis through dynamic light scattering using a Zetasizer 3000HSA (Malvern Instruments, Malvern, UK), at a fixed angle of 90° and at 25°C. The SLN dispersions were diluted in distilled and filtered water (cellulose ester membrane, 0.45 μM, Millipore, Billerica, MA) up to a count rate of 50 to 300 Kcps (1000 counts per second). The data reported were particle size, evaluated as the intensity obtained from three repeat measurements, and the polydispersity index.Citation19
Zeta potential
Zeta potential measurements were carried out by the electrophoretic mobility determination at 90° and 25°C. Before the measurements, SLN dispersions were diluted in filtered 1 mM NaCl solution (cellulose ester membrane, 0.45 mm, Millipore) up to a count rate of 100 to 1000 Kcps. All measurements were performed in triplicate using a Zetasizer 3000HSA (Malvern Instruments).
Drug-encapsulation efficiency
Encapsulation efficiency (EE) for RA in SLNs was determined according to the method previously described.Citation19 This method was based on the determination of RA concentration in the SLNs before (total RA) and after filtration (cellulose ester membrane, 0.45 μM, Millipore). The RA crystals, usually measuring a few micrometers across, present characteristic forms and can be easily distinguished from SLNs.Citation14 RA concentration in the external aqueous phase of the SLNs was determined by ultrafiltration method (Amicon® 100 k, Millipore) with a 100 kDa molecular weight cutoff membrane. RA concentration in the aqueous phase was negligible, owing to its low solubility in water.Citation15
RA concentration in SLNs (before and after filtration) or in the aqueous phase was determined according to the method described by Castro et al.Citation18 Briefly, an aliquot of the SLN dispersion was dissolved in tetrahydrofuran and later diluted in a mixture of acetonitrile, distilled water, and phosphoric acid (80:19.9:0.1). This mixture keeps the RA in solution (dissolved), but causes lipid precipitation. This dispersion was filtered in a 0.45 μM Millex HV filter (Millipore) and analyzed by HPLC. EE was calculated using the following formula: EE (%) = (filtered RA/total RA) × 100.
To investigate the influence of the amines on stability (RA retention in lipid matrix), two SLN formulations loaded with lipophilic BA or hydrophilic TA were prepared. The two SLN formulations were injected into 10 mL glass containers within a nitrogen atmosphere and were stored at 4°C. Sampling aliquots were withdrawn at 30 days, and the EE was determined as previously described.
High performance liquid chromatography (HPLC) consisted of a Waters 515 HPLC Pump (Milford, MA), a Waters 717 Plus Auto-sampler, and a photodiode array detector (Waters 2996). A C18 reverse-phase column (125 mm of length, 4 mm of width, and particles of 5 μm) (LichroCart 125–4, Merck, Darmstadt, Germany) was used. The mobile phase was a mixture of acetonitrile, distilled water, and phosphoric acid (80:19.9:0.1). The detection was carried out at 340 nm, with a flow rate of 1.0 mL/minute and 20 μL of sample. The five-point (0.25, 0.5, 1.0, 2.0, and 5.0 μg/mL) linear regression analysis resulted in the following linear equation: y = −2435 + 137200 × (r = 0.9998).
Cell cultures
Cell viability studies were conducted against both normal and cancer cells. Cancer cell lines (Jurkat, HL-60, HCT-116, and MCF-7) were cultured in RPMI or Dulbecco’s modified Eagle’s medium containing fetal bovine serum (10%), 200 mM glutamine, and antibiotics (100 μg/mL streptomycin and 100 UI/mL penicillin). All cultures were kept in a humidified incubator with 5% CO2 at 37°C. Normal cells (peripheral blood mononuclear cells; PBMCs) were obtained through agreement with the Minas Gerais Hematology and Hemotherapy Center Foundation – HEMOMINAS – (protocol n° 105/2004) from healthy adult volunteers of both sexes by centrifugation of heparinized venous blood over a Ficoll cushion. PBMCs were collected from the interphase after Ficoll separation and washed three times in RPMI before further processing.Citation20 All PBMC cultures were carried out in RPMI medium, supplemented with 5% (v/v) heat-inactivated, pooled AB sera, and 2 mM L-glutamine. An antibiotic/antimicotic solution containing 1000 U/mL penicillin, 1000 μg/mL streptomycin, and 25 μg/mL fungizone was added to control fungal and bacterial contamination.
Analysis of cell viability
Cell proliferation was measured by MTT assay based on the reduction of tetrazolium salt to formazan crystals by living cells.Citation21 Briefly, aliquots containing 7.0 × 103 (MCF-7), 9 × 103 (HL60), 1.8 × 104 (Jurkat and HCT-116), or 3.6 × 104 (PBMC) cells/well were seeded into 96-well plates. After 24 hours of incubation at 37°C and 5% CO2, freshly prepared solutions of free RA and SLNs were added to the wells (RA concentration ranged from 0.78 μM to 100 μM). Free RA was dissolved in absolute ethanol (4 mM) prior to dilution. After 48 hours of incubation at 37°C and 5% CO2, 20 μL of the 5 mg/mL MTT solution was added to each plate. Plates were incubated at 37°C for 4 hours, and then the medium was replaced by 200 μL of 0.04 M HCl solution in isopropanol. Cell viability was estimated by measuring the rate of mitochondrial reduction of MTT, determined by evaluating the absorbance of the converted dye at a wavelength of 595 nm. Absorbance values of the wells in which the cells were maintained in medium alone were considered as 100% of cell viability. Control groups included treatment with ethanol (negative control) and staurosporine (positive control). Cell viability was found to be 100% after treatment with negative control (ethanol), while staurosporine was effective in promoting cell-growth inhibition. Data were expressed as percentage of cell viability compared to the control (mean ± standard deviation [SD]). At least three independent experiments were performed.
Subdiploid DNA content and cell-cycle analysis
A flow-cytometric DNA fragmentation assay was employed as a quantitative measure of subdiploid content and phases of the cell cycle.Citation22 MCF-7 cells were plated at a density of 5 × 104 cells/well on 24-well plates and treated with free RA, RA-loaded SLNs, or blank SLNs for 48 hours (RA concentration was 25 μM). After this time, cells were centrifuged at 200 g (gravities) for 5 minutes at room temperature, and the culture medium was aspirated off. The pellet was gently resuspended in 300 μL of hypotonic fluorochrome solution containing 0.5% Triton X-100 and 50 μg/mL propidium iodide. Cells were incubated in the dark at 4°C for 4 hours and analyzed with a Guava® EasyCyte™ 6-2L Base System cytometer (Millipore). Data analysis was performed with FlowJo™ 7.6.5 (Tree Star Inc, Ashland, OR, USA), to determine percentages of subdiploid content and phases of the cell cycle.
Data analysis
Analysis of the cytotoxicity and cell-cycle studies were carried out using one-way ANOVA followed by Tukey’s test. For all analyses, the difference was considered significant when the P value was <0.05.
Results
Preparation and characterization of SLNs
The main characteristics of RA-loaded SLNs prepared with lipophilic (SA or BA) and hydrophilic (TA) amines are listed in . The mean particle size decreased from 155 ± 1 nm (SLNs without amine) to 104 ± 4 nm and 95 ± 1 for SLNs loaded with SA and BA lipophilic amines, respectively. Negatively charged particles were obtained for SLNs without amine (−30 ± 1 mV) and SLNs loaded with BA (zeta potential of −39 ± 0.2 mV). In contrast, using SA resulted in positively charged particles with a zeta potential of 20 ± 2 mV. These differences in particle charge can be explained by the fact that SA presents a higher potential for interfacial adsorption, in comparison to BA. In fact, the lipophilicity of SA is higher than that of BA (). It is noteworthy that EE for RA in SLNs increased from 36% ± 6% (SLNs without amine) to 97% ± 2% and 100% ± 1% for SLNs loaded with SA and BA, respectively. Therefore, SA and BA promoted a significant increase of EE for RA in SLNs. A plausible explanation for the EE increase could be the formation of an ion pairing between RA and the lipophilic amines. The ion pairing increases the lipophilic properties of the drug, making its incorporation into the lipid matrix easier. These findings are in agreement with previous observations that showed that the loading capacity in SLNs is related to drug lipophilicity.Citation23,Citation24
Table 1 Influence of the amine type on the particle size, polydis-persion index, zeta potential and EE of RA in SLNs
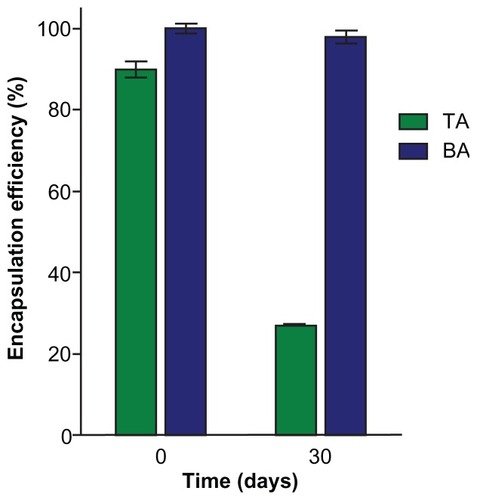
For the SLNs prepared with TA, a reduction in the particle diameter to 96 ± 1 nm was also observed. These SLNs were negatively charged (zeta potential of −33 ± 4 mV), and this can be explained by the fact that interfacial adsorption of hydrophilic TA is unlikely. TA also promoted a significant increase in EE for RA in SLNs (90% ± 2%), when compared with SLNs without amine (). However, as shown in , after 30 days of storage at 4°C, a dramatic decrease in EE for RA in RA-TA-loaded-SLNs was observed (from 90% ± 2% to 27% ± 0.1%). In significant contrast, the EE for BA-loaded SLNs was high immediately after preparation and remained constant after 30 days (98% ± 2%). These findings are in agreement with our previous data, which showed that it was possible to produce RA-loaded SLNs with high EE and stability by employing RA-amine ion pairing. Increasing the lipophilicity of the amine increases the drug-loading capacity in SLNs due to the increase in lipophilicity of the ion pair formed.Citation19
Cell viability studies
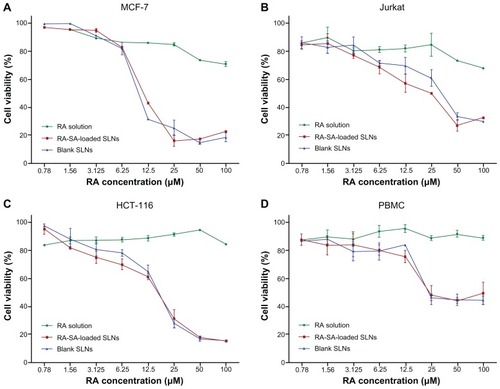
To investigate whether cytotoxicity of RA was affected by loading in SLNs containing amines, the cell viability assay using MTT was investigated in cancer and normal cells. First, the cell viability of SLNs loaded with lipophilic SA was assayed. Normal and human cancer cells were incubated with free RA, unloaded (blank) SLNs, or RA-SA-loaded SLNs and analyzed for their viability. The data obtained, expressed as cell viability (percentage), are shown in . The lowest antitumor activity was observed for the free RA (cell viability was approximately 80%), independent of the cell lines evaluated. MCF-7 cells were more sensitive to free RA, while the Jurkat and HCT-116 cells were the least sensitive. In contrast, the activity of SA-RA-loaded SLNs was much higher than that observed for the free RA, with MCF-7 cells showing greater sensitivity, compared with other cancer cells. However, the cytotoxic effects of unloaded (blank) SLNs against tumor cells were also high. It is interesting to note that these cytotoxic effects were also observed against normal cells (PBMCs). The cytotoxicity of the blank SLNs was attributed to the presence of the cationic lipophilic SA. In fact, blank SLNs without SA were prepared and their cytotoxic effects against MCF-7 cells investigated. These effects had disappeared completely (data not shown). Considering that the MCF-7 cells were more sensitive to RA, these cells were selected for further studies.
Figure 3 Cell viability studies, as evaluated by MTT assay, of blank SLNs, RA-SA-loaded SLNs, and free RA in cancer and normal cells, MCF-7 (A), Jurkat (B), HCT-116 (C), and PBMCs (D), after 48 hours of exposure.
Notes: Data were expressed as mean ± SD of three independent experiments. The blank SLNs were diluted at the same proportion as RA-loaded SLNs.
Abbreviations: MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; RA, all-trans retinoic acid; PBMC, peripheral blood mononuclear cells; SA, stearylamine; SD, standard deviation; SLN, solid lipid nanoparticle.
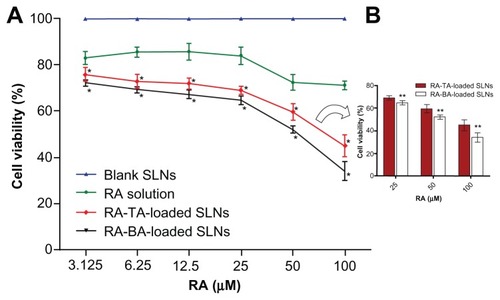
To investigate whether SLNs loaded with hydrophilic TA or lipophilic BA, which were used as amines for the in situ formation of an ion pairing with RA, would show the same pattern of activity, MCF-7 cancer cells were incubated with unloaded (blank) SLNs, RA-TA-loaded SLNs, RA-BA-loaded SLNs, or free RA and analyzed for their metabolic viability. The data obtained, expressed as percentage of MTT metabolism, are shown in . Interestingly, blank SLNs loaded with TA or BA showed no inhibitory effect, with cell viability near 100%. These data demonstrate that blank SLNs loaded with TA or BA present negligible cytotoxicity, when compared with the blank SLNs loaded with SA, and they are a potential new alternative for development of formulations for intravenous administration of RA. In addition, RA-loaded SLNs containing BA or TA presented cytotoxic effects significantly higher than those observed for the free RA in all drug concentrations tested; a dose-dependent relationship between drug concentration and cell viability was clearly observed. The maximum reductions in cell viability for RA-TA-loaded SLNs, RA-BA-loaded SLNs, and free RA, observed at the concentration 100 μM, were 45% ± 5%, 34% ± 4%, and 71% ± 2%, respectively. The differences between BA-loaded SLNs and TA-loaded SLNs were significant.
Figure 4 Cell viability studies, as evaluated by MTT assay, of blank SLNs, RA-TA-loaded SLNs, RA-BA-loaded SLNs and free RA in MCF-7 cells after 48-hour exposure.
Notes: Data were expressed as mean ± SD of three independent experiments. The data for blank SLNs, which were diluted at the same proportion as RA-loaded SLNs, represent overlapping values for TA-based SLNs and BA-based SLNs. aSignificant difference compared to free RA; bsignificant difference between TA-based SLNs and BA-based SLNs.
Abbreviations: MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; BA, benethamine; RA, all-trans retinoic acid; SLN, solid lipid nanoparticle; TA, triethylamine.
Subdiploid DNA content and cell-cycle analysis
In order to determine whether the improvement of the cytotoxic activity of RA after incorporation into BA-loaded SLNs was associated with alterations in cell-cycle progression and DNA fragmentation, we performed flow cytometry studies. We used the protocol described by Riccardi and Nicoletti,Citation22 that is based on the principle that apoptotic cells, among other typical features, are characterized by DNA fragmentation and, consequently, loss of nuclear DNA content. The use of a fluorochrome, such as propidium iodide, that is capable of binding and labeling DNA makes it possible to obtain a rapid and precise evaluation of cellular DNA content by flow-cytometric analysis and subsequent identification of hypodiploid cells. Representative histograms of DNA content after PI staining are shown in . The data are summarized in . The increase in subdiploid DNA content was negligible for the treatment with RA-TA-loaded SLNs (0.5% ± 0.20%) and free RA (0.4% ± 0.05%), when compared with the control. After 48 hours of treatment, the subdiploid DNA content for RA-BA-loaded SLNs (6.1% ± 0.65%) was significantly increased, inducing DNA fragmentation 12 and 17 times higher than that observed for the RA-TA-loaded SLNs and free RA, respectively. Blank SLNs (with TA or BA) showed no significant increase in subdiploid content in comparison with the control. These data suggest that BA-containing SLNs show more advantages as carriers of RA than the TA-loaded SLNs, as observed in the improvement of their cytotoxic effects.
Table 2 Effects of different treatments of MCF-7 cancer cells on DNA fragmentation and cell-cycle stage distribution
Figure 5 DNA fluorescence histograms of PI-stained MCF-7 cells after 48 hours of incubation under various experimental conditions: medium alone (A), blank SLNs (B), free RA (C), or BA-RA-loaded SLNs (D).
Notes: RA was used at 25 μM. BA-RA-loaded SLNs induce cell-cycle arrest accompanied by reduction in the S phase in MCF-7 cells. Data from one representative experiment that represents 20,000 events (cells) are shown.
Abbreviations: BA, benethamine; PI, propidium iodide; RA, all-trans retinoic acid; SLN, solid lipid nanoparticle.
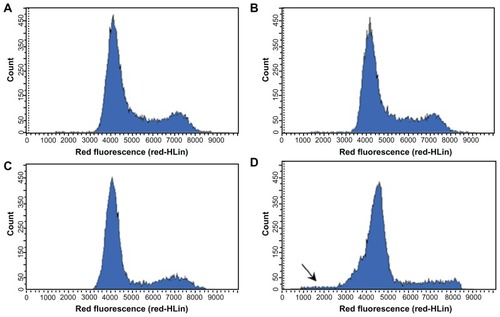
Data concerning the cell-cycle stage distribution clearly showed a significant increase in the G0/G1 phase after treatment with RA-loaded SLNs (73.0% ± 0.5% and 74% ± 0.6% for SLNs with TA and BA, respectively), when compared with the control (60.5% ± 1.0%). This increase was higher than that observed for the treatment with free RA (70.6% ± 0.5%). Also, the frequency of MCF-7 cells in the S-phase range of DNA content decreased. This reduction was more evident in SLNs with BA (8% ± 0.3%) than in SLNs with TA (12.4% ± 0.6%), when compared with the control (14.8% ± 0.7%). Free RA also showed a significant decrease in the S-phase (10.8% ± 0.1%), which was revealed to be lower than that observed for the treatment with RA-BA-loaded SLNs. These findings are in agreement with previous observations that showed that RA treatment of MCF-7 cells induces G1 arrest before inducing apoptosis.Citation24,Citation25
Discussion
SLNs have gained attention as particulate systems that improve the delivery of lipophilic drugs, such as RA, due to the high affinity of these molecules for the lipid matrix.Citation11,Citation12 However, unexpectedly, the EE for RA in SLNs is usually low, and the in situ formation of an ion pairing between RA and an amine provides an interesting alternative to enhance the drug encapsulation.Citation19 Therefore, this work aimed to develop, characterize, and evaluate the in vitro antitumor activity of SLNs loaded with RA and different amines. Three amines were evaluated: one hydrophilic (TA) and two lipophilic amines (SA and BA).
Marked differences between formulations were observed when the data concerning the RA encapsulation in SLNs were evaluated. Our data clearly show that EEs was significantly increased in SLNs containing SA, TA, or BA, when compared with SLNs without amines. Comparing the amines, the data show that the EEs for RA in SLNs loaded with lipophilic amines (BA and SA) were higher than that observed for SLNs containing TA. In addition, the stability (drug retention) of SLNs loaded with lipophilic BA was much higher than that observed for SLNs containing TA. Although TA is considered a stronger base than BA or SA (the pKb value for TA is lower than that for other amines), the differences in basicity among amines are relatively low. On the other hand, our data showed that RA retention in lipid matrix increases with the hydrophobicity of the counterion used for the ion pair formation, revealing that lipophilicity rather than basicity was the most relevant parameter. These findings are consistent with our previous observations, which showed that EE for RA in SLNs significantly increased when the lipophilicity of the amine increased, producing more-stable SLNs.Citation18 These findings demonstrate that the lipophilic amine triggered an intense interaction of RA with the lipid matrix.
Cytotoxic effects against both cancer and normal cells were investigated. It was clearly observed that in vitro antitumor activity of RA-SA-loaded SLNs was higher than that observed for free RA. Data for free RA compare with findings previously described.Citation25,Citation26 However, cytotoxic effects for unloaded (blank) SLNs were also high against both cancer and normal cells. This phenomenon was attributed to the cationic lipid (SA), since blank SLNs without SA showed no cytotoxic effects against MCF-7 cells. These data are in agreement with previous findings, which demonstrated that cationic lipid-stabilized SLNs or liposomes show cytotoxic effects. The proposed mechanisms of the cytotoxicity are the electrostatic interactions of these cationic amphiphilic molecules, with the anionic phospholipids of the cell membrane leading to membrane damage.Citation27–Citation29
To reduce the toxicity, SLNs loaded with TA or BA were produced and tested in MCF-7 cells. Blank SLNs, which displayed negatively charged particles, revealed no cytoxicity against the tested cancer cells. In contrast, RA-loaded SLNs showed a remarkable increase in antitumor activity against the human breast cancer cell line MCF-7, in comparison with the free RA. The reduction in cell viability observed for BA-loaded SLNs were significantly higher than that observed for TA-loaded SLNs, and differences between the two formulations were even more prominent when the data for subdiploid DNA content were evaluated. Furthermore, it was observed that RA-loaded SLNs were able to decrease the frequency of MCF-7 cells in the S-phase and to accumulate cells in the G0/G1-phases. These effects were more pronounced than those observed for free RA and are consistent with data reported previously.Citation30,Citation31
Previous studies showed no significant increase in the anticancer activity of RA-loaded lipid nanocarriers when compared to free RA.Citation8,Citation32 Su and colleaguesCitation33 showed that the cytoxicity of RA-loaded tributyrin nanoemulsion against hepatic or colonic cancer cells was higher than that of free RA. However, the combination of RA with tributyrin, a prodrug of butyric acid, was reported to have synergistic anticancer effects.Citation34,Citation35 Therefore, it is difficult to separate the effects of the combination of RA and tributyrin from those of drug encapsulation.
The present study reported for the first time a remarkable increase in anticancer activity of RA-BA-loaded SLNs in comparison with free RA. The in situ formation of an ion pairing between RA and BA favors drug retention in lipid matrix. After dilution of the nanoparticles in the culture medium, two opposing scenarios can occur: RA may be released (bound or not to the amine) or remain associated with the SLNs. If RA is released from SLNs as an ion pair, reverse ion exchange can occur, with ions from the culture medium substituting the counterions (amines), resulting in the reformation of the parent drug.Citation36 In this case, it would be expected that the activity of the SLNs was similar to that observed for the free RA. Considering that our data showed a higher activity for the SLNs, the most probable hypothesis is that RA remained associated with the lipid matrix of the SLNs after dilution in the medium, enabling improved drug uptake by the tumor cells.Citation37 Previous studies have reported enhanced – despite negatively charged particles – intracellular levels of anticancer drugs loaded in SLNs.Citation38
Finally, studies have pointed out the important role RA plays in the control of metabolic diseases such as diabetes and obesity through its activation of the retinoid X receptor and its heterodimers pathways. Treatment with RA can reduce body weight and adiposity, and enhance glucose tolerance and insulin sensitivity.Citation39,Citation40 To improve bioavailability, RA has been administered orally as an emulsion and subcutaneously as an oily solution. For novel formulations, the ion pair formation in SLNs can be explored as a strategy to improve the efficacy and bioavailability of RA in the treatment of obesity and diabetes.
Conclusion
In summary, an SLN formulation loaded with RA, a lipophilic acid, and a lipophilic amine (BA) was designed and evaluated. It was possible to obtain high encapsulation efficiency (almost 100%) for RA in SLNs. Moreover, RA-BA-loaded SLNs promoted enhanced in vitro antitumor activity when compared to the free RA and other SLN formulations. These findings suggest that the RA-BA-loaded SLN formulation is a promising alternative for the administration of RA in the treatment of cancer.
Acknowledgments
This study was supported by the Minas Gerais State Agency for Research and Development (FAPEMIG, Brazil) and by the Brazilian agencies CAPES and CNPq. The authors wish to thank Glenn Hawes from the American Language Program of the University of Georgia for editing this manuscript.
Disclosure
The authors state no conflict of interest and have received no payment in preparation of this manuscript. All authors have approved the final article.
References
- TangXHGudasLJRetinoids, retinoic acid receptors, and cancerAnnu Rev Pathol2011634536421073338
- NilesRMSignaling pathways in retinoid chemoprevention and treatment of cancerMutat Res20045551–2819615476854
- BushueNWanYJRetinoid pathway and cancer therapeuticsAdv Drug Deliv Rev201062131285129820654663
- AdamsonPCPitotHCBalisFMRubinJMurphyRFPoplackDGVariability in the oral bioavailability of all-trans-retinoic acidJ Natl Cancer Inst199385129939968388479
- MuindiJRFrankelSRHuseltonCClinical pharmacology of oral all-trans retinoic acid in patients with acute promyelocytic leukemiaCancer Res1992528213821421559217
- AbdulmajedKHeardCMTopical delivery of retinyl ascorbate co-drug. 1. Synthesis, penetration into and permeation across human skinInt J Pharm20042801–211312415265552
- ChungKDJeongYIChungCWKim doHKangDHAnti-tumor activity of all-trans retinoic acid-incorporated glycol chitosan nanoparticles against HuCC-T1 human cholangiocarcinoma cellsInt J Pharm20124221–245446122093956
- LimSJLeeMKKimCKAltered chemical and biological activities of all-trans retinoic acid incorporated in solid lipid nanoparticle powdersJ Control Release20041001536115491810
- ChansriNKawakamiSYamashitaFHashidaMInhibition of liver metastasis by all-trans retinoic acid incorporated into O/W emulsions in miceInt J Pharm20063211–2424916790329
- KawakamiSSuzukiSYamashitaFHashidaMInduction of apoptosis in A549 human lung cancer cells by all-trans retinoic acid incorporated in DOTAP/cholesterol liposomesJ Control Release2006110351452116360957
- MullerRHMaderKGohlaSSolid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the artEur J Pharm Biopharm200050116117710840199
- JenningVLippacherAGohlaSHMedium scale production of solid lipid nanoparticles (SLN) by high pressure homogenizationJ Microencapsul200219111011811751
- JoshiMDMullerRHLipid nanoparticles for parenteral delivery of activesEur J Pharm Biopharm200971216117218824097
- JenningVGohlaSHEncapsulation of retinoids in solid lipid nanoparticles (SLN)J Microencapsul200118214915811253932
- LimSJKimCKFormulation parameters determining the physicochemical characteristics of solid lipid nanoparticles loaded with all-trans retinoic acidInt J Pharm20022431–213514612176302
- HuLTangXCuiFSolid lipid nanoparticles (SLNs) to improve oral bioavailability of poorly soluble drugsJ Pharm Pharmacol200456121527153515563759
- KayaliIWardAJSuheryTFribergSESimionARheinLDInteractions of retinoic acid with a model of stratum corneum lipidsJ Dermatol Clin Eval Soc19912717
- CastroGAOreficeRLVilelaJMAndradeMSFerreiraLADevelopment of a new solid lipid nanoparticle formulation containing retinoic acid for topical treatment of acneJ Microencapsul200724539540717578730
- CastroGACoelhoALOliveiraCAMahechaGAOreficeRLFerreiraLAFormation of ion pairing as an alternative to improve encapsulation and stability and to reduce skin irritation of retinoic acid loaded in solid lipid nanoparticlesInt J Pharm20093811778319647057
- Souza-FagundesEMQueirozABMartins FilhoOAScreening and fractionation of plant extracts with antiproliferative activity on human peripheral blood mononuclear cellsMem Inst Oswaldo Cruz20029781207121212563491
- MosmannTRapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assaysJ Immunol Methods1983651–255636606682
- RiccardiCNicolettiIAnalysis of apoptosis by propidium iodide staining and flow cytometryNat Protoc2006131458146117406435
- MunsterUNakamuraCHaberlandARU 58841-myristate – prodrug development for topical treatment of acne and androgenetic alopeciaPharmazie200560181215700772
- Schafer-KortingMMehnertWKortingHCLipid nanoparticles for improved topical application of drugs for skin diseasesAdv Drug Deliv Rev200759642744317544165
- FanjulANBouterfaHDawsonMPfahlMPotential role for retinoic acid receptor-gamma in the inhibition of breast cancer cells by selective retinoids and interferonsCancer Res1996567157115778603404
- HongTKLee-KimYCEffects of retinoic acid isomers on apoptosis and enzymatic antioxidant system in human breast cancer cellsNutr Res Pract200932778320016705
- RobertsWRAddyMComparison of the in vivo and in vitro antibacterial properties of antiseptic mouthrinses containing chlorhexidine, alexidine, cetyl pyridinium chloride and hexetidine. Relevance to mode of actionJ Clin Periodontol1981842953106947993
- LappalainenKJaaskelainenISyrjanenKUrttiASyrjanenSComparison of cell proliferation and toxicity assays using two cationic liposomesPharm Res1994118112711317971713
- ScholerNOlbrichCTabattKMullerRHHahnHLiesenfeldOSurfactant, but not the size of solid lipid nanoparticles (SLN) influences viability and cytokine production of macrophagesInt J Pharm20012211–2576711397567
- ZhuWYJonesCSKissAMatsukumaKAminSDe LucaLMRetinoic acid inhibition of cell cycle progression in MCF-7 human breast cancer cellsExp Cell Res199723422932999260897
- MangiarottiRDanovaMAlbericiRPellicciariCAll-trans retinoic acid (ATRA)-induced apoptosis is preceded by G1 arrest in human MCF-7 breast cancer cellsBr J Cancer19987721861919460987
- HwangSRLimSJParkJSKimCKPhospholipid-based microemulsion formulation of all-trans-retinoic acid for parenteral administrationInt J Pharm20042761–217518315113624
- SuJZhangNHoPCEvaluation of the pharmacokinetics of all-trans-retinoic acid (ATRA) in Wistar rats after intravenous administration of ATRA loaded into tributyrin submicron emulsion and its cellular activity on caco-2 and HepG2 cell linesJ Pharm Sci20089772844285317879972
- ChenZXBreitmanTRTributyrin: a prodrug of butyric acid for potential clinical application in differentiation therapyCancer Res19945413349434998012972
- GiermaszANowisDJaliliAAntitumor activity of tributyrin in murine melanoma modelCancer Lett2001164214314811179828
- LengsfeldCSPiteraDManningMRandolphTWDissolution and partitioning behavior of hydrophobic ion-paired compoundsPharm Res200219101572157612425478
- MartinsSCosta-LimaSCarneiroTCordeiro-da-SilvaASoutoEBFerreiraDCSolid lipid nanoparticles as intracellular drug transporters: an investigation of the uptake mechanism and pathwayInt J Pharm20124301–221622722465548
- SerpeLCatalanoMGCavalliRCytotoxicity of anticancer drugs incorporated in solid lipid nanoparticles on HT-29 colorectal cancer cell lineEur J Pharm Biopharm200458367368015451544
- AltucciLLeibowitzMDOgilvieKMde LeraARGronemeyerHRAR and RXR modulation in cancer and metabolic diseaseNat Rev Drug Discov200761079381017906642
- BonetMLRibotJPalouALipid metabolism in mammalian tissues and its control by retinoic acidBiochim Biophys Acta20121821117718921669299