 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A hyperbranched cationic polysaccharide derivative-mediated small interfering (si)RNA interference strategy was proposed to inhibit nuclear transcription factor-kappa B (NF-κB) activation in human retinal pigment epithelial (hRPE) cells for the gene therapy of diabetic retinopathy. Two hyperbranched cationic polysaccharide derivatives containing the same amount of cationic residues, but with different branching structures and molecular weights, including 3-(dimethylamino)-1-propylamine-conjugated glycogen (DMAPA-Glyp) and amylopectin (DMAPA-Amp) derivatives, were developed for the efficient delivery of NF-κB siRNA into hRPE cells. The DMAPA-Glyp derivative showed lower toxicity against hRPE cells. Furthermore, the DMAPA-Glyp derivative more readily condensed siRNA and then formed the nanoparticles attributed to its higher branching architecture when compared to the DMAPA-Amp derivative. Both DMAPA-Glyp/siRNA and DMAPA-Amp/siRNA nanoparticles were able to protect siRNA from degradation by nuclease in 25% fetal bovine serum. The particle sizes of the DMAPA-Glyp/siRNA nanoparticles (70–120 nm) were smaller than those of the DMAPA-Amp/siRNA nanoparticles (130–180 nm) due to the higher branching architecture and lower molecular weight of the DMAPA-Glyp derivative. In addition, the zeta potentials of the DMAPA-Glyp/siRNA nanoparticles were higher than those of the DMAPA-Glyp/siRNA nanoparticles. As a result, siRNA was much more efficiently transferred into hRPE cells using the DMAPA-Glyp/siRNA nanoparticles rather than the DMAPA-Amp/siRNA nanoparticles. This led to significantly high levels of suppression on the expression levels of NF-κB p65 messenger RNA and protein in the cells transfected with DMAPA-Glyp/siRNA nanoparticles. This work provides a potential approach to promote hyperbranched polysaccharide derivatives as nonviral siRNA vectors for the inhibition of NF-κB activation in hRPE cells.
Introduction
Diabetic retinopathy is a common complication of diabetes that can cause retinal damage eventually leading to blindness, and retinal neovascularization is regarded as one of the most important causes of this complication.Citation1 Clinically, the available therapeutic regimens are laser photocoagulation and blocking neovascularization through multiple intravitreal injections of drugs such as antivascular endothelial growth factor agents, protein kinase C inhibitors, and triamcinolone acetonide.Citation2–Citation5 But these effects are transient and require multiple reinjections. As a result, these therapies are usually associated with several undesirable complications such as vitreous hemorrhage, glaucoma, retinal detachment, and endophthalmitis.Citation2,Citation6
An RNA interference approach, especially using small interfering (si)RNAs, has recently received much attention in treating ocular diseases.Citation7,Citation8 As a therapeutic method to block neovascularization, the siRNA approach has incomparable long-term effects and safety effects. It was reported that nuclear transcription factor-kappa B (NF-κB) played an important role in the angiogenesis of diabetic retinopathy.Citation9–Citation11 On the other hand, human retinal pigment epithelial (hRPE) cells form a monolayer between the neuroretina and the choriocapillaris, which are the essential components of the outer blood retinal barrier that maintain physiological and structural balance within the retina.Citation12 Previous work showed that hRPE cells could synthesize the secreted angiogenic peptide vascular permeability factor/vascular endothelial growth factor,Citation13 and it played a major role in proliferative diabetic retinopathy.Citation14 Therefore, hRPE cells may serve as a potential target for drug delivery and gene transfer leading to diabetic retinopathy.Citation6,Citation12,Citation15 With all the aforementioned research in mind, the decision was taken to investigate the inhibition of NF-κB activation through the siRNA strategy in hRPE cells.
Although siRNA has a small size, siRNA transportation across the cellular membrane is hindered due to its hydrophilicity and negative charge. In addition, siRNA is quickly cleared during in vivo circulation before reaching the target disease site.Citation16 Therefore, successful siRNA-mediated therapy would heavily rely on the development of effective siRNA delivery vectors with high transfection efficiencies and minimal cytotoxicities.Citation16,Citation17 Adeno-associated virus-mediated gene therapy has shown promise in several retinal pigment epithelium (RPE) clinical trials, but adeno-associated virus has a limited payload capacity and potential immunogenicity.Citation18 Nonviral gene delivery vectors, especially cationic polymers (eg, polyethyleneimine, chitosan, and polylysine peptides) and liposomes, have attracted much attention in the RPE gene therapeutic strategy.Citation18 Recent work has established that the architecture of cationic polymers affects the stability, delivery, cytotoxicity, and transfection efficiencies of their complexes with genes.Citation19–Citation22 Compared to linear cationic polymers, hyperbranched cationic polymers exhibit higher levels of gene expression and lower cytotoxicity.Citation19–Citation22 Ahmed et alCitation23 reported that hyperbranched glycopolymers were hemocompatible in vitro and featured less toxicity in response to hyperbranched polymers. Amylopectin and glycogen are naturally hyperbranched polysaccharides with nontoxicity and good biocompatibility, and they are also biodegradable.Citation24–Citation27 Both of them are composed of α-D-(1→4) glucose units of the linear chains, which are interlinked by α-D-(1→6) glycosidic linkage forming the branched structure.Citation24–Citation27 In contrast to amylopectin, the fraction of branching units for glycogen is higher at 8%, whereas the value is approximately 5% for amylopectin.Citation25–Citation27 Additionally, the weight average molecular weight (Mw) of glycogen (106−107g/mol) is usually smaller than that of amylopectin (106−108 g/mol).Citation24–Citation26 We recently reported that both hyperbranched cationic amylopectin and glycogen derivatives conjugated with 3-(dimethylamino)-1-propylamine (named as DMAPA-Amp and DMAPA-Glyp, respectively; ) exhibited good blood compatibility and low cytotoxicity, and could effectively deliver plasmid DNA in vitro.Citation28,Citation29 However, whether hyperbranched polysaccharide derivatives can effectively deliver siRNA into hRPE cells is unclear.
Figure 1 A speculated hyperbranched structure and chemical structure of the cationic polysaccharide derivatives.
Notes: (A) A speculated hyperbranched structure and (B) chemical structure of the cationic polysaccharide derivatives (n=9–13 for the DMAPA-Glyp derivative, and n=19–24 for the DMAPA-Amp derivative. The n-values were based on those of glycogen and amylopectin).Citation24,Citation27
Abbreviations: n, number; DMAPA-Glyp, 3-(dimethylamino)-1-propylamine-conjugated glycogen; DMAPA-Amp, 3-(dimethylamino)-1-propylamine-conjugated amylopectin.

In this work, the siRNA approach was used to knockdown the NF-κB p65 subunit and inhibited the function of NF-κB, since p65 has been shown to be a key active subunit in NF-κB transcription on hRPE cells.Citation9–Citation11 Nanoparticles based on two hyperbranched cationic polysaccharide derivatives containing the same amount of cationic residues, but different branching structures and molecular weights, including DMAPA-Glyp and DMAPA-Amp derivatives, were developed for the delivery of siRNA into hRPE cells. To optimize one hyperbranched cationic polysaccharide derivative as a potential siRNA delivery vector, the effects of branching structures and molecular weights of these derivatives on cytotoxicity, siRNA delivery efficacy, and NF-κB gene silencing efficiency were investigated.
Materials and methods
Materials
Glycogen (from oysters), amylopectin (from maize), and branched polyethylenimine (bPEI) (Mw =2.5×104 g/mol) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). The Mw values of glycogen and amylopectin were determined to be 1.5×106 g/mol and 3.1×107 g/mol by static light scattering, respectively. Synthesis of two hyperbranched cationic polysaccharide derivatives (ie, DMAPA-Amp and DMAPA-Glyp) was described in detail in the Supplementary materials. The degree of substitution of DMAPA residues on the polysaccharide, which is defined as the number of DMAPA residues per glucose unit of polysaccharides, was determined to be 2.8 for both polysaccharide derivatives using 1H nuclear magnetic resonance spectroscopy. The weight average molecular weights of the DMAPA-Glyp and DMAPA-Amp were determined to be 4.1×106 g/mol and 8.6×107 g/mol according to the degrees of substitution and the Mw values of the native of glycogen and amylopectin ().
Table 1 Characterization of the cationic hyperbranched polysaccharide derivatives and properties of the polysaccharide derivative/siRNA nanoparticles
Lipofectamine 2000 (Lip2000), fetal bovine serum (FBS), Dulbecco’s Modified Eagle’s Medium (DMEM), penicillin–streptomycin, trypsin, and Opti-MEM® were purchased from Invitrogen Co. (Carlsbad, CA, USA). Hoechst 33258 was purchased from the Beyotime Institute of Biotechnology (Shanghai, People’s Republic of China), and Cell Counting Kit-8 (CCK-8) from Dojindo Laboratories (Kumamoto, Japan). A siRNA duplex was designed to target human the NF-κB p65 gene based on the public GenBank and applied by Guangzhou RiboBio Co., Ltd. (Guangzhou, People’s Republic of China). It is a 21 bp double-stranded RNA oligos with dTdT 3′ overhangs and has sequences as follows: (sense) 5′-GGACAUAUGAGACCUUCAAdTdT-3′; and (antisense) 5′-UUGAAGGUCUCAUAUGUCCdTdT-3′. A 5′-cy5-labeled nonspecific siRNA duplex (cy5-siRNA) was prepared as a control in the sequence as follows: (sense) 5′-cy5-UUCUCCGAACGUGUCACGUdTdT-3′; and (antisense) 5′-ACGUGACACGUUCGGAGAAdTdT-3′. A nonspecific siRNA duplex was also prepared in the same sequence without cy5 labeling.
Preparation of the hyperbranched polysaccharide derivatives/siRNA complexes
The cationic polysaccharide derivatives were dissolved in RNase-free water (Takara Bio Inc, Kyoto, Japan) at a concentration of 1 mg/mL. Lyophilized siRNA was dissolved in RNase-free water to form a stock solution of 20 μM. A portion of siRNA solution (2.5 μL) was diluted in 200 μL of Opti-MEM® and incubated at room temperature for 5 minutes. Then, the polysaccharide derivative solutions at different polysaccharide derivative/siRNA weight ratios were added to this solution. The resulting mixtures were gently agitated for 10 seconds on a vortex agitator before being incubated at room temperature for 20 minutes prior to use. The nanoparticles containing cy5-siRNA were prepared in the same way in the dark circumstance.
Agarose gel electrophoresis
To assess the condensation ability of the cationic polysaccharide derivatives to siRNA, electrophoresis tests were performed. Ten microliters of the polysaccharide derivative/siRNA nanoparticles with different polysaccharide derivative/siRNA weight ratios in the range of 0.5–20 and naked siRNA were loaded onto 2% agarose gels containing 3 μL of Goldview fluorescence reagent (SBS Genetech Co. Ltd., Beijing, People’s Republic of China) and run with Tris-acetate running buffer at 120 V for 15 minutes. siRNA retardation was then observed and photographed under ultraviolet illumination using an INFINITY 3026 gel image machine (Vilber Lourmat Deutschland GmbH, Eberhardzell, Germany).
Serum stability study
To determine the protective property of the cationic polysaccharides against siRNA degradation, the cationic polysaccharide derivative/siRNA nanoparticles with several weight ratios ranging from 5–20 were incubated with 25% FBS at 37°C for 24 hours, based on the method used in the literature.Citation30 Meanwhile, free siRNA was incubated with 25% FBS as the negative control. Samples were then incubated for 1 hour with excess heparin solution at a heparin/siRNA weight ratio of 5 to ensure complete release of siRNA from the nanoparticles. The samples, as well as an equal weight of intact siRNA without FBS addition, as well as FBS of the same volume, were assessed via agarose gel electrophoresis assay, as described earlier, to examine the integrity of siRNA.
Characterization of the cationic polysaccharide derivative/siRNA nanoparticles
The solutions of polysaccharide derivative/siRNA nanoparticles with different weight ratios (2 mL) were prepared in double-distilled water to a final concentration of 1.0 μg/mL for siRNA, as mentioned previously. Following filtration through a membrane filter (nominal pore size of 0.45 μm), the nanoparticle sizes were measured by dynamic light scattering using ZetaPALS (Brookhaven Instruments Corporation, Holtsville, NY, USA) at 25°C with a 90° scattering angle. The zeta potentials were also evaluated using the same instrument. All of the measurements were performed in triplicate. The morphology of the polysaccharide derivative/siRNA nanoparticles (w/w=20) was observed on an S-4800 scanning electron microscope (HI-9056-0003; Hitachi Ltd., Tokyo, Japan), after the samples were sputter-coated with gold in an E-1045 ion sputter (Hitachi Ltd.).
Cell culture
D407 hRPE cells were provided by the ophthalmic laboratory of the Zhongshan Ophthalmic Center of Sun Yat-sen University (Guangzhou, People’s Republic of China), and this study was approved by the Sun Yat-sen Memorial Hospital’s Ethics Committee (ethics number: 2010-06). The cells were cultured in DMEM containing 10% FBS and 1% antibiotic mixtures of 100 U/mL penicillin G and 100 μg/mL streptomycin (Gibco®; Thermo Fisher Scientific). Before performing a transfection experiment, the cells, which were further identified by immunocytochemical staining, as described in detail in , were grown to 30%–40% confluency in a humidified 5% CO2 environment at 37°C.
Cytotoxicity assay
Cytotoxicity of the polysaccharide derivatives and the polysaccharide derivative/siRNA nanoparticles was evaluated using the CCK-8 assay.Citation31 Detailed information was provided in the Supplementary materials.
Evaluation of cell uptake efficiency
To evaluate hRPE cell uptake efficiency of the polysaccharide derivative/cy5-siRNA nanoparticles, flow cytometry assay and laser confocal microscopy were employed. The detailed information was provided in the Supplementary materials.
Evaluation of suppression on NF-κB p65 gene expression
hRPE cells were plated in six-well plates and cultured for 24 hours, following incubation with the polysaccharide derivative/siRNA nanoparticles (w/w=10) for 6 hours, as mentioned previously. Afterward, the medium was changed with fresh DMEM containing 10% FBS. Following an additional 24 hours, 48 hours, and 72 hours of incubation, the medium was removed, and the cells were collected for RNA extraction. Normally, cultured hRPE cells without any treatment were used as the control, and hRPE cells incubated with the Lip2000/nonspecific siRNA complex served as the negative control. The expression levels of NF-κB p65 mRNA and protein were then evaluated in hRPE cells, using semiquantitative and quantitative real-time reverse transcription polymerase chain reaction (PCR) assays, as well as Western blot assay. Details were supplied in the Supplementary materials.
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (SPSS software, version 13.0; IBM Corporation, Armonk, NY, USA). Results were expressed as the mean ± standard deviation, and a value of P<0.05 was considered statistically significant.
Results and discussion
Formation of the cationic polysaccharide derivative/siRNA complexes
Hyperbranched cationic polymers have recently received much attention as a nonviral gene delivery vector because of their high levels of gene expression.Citation19–Citation23 With this in mind, two hyperbranched cationic polysaccharide derivatives containing the same amount of cationic residues, but different branching structures and molecular weights, were developed for delivery of siRNA into hRPE cells to silence NF-κB gene expression ( and ).
The formation of the cationic polysaccharide derivative/siRNA complexes was examined by agarose gel electrophoresis assay using naked siRNA as a control. As shown in , the migration of siRNA was completely retarded when the DMAPA-Amp derivative/siRNA and the DMAPA-Glyp derivative/siRNA weight ratios exceeded 10 and 5, respectively. The results indicated that both hyperbranched cationic polysaccharide derivatives could condense siRNA to the complexes, which may facilitate cell uptake while providing protection from nuclease degradation. The DMAPA-Glyp derivative formed the complex with siRNA at lower polysaccharide derivative/siRNA weight ratios, attributed to its higher branching architecture, when compared to the DMAPA-Amp derivative. This was in agreement with the literature, which reported that higher branched polymers showed stronger complexation and condensation of nucleic acid.Citation19,Citation21
Figure 2 Agarose gel electrophoresis retardation assay and protection and release assay of siRNA.
Notes: (A) Agarose gel electrophoresis retardation assay of the polysaccharide derivative/siRNA complexes at different weight ratios: (a) DMAPA-Amp/siRNA and (b) DMAPA-Glyp/siRNA; (B) Protection and release assay of siRNA in FBS (25%). (a) naked siRNA; (b) naked siRNA incubated with FBS; (c) FBS; (d–f) the DMAPA-Amp/siRNA complexes (w/w=10, w/w=15, and w/w=20) incubated with FBS; (g–i) the DMAPA-Glyp complexes (w/w=5, w/w=10, and w/w=20) incubated with FBS.
Abbreviations: siRNA, small interfering RNA; DMAPA-Amp, 3-(dimethylamino)-1-propylamine-conjugated amylopectin; DMAPA-Glyp, 3-(dimethylamino)-1-propylamine-conjugated glycogen; FBS, fetal bovine serum.

Zeta potentials and particle sizes
As shown in , the zeta potentials became positive after the polysaccharide derivative/siRNA complexes formed. Moreover, the zeta potentials of the polysaccharide derivative/siRNA complexes increased when increasing their weight ratios in the range of +30–+40 mV, which is amenable to the effective condensation of siRNA. Meanwhile, the volumes of the polysaccharide derivative/siRNA complexes decreased when increasing the weight ratios in the range of 100–200 nm ie, the complexes became progressively smaller during the siRNA condensation process (). The particle size of the DMAPA-Glyp/siRNA complexes was smaller than that of the DMAPA-Amp/siRNA complexes due to the higher branching architecture and lower molecular weight of the DMAPA-Glyp derivative ().
Figure 3 Zeta potentials and particle sizes of hyperbranched polysaccharide derivative/siRNA complexes at various weight ratios, as well as SEM images siRNA complexes.
Notes: (A) Zeta potentials and (B) particle sizes of the hyperbranched polysaccharide derivative/siRNA complexes at various weight ratios. (C) SEM images of (a) the DMAPA-Glyp/siRNA complexes and (b) the DMAPA-Amp/siRNA complexes (w/w=10).
Abbreviations: DMAPA-Amp, 3-(dimethylamino)-1-propylamine-conjugated amylopectin; siRNA, small interfering RNA; DMAPA-Glyp, 3-(dimethylamino)-1-propylamine-conjugated glycogen; SEM, scanning electron microscopy.
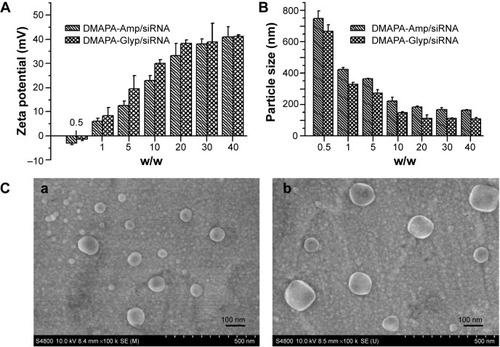
The morphology of polysaccharide derivative/siRNA complexes was investigated by scanning electron microscopy. As shown in , the complexes at a weight ratio of 20 were observed as nanoparticles with a spherical shape. The size of the DMAPA-Glyp/siRNA nanoparticles (70–120 nm) was significantly smaller than that of the DMAPA-Amp/siRNA nanoparticles (130–180 nm), which is in agreement with the results of the particle size analysis from .
Serum stability
It is considered that, as with cationic polymer/siRNA complexes, high serum stability and low cytotoxicity are essential for successful siRNA delivery both in vitro and, especially, in vivo.Citation30 The serum stability of hyperbranched polysaccharide/siRNA complexes was assessed in 25% FBS (). Naked siRNA was completely degraded by nuclease with 24-hour incubation (). However, the migration of siRNA was observed after the polysaccharide derivatives/siRNA complexes were incubated with FBS and treated with heparin solution (). This demonstrated that both hyperbranched polysaccharides were able to efficiently protect siRNA from degradation by nuclease in serum and release complexed siRNA in the presence of heparin. On the other hand, the weak bands were observed during the siRNA migration process (), which were probably attributed to some components existing in FBS when compared with the weak bands of FBS in .
Cytotoxicity
Cytotoxicity of the polysaccharide derivatives was evaluated in hRPE cells using the CCK-8 assay (). Both the DMAPA-Glyp and DMAPA-Amp derivatives showed significantly lower cytotoxicity against hRPE cells when compared to bPEI. In comparison, the DMAPA-Glyp derivative showed lower toxicity against hRPE cells. This may be related to the lower molecular weight of the DMAPA-Glyp derivative and the different biocompatibilities of two native polysaccharides as well. That is, glycogen is from animals and exhibits better biocompatibility for hRPE cells than does amylopectin, which comes from plants.
Figure 4 Viability and morphology of hRPE cells.
Notes: (A) Viability of hRPE cells incubated with the hyperbranched polysaccharide derivatives and bPEI at various concentrations for 24 hours. Both the DMAPA-Glyp and DMAPA-Amp derivatives showed significantly lower cytotoxicities at different concentrations in the range from 10–1,000 μg/mL (n=5; *P<0.05, when compared with the bPEI at the same concentration). (B) Viability of hRPE cells incubated with the hyperbranched polysaccharide derivative/siRNA complexes and the bPEI/siRNA complex at various weight ratios for 24 hours. Both the DMAPA-Glyp/siRNA and DMAPA-Amp/siRNA nanoparticles showed significantly lower cytotoxicities at the w/w ratios above 5 in the hRPE cells. (n=5; *P<0.05, when compared with the bPEI/siRNA complex at the same weight ratio). (C) Morphology of the hRPE cells incubated with the polymer/siRNA complexes for 24 hours (w/w=10, ×100): (a) the control group; (b) the DMAPA-Glyp/siRNA complex group; (c) the DMAPA-Amp/siRNA complex group; and (d) the bPEI/siRNA complex group.
Abbreviations: DMAPA-Glyp, 3-(dimethylamino)-1-propylamine-conjugated glycogen; DMAPA-Amp, 3-(dimethylamino)-1-propylamine-conjugated amylopectin; bPEI, branched polyethylenimine; siRNA, small interfering RNA; hRPE, human retinal pigment epithelial; n, number.
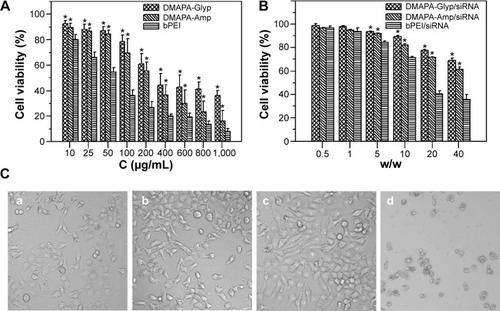
Although a positive charge was known to facilitate the cell uptake of nanoparticles through a nonspecific electrostatic interaction between the nanoparticles and negatively-charged cell membranes, it was also considered a major cause for cytotoxicity.Citation32 Therefore, it was important to evaluate the cytotoxicity of the polysaccharide derivative/siRNA nanoparticles formed at different w/w ratios. As shown in , the polysaccharide derivative/siRNA nanoparticles exhibited much lower cytotoxicity than did the bPEI/siRNA complexes in the hRPE cells, determined using the CCK-8 assay, owing to lower cytotoxicities of the polysaccharide derivatives. For example, as the w/w ratio reached 20, cells transfected with the polysaccharide derivative/siRNA nanoparticles still retained a relatively high viability of about 80%, while the cell viability reduced to 40% in the cells transfected with the bPEI/siRNA complex. Furthermore, it was found that the cytotoxicity of the DMAPA-Glyp/siRNA nanoparticles were lower than those of the DMAPA-Amp/siRNA nanoparticles due to the lower cytotoxicity of the DMAPA-Glyp derivative.
The morphology of hRPE cells incubated with the polysaccharide derivative/siRNA nanoparticles and the bPEI/siRNA complex is shown in . The untreated hRPE cells were used as the control (). hRPE cells incubated with both the DMAPA-Glyp/siRNA and DMAPA-Amp/siRNA nanoparticles showed no obvious change () in contrast with the control. However, hRPE cells incubated with the bPEI/siRNA complex were destroyed or shrunken, suggesting strong cytotoxicity ().
Study on cell uptake
The cell uptake of cationic polymers/gene complexes is assumed to be dependent on their surface properties and particle sizes. It has been reported that cationic polymers/gene complexes with a positive surface charge and with sizes between 50 nm to several hundred nanometers would be suitable for the endocytosis of complexes and efficient gene delivery.Citation33 Based on the aforementioned results, the zeta potentials and particle sizes of both polysaccharide derivative/siRNA nanoparticles were in the range of +30–+40 mV and 100–200 nm, respectively. As expected, these properties enabled the polysaccharide derivative/siRNA nanoparticles to enter the hRPE cells feasibly. The hRPE cell uptake of the polysaccharide/siRNA nanoparticles was evaluated using flow cytometry; this was compared with the bPEI/cy5-siRNA complex and the Lip2000/cy5-siRNA complex as the positive controls, and the naked cy5-siRNA as the negative control ( and ). It was found that there were significantly more fluorescent cells than nonfluorescent cells in , and in , indicating that cy5-siRNA could be effectively transferred into hRPE cells mediated by the DMAPA-Glyp and DMAPA-Amp derivatives. Cell uptake efficiency was then quantitative determined by scoring the percentage of cy5-positive hRPE cells, as shown in . The results indicated that the cell uptake efficiency generally increased when increasing the weight ratios of the polymer/siRNA nanoparticles. Moreover, siRNA was much more efficiently transferred into hRPE cells using the DMAPA-Glyp/siRNA nanoparticles rather than the DMAPA-Amp/siRNA nanoparticles. This is attributed to the smaller sizes and higher zeta potentials of the DMAPA-Glyp/siRNA nanoparticles than those of the DMAPA-Amp/siRNA nanoparticles. It should be noted that the cell uptake efficiency of the DMAPA-Glyp/cy5-siRNA nanoparticles (w/w=20) was close to that of the Lip2000/cy5-siRNA complex and the bPEI/cy5-siRNA complex (w/w=20), implying the high cell uptake efficiency of DMAPA-Glyp/cy5-siRNA nanoparticles.
Figure 5 Flow cytometric analysis, quantitative determination of cell uptake efficiency, and laser scanning confocal images of hRPE cells.
Notes: (A) Flow cytometric analysis of cy5-positive hRPE cells following incubation of hRPE cells with the different complexes at a cy5-siRNA final concentration of 50 nM: (a) the DMAPA-Glyp/cy5-siRNA complexes (w/w=20); (b) the DMAPA-Amp/cy5-siRNA complexes (w/w=20); (c) the bPEI/cy5-siRNA complex (w/w=20); (d) the Lip2000/cy5-siRNA complex; and (e) the naked cy5-siRNA. Q1, dead and nonfluorescent cells; Q2, dead and cy5 fluorescent cells; Q3, live and nonfluorescent cells; and Q4, live and cy5 fluorescent cells. (B) Quantitative determination of cell uptake efficiency by the scoring the percentage of cy5-positive hRPE cells using flow cytometry (n=3). The cell uptake efficiencies of the DMAPA-Amp group and the DMAPA-Glyp group had significant differences at different weight ratios (n=3; *P<0.05). (C) Laser scanning confocal images of hRPE cells with different incubation times following a 6-hour period of incubation with the DMAPA-Glyp/cy5-siRNA complexes (w/w=10): (a) nuclei: stained blue with Hoechst 33258; (b) red fluorescence: cy5-siRNA; (c) the images (a and b) were merged (×400).
Abbreviations: SSC-A, side scatter-area; APC-A, allophycocyanin- area; PI-A, propidium iodide- area; DMAPA-Amp, 3-(dimethylamino)-1-propylamine-conjugated amylopectin; siRNA, small interfering RNA; DMAPA-Glyp, 3-(dimethylamino)-1-propylamine-conjugated glycogen; bPEI, branched polyethylenimine; Lip2000, lipofectamine 2000; hRPE, human retinal pigment epithelial; n, number.
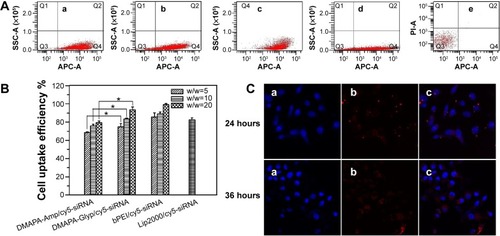
The cell uptake was further confirmed by the accumulation and spread of red fluorescence of cy5-siRNA in the cytoplasm in the confocal images (), when hRPE cells were incubated for 24 hours and 36 hours, following 6 hours of incubation, with the DMAPA-Glyp/Cy5-siRNA nanoparticles (w/w=10). The spread of red fluorescence of cy5-siRNA in the cytoplasm suggested the endocytosis and endosome escape of the DMAPA-Glyp/siRNA nanoparticles, which could be explained by the good buffer capability of the DMAPA-Glyp derivative.Citation29
Suppression on NF-κB p65 gene expression
NF-κB activation is thought to play an important role in the angiogenesis of diabetic retinopathy, since it leads to the high expression of angiogenesis factors such as cyclooxygenase-2, interleukin-8, and tumor necrosis factor-α, resulting in retinal neovascularization.Citation9–Citation11 Herein, we attempted to block retinal neovascularization through the inhibition of the signal pathway of NF-κB in hRPE cells using a siRNA strategy. siRNA was designed and used to knockdown the NF-κB p65 subunit and inhibited the function of NF-κB, since p65 has been shown to be a key active subunit in NF-κB transcription on hRPE cells.Citation9–Citation11
According to the proposed molecular mechanism of the RNA interference approach, cells transfected with effective siRNA can exhibit a reduction in the amount of targeted mRNA and in the amount of protein of NF-κB p65.Citation34 To assess whether polysaccharide derivative/siRNA nanoparticle-mediated gene silencing occurred, the expression levels of NF-κB p65 mRNA and protein were evaluated in hRPE cells. As shown in , the NF-κB p65 mRNA bands progressively weakened when prolonging the incubation times in hRPE cells for both polysaccharide derivative/siRNA groups, as compared to the bands that appeared in the normally cultured control cells and the cells incubated with the Lip2000/nonspecific siRNA complex. The result indicated that the NF-κB p65 mRNA expression was effectively suppressed by both polysaccharide derivative/siRNA nanoparticles. This was further confirmed by quantitative real-time PCR assay, as shown in . It was found that the cells incubated with the DMAPA-Glyp/siRNA nanoparticles showed obviously lower levels of NF-κB p65 mRNA when compared to the cells incubated with the DMAPA-Amp/siRNA nanoparticles.
Figure 6 Efficacy of the polysaccharide derivative/siRNA complexes (w/w=10) on suppressing NF-κB p65 gene expression in hRPE cells with different incubation time following a 6-hour period of incubation.
Notes: (A) Suppression on the NF-κB p65 mRNA levels assessed by semiquantitative RT-PCR analysis. (B) Suppression on the NF-κB p65 mRNA levels quantified by quantitative real-time RT-PCR analysis (n=3; *P<0.01, compared with the control group and the nonspecific siRNA group). (C, D) Suppression on the protein expression of the NF-κB p65 gene evaluated by Western blot analysis (n=3; *P<0.01, compared with the control group and the nonspecific siRNA group).
Abbreviations: GAPDH, glyceraldehydes 3-phosphate dehydrogenase; NF-κB, nuclear transcription factor-kappa B; DMAPA-Glyp, 3-(dimethylamino)-1-propylamine-conjugated glycogen; siRNA, small interfering RNA; DMAPA-Amp, 3-(dimethylamino)-1-propylamine-conjugated amylopectin; mRNA, messenger RNA; hRPE, human retinal pigment epithelial; RT-PCR, reverse transcription polymerase chain reaction; n, number.
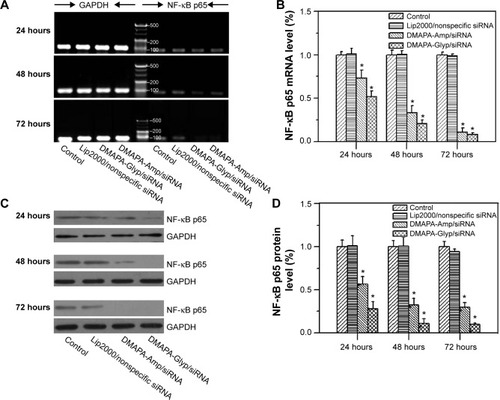
The determination of suppression at the NF-κB p65 protein level yielded consistent results in the hRPE cells when using Western blot assay, as shown in . hRPE cells incubated with the polysaccharide derivative/siRNA nanoparticles showed extremely lower levels of NF-κB p65 protein when prolonging the incubation times, as compared with the control group and the nonspecific siRNA group. Furthermore, the extremely lower expression level of the NF-κB p65 protein in the DMAPA-Glyp/siRNA nanoparticle group suggested that the DMAPA-Glyp/siRNA nanoparticles showed significantly higher efficacies of suppression on NF-κB p65 than did the DMAPA-Amp/siRNA nanoparticles. For example, the NF-κB p65 protein level in the DMAPA-Glyp/siRNA nanoparticle group was approximately one-third of that in the DMAPA-Amp/siRNA nanoparticle group following 72 hours of incubation in the hRPE cells. This result indicates that the suppression efficacy on gene expression is strongly dependent on cell uptake, which is related to the sizes and charges of the polysaccharide derivative/siRNA nanoparticles.
Conclusion
Two hyperbranched cationic polysaccharide derivatives containing the same amount of cationic residues, but different branching structures and molecular weights, were investigated for the delivery of siRNA into hRPE cells to silence NF-κB gene expression. The DMAPA-Glyp derivative could more efficiently condense siRNA, which was attributed to its higher branching architecture when compared to the DMAPA-Amp derivative. Furthermore, the DMAPA-Glyp derivative showed lower toxicity against hRPE cells. Both hyperbranched cationic polysaccharide derivatives could condense siRNA to form nanoparticles with zeta potentials and particle sizes in the range of +30–+40 mV and 100–200 nm, respectively, which facilitated the protection of siRNA from degradation by nuclease in 25% FBS. The DMAPA-Glyp/siRNA nanoparticles more effectively transferred siRNA to the hRPE cells because of their smaller sizes and higher zeta potentials when compared to the DMAPA-Amp/siRNA nanoparticles. This led to significantly higher levels of suppression on the expression levels of NF-κB p65 mRNA and protein in the cells transfected with DMAPA-Glyp/siRNA nanoparticles.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21244005, 20974130) and the Science and Technology Planning Project of Guangzhou, Guangdong Province, People’s Republic of China (201300000149).
Supplementary materials
Synthesis of the hyperbranched cationic polysaccharide derivatives
N,N′-carbonyldiimidazole (CDI), 3-(dimethylamino)-1- propylamine (DMAPA), and dimethyl sulfoxide (DMSO) were bought from Aladdin Reagent Co. Ltd. (Shanghai, People’s Republic of China). DMSO was dried for 1 week prior to use by soaking in molecular sieves and calcium hydride. All other chemical reagents were used without further purification. The DMAPA-conjugated amylopectin (DMAPA-Amp) and DMAPA-conjugated glycogen (DMAPA-Glyp) derivatives were synthesized following the methods used in our previous work.Citation1,Citation2 In brief, amylopectin or glycogen (0.1000 g; 0.62 mmol glucose units) was dissolved in 10 mL of water-free DMSO, and was then activated by adding CDI (0.9045 g; 5.58 mmol) and stirred for 1 hour in a nitrogen atmosphere at room temperature. DMAPA (3.8011 g; 37.2 mmol) was added to the amylopectin or glycogen reaction solution. The reaction was allowed to proceed for 24 hours in a nitrogen atmosphere at room temperature. The reaction solution was then dialyzed against the distilled water in a dialysis bag (molecular weight cutoff: 14,000 D) for 3 days, and lyophilized to yield the solid products.
Fourier transform infrared spectroscopy (FTIR) measurement was performed with an FTIR Analyzer (Nicolet/Nexus 670, Thermo Nicolet Corporation, Wisconsin, USA) at a resolution of 4 cm−1 using the KBr method. The assignments of FTIR peaks are listed as follows: 3,325 cm−1 (ν–OH), 2,946 cm−1 (ν–N[CH3]2), 1,709 cm−1, 1,548 cm−1, and 1,258 cm−1 (ν–C=O, δ–NH– and ν–C–N of carbamate groups), 1,462 cm−1 (deformation vibration of –CH2- and –CH3 groups of DMAPA residues), 1,153 cm−1, and 1,036 cm−1 (ν–C–O–C– of polysaccharide).
1H nuclear magnetic resonance (NMR) analysis was carried out on an NMR spectrometer (Mercury Plus 300; Varian Medical Systems, Palo Alto, CA, USA) at 50°C using D2O as a solvent. The signal at δ 4.50 ppm for HDO was used as the internal standard.Citation1,Citation2 The signal assignments are listed as follows: δ=5.00–6.00 (glucose unit, H1); δ=4.50–3.50 (glucose unit, H2–H6); δ=3.27 (–CONH–CH2−); δ=2.55 (–CH2–N<); δ=2.39 (–N[CH3]2), δ=1.83 (–CH2–). The degree of substitution of DMAPA residues on the polysaccharide, which is defined as the number of DMAPA residues per glucose unit of polysaccharides, was determined by the integration of H1 of polysaccharides and protons of DMAPA residues. Degrees of substitution =2.8 for both polysaccharide derivatives.
hRPE cell identification
All human retinal pigment epithelial (hRPE) cells between the third to fifth passages were harvested and cultured on glass coverslips, underwent a process involving a wash three times with phosphate buffered saline (PBS) solution (pH 7.4), fixation with 4% paraformaldehyde for 15 minutes, and a wash three times with PBS solution (pH 7.4) again, before subsequent treatment in 0.1% Triton™ X-100 and 1% bovine serum albumin (50 μL) for 30 minutes. The treated cells were incubated with the primary antibody of anticytokeratin at 4°C overnight, followed by incubation with a second antibody of fluorescein isothiocyanate (FITC)-conjugated goat antimouse antibody immunoglobulin (Ig)G for 30 minutes in darkness; then, the cells were washed three times with PBS solution, the nucleus was stained with Hoechst 33258 (Beyotime Institute of Biotechnology, Shanghai, People’s Republic of China) for 10 minutes, and they were washed three times with PBS solution. Controlled cells were performed using PBS instead of the primary antibody. All hRPE cells were observed with a Zeiss LSM510 confocal microscope (Carl Zeiss Meditec AG, Jena, Germany).
Cytotoxicity assay
hRPE cells (1×104 per well) were seeded in 96-well plates and cultured in a humidified incubator (37°C; 5% CO2) for 24 hours for adherence. The medium was then replaced with 100 μL of fresh medium containing 10 μL of solutions of the polysaccharide derivatives and branched polyethylenimine (bPEI) at different concentrations. In addition, 10 μL of the polysaccharide derivative/small interfering (si)RNA nanoparticles with different weight ratios were added for the cytotoxicity assay, and the amount of siRNA (0.13 μg per well) was constant. The bPEI and the bPEI/siRNA complex were used as the controls. After the cells were incubated for an additional 24 hours, the culture medium was replaced, and then 10 μL of Cell Counting Kit (CCK)-8 reagent was added to each well, following further incubation for 1 hour. The absorbance values of the samples were measured at 450 nm, using a Wellscan MK3 multifunction microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). Cell viability (%) was then calculated according to EquationEquation 1(1) :
The morphology of hRPE cells incubated with the polysaccharide/siRNA nanoparticles and the bPEI/siRNA complex (w/w=10) were observed on a CKX31 inverted phase contrast microscope (Olympus Corporation, Tokyo, Japan).
Flow cytometry analysis
hRPE cells were plated in six-well plates at a density of 4×104 cells/well in 1 mL of Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS), and they were subsequently cultured in a humidified incubator (37°C; 5% CO2) for 24 hours. The solutions of the polysaccharide derivative/cy5-siRNA nanoparticles at different weight ratios (w/w=5, w/w=10, and w/w=20) were prepared and incubated with cells in each well at a cy5-siRNA final concentration of 50 nM. The bPEI/cy5-siRNA and lipofectamine 2000/cy5-siRNA complexes were used as the positive controls. Meanwhile, the naked cy5-siRNA was used as the negative control. The cells were incubated with the complexes in 10% FBS-containing DMEM for 6 hours at 37°C. The media were then changed with fresh DMEM containing 10% FBS. Following further incubation for 24 hours, the cells were washed twice with PBS (pH 7.4) and trypsinized by 0.25% trypsin and suspended in PBS. The cell uptake of cy5-siRNA was detected by an FACSAria™ flow cytometer (BD Biosciences, San Jose, CA, USA). The data were analyzed with CellQuest™ 3.0 software (BD Biosciences). The cell uptake efficiency was then valuated by scoring the percentage of cy5-positive hRPE cells. All of the experiments were conducted in triplicate.
Laser confocal microscopy
hRPE cells (1×104 cells/well) were plated in glass bottom culture dishes for confocal assay and subsequently cultured for 24 hours. The polysaccharide derivative/cy5-siRNA nanoparticles at a weight ratio of 10 were incubated with cells, as mentioned in the experiment of flow cytometry. Following further incubation for 24 hours and 36 hours, the cells were washed three times with PBS and fixed with 4% formaldehyde. They were then stained with Hoechst 33258 (0.1%, w/v) for the nucleus. The intracellular environment of the cy5-siRNA nanoparticles was observed with a Zeiss LSM510 confocal microscope (Carl Zeiss Meditec AG, Jena, Germany).
Semiquantitative RT-PCR assay for the mRNA level of the NF-κB p65 gene
hRPE cells were plated in six-well plates and cultured for 24 hours, following incubation with the polysaccharide derivative/siRNA nanoparticles (w/w=10) for 6 hours, as mentioned previously. Afterward, the medium was changed with fresh DMEM containing 10% FBS. Following an additional 24 hours, 48 hours and 72 hours of incubation, the medium was removed, and the cells were collected for RNA extraction. Normally cultured hRPE cells without any treatment were used as the control, and hRPE cells incubated with nonspecific siRNA served as the negative control.
The messenger (m)RNA level of NF-κB p65 gene was then evaluated using semiquantitative reverse transcription polymerase chain reaction (RT-PCR) assay. Total RNA was isolated from the cells using the RNAiso Plus (Takara Bio Inc., Kyoto, Japan) according to the manufacturer’s manual. Complementary (c)DNA was synthesized by reverse transcription from 1.0 μg of total RNA from each sample using a PrimeScript™ RT reagent Kit (Takara Bio Inc.). The cDNA mixture (1 μL) was used for the semiquantitative RT-PCR amplification mixture (50 μL) containing 25 μL of Premix Ex Taq (Takara Bio Inc.), 1 μL of sense primers (20 μM), and 1 μL of antisense primers (20 μM). Semiquantitative RT-PCR was performed on two genes, NF-κB p65 and GAPDH. The GAPDH gene was measured in each sample as an internal normalization standard. The forward and reverse primers targeting the NF-κB p65 sequence were 5′-TCTCCCTGGTCACCAAGGAC-3′ and 5′-TCATAGAAGCCATCCCGGC-3′, respectively. The forward and reverse primers of GAPDH were 5′-CACCAACTGCTTAGCACCCC-3′ and 5′-TCTTCTGGGTGGCAGTGATG-3′, respectively. For the polymerase chain reaction (PCR), we ran 30 cycles of 98°C for 10 seconds, 68°C for 30 seconds, and 72°C for 30 seconds, and we ran a final extension for 10 minutes at 72°C. All reactions were performed in triplicate. The amplified products were subjected to electrophoresis on 2% agarose gels containing Goldview fluorescence reagent and visualized under an ultraviolet light using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Quantitative real-time RT-PCR assay for the mRNA level of the NF-κB p65 gene
hRPE cells underwent the same siRNA transfection, total RNA extraction, and reverse transcription processes as described previously. Quantitative real-time RT-PCR was performed with LightCycler® 480 SYBR Green I Master reagent and the program was run in a LightCycler® 480 Real-Time PCR System (Hoffman-La Roche Ltd., Basel, Switzerland). Thermal cycling conditions included 5 minutes at 95°C for preincubation, followed by 46 cycles of 10 seconds at 95°C for denaturing, annealing for 15 seconds at 63°C, and extending at 72°C for 10 seconds. Melting-curve analysis was used to confirm the specificity of the amplification reactions. All experiments were carried out in triplicate.
Evaluation of NF-κB p65 gene silencing efficacy by Western blot
hRPE cells underwent the same siRNA transfection process in the experiment of real-time PCR, as was just described. The total protein in the different groups was extracted at 24 hours, 48 hours, and 72 hours after siRNA transfection, and they were then separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. The membranes were blocked in 5% solution of nonfat milk powder for 1 hour, followed by incubation with the primary antibody of monoclonal rabbit anti-NF-κB p65 (1:600 dilution; Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight. Horseradish peroxidase-conjugated antirabbit IgG (1:8,000 dilution; SouthernBiotech, Birmingham, AL, USA) was used as the secondary antibody, which was further incubated at 37°C for 1 hour. The bands were visualized by the Immobilon Western Chemiluminescent HRP Substrate (EMD Millipore, Billerica, MA, USA). The results were photodocumented and normalized by the GAPDH signal. The percent knockdown of NF-κB p65 was calculated using densitometry from the Western blot image with an ultraviolet light using ImageJ. All experiments were conducted in triplicate.
Figure S1 Laser scanning confocal images.
Notes: (A) Hoechst 33258 staining of hRPE cells; (B) FITC staining of hRPE cells following immunocytochemical reaction; and (C) images (A and B) were merged (×400).
Abbreviations: hRPE, human retinal pigment epithelial; FITC, fluorescein isothiocyanate.
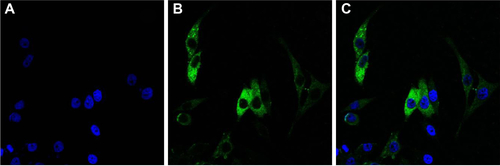
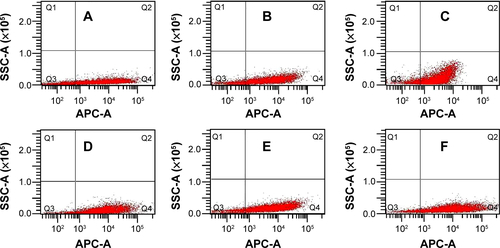
Figure S2 Flow cytometric analysis of cy5-positive hRPE cells following incubation of hRPE cells.
Notes: Incubation of hRPE cells with (A) the DMAPA-Glyp/cy5-siRNA complex (w/w = 5), (B) the DMAPA-Amp/cy5-siRNA complex (w/w = 5), (C) the bPEI/cy5-siRNA complex (w/w =5), (D) the DMAPA-Glyp/cy5-siRNA complex (w/w =10), (E) the DMAPA-Amp/cy5-siRNA complex (w/w =10), (F) the bPEI/cy5-siRNA complex (w/w =10). (a) w/w=5; (b) w/w=10. Q1, dead and nonfluorescent cells; Q2, dead and cy5 fluorescent cells; Q3, live and nonfluorescent cells; and Q4, live and cy5 fluorescent cells.
Abbreviations: SSC-A, side scatter-area; APC-A, allophycocyanin- area; hRPE, human retinal pigment epithelial; DMAPA-Glyp, 3-(dimethylamino)-1-propylamine-conjugated glycogen; siRNA, small interfering RNA; DMAPA-Amp, 3-(dimethylamino)-1-propylamine-conjugated amylopectin; bPEI, branched polyethylenimine.
References
- ZhouYYangBRenXHyperbranched cationic amylopectin derivatives for gene deliveryBiomaterials201233184731474022445252
- LiangXRenXLiuZAn efficient nonviral gene-delivery vector based on hyperbranched cationic glycogen derivativesInt J Nanomedicine2014941943524520193
Disclosure
The authors report no conflicts of interest in this work.
References
- ChistiakovDADiabetic retinopathy: pathogenic mechanisms and current treatmentsDiabetes Metab Syndr20115316517222813573
- PortaMBandelloFDiabetic retinopathyA clinical updateDiabetologia200245121617163412488951
- NicholsonBPSchachatAPA review of clinical trials of anti-VEGF agents for diabetic retinopathyGraefes Arch Clin Exp Ophthalmol2010248791593020174816
- FrankRNPotential new medical therapies for diabetic retinopathy: protein kinase C inhibitorsAm J Ophthalmol2002133569369811992868
- JermakCMDellacroceJTHeffezJPeymanGATriamcinolone acetonide in ocular therapeuticsSurv Ophthalmol200752550352217719372
- ZhouHYangLLiHDownregulation of VEGF mRNA expression by triamcinolone acetonide acetate-loaded chitosan derivative nanoparticles in human retinal pigment epithelial cellsInt J Nanomedicine201274649466022942646
- FattalEBochotAOcular delivery of nucleic acids: antisense oligonucleotides, aptamers and siRNAAdv Drug Deliv Rev200658111203122317097190
- OshitariTBrownDRoySSiRNA strategy against overexpression of extracellular matrix in diabetic retinopathyExp Eye Res2005811323715978252
- YoshidaAYoshidaSKhalilAKIshibashiTInomataHRole of NF-kappaB-mediated interleukin-8 expression in intraocular neovascularizationInvest Ophthalmol Vis Sci1998397109711069620068
- SchmedtjeJFJrJiYSDuBoisRNRungeMSHypoxia induces cyclooxygenase-2 via the NF-kappaB transcription factor in human vascular endothelial cellsJ Biol Chem199727216016088995303
- KroonMEKoolwijkPvan der VechtBvan HinsberghVWHypoxia in combination with FGF-2 induces tube formation by human microvascular endothelial cells in a matrix: involvement of at least two signal transduction pathwaysJ Cell Sci2001114Pt 482583311171387
- SongMKSalamNKRoufogalisBDHuangTHLycium barbarum (Goji Berry) extracts and its taurine component inhibit PPAR-γ-dependent gene transcription in human retinal pigment epithelial cells: possible implications for diabetic retinopathy treatmentBiochem Pharmacol20118291209121821820420
- AdamisAPShimaDTYeoKTSynthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cellsBiochem Biophys Res Commun199319326316388512562
- HiscottPGrayRGriersonIGregorZCytokeratin-containing cells in proliferative diabetic retinopathy membranesBr J Ophthalmol19947832192227511932
- KimYSJungDHKimNHLeeYMKimJSEffect of magnolol on TGF-beta1 and fibronectin expression in human retinal pigment epithelial cells under diabetic conditionsEur J Pharmacol20075621–2121917321517
- LeeSJSonSYheeJYStructural modification of siRNA for efficient gene silencingBiotechnol Adv201331549150322985697
- VarkouhiAKScholteMStormGHaismaHJEndosomal escape pathways for delivery of biologicalsJ Control Release2011151322022821078351
- KoiralaAConleySMNaashMIA review of therapeutic prospects of non-viral gene therapy in the retinal pigment epitheliumBiomaterials201334297158716723796578
- WangRZhouLZhouYSynthesis and gene delivery of poly(amido amine)s with different branched architectureBiomacromolecules201011248949520047311
- MalmoJVårumKMStrandSPEffect of chitosan chain architecture on gene delivery: comparison of self-branched and linear chitosansBiomacromolecules201112372172921294570
- FischerDvon HarpeAKunathKPetersenHLiYKisselTCopolymers of ethylene imine and N-(2-hydroxyethyl)-ethylene imine as tools to study effects of polymer structure on physicochemical and biological properties of DNA complexesBioconjug Chem20021351124113312236795
- SchallonAJérômeVWaltherASynatschkeCVMüllerAHEFreitagRPerformance of three PDMAEMA-based polycation architectures as gene delivery agents in comparison to linear and branched PEIReactive and Functional Polymers2010701110
- AhmedMLaiBFKizhakkedathuJNNarainRHyperbranched glycopolymers for blood biocompatibilityBioconjug Chem20122351050105822500726
- PutauxJLPotocki-VéronèseGRemaud-SimeonMBuleonAAlpha-D-glucan-based dendritic nanoparticles prepared by in vitro enzymatic chain extension of glycogenBiomacromolecules2006761720172816768390
- Rolland-SabatéAMendez-MontealvoMGColonnaPPlanchotVOnline determination of structural properties and observation of deviations from power law behaviorBiomacromolecules2008971719173018547102
- Rolland-SabatéAColonnaPMendez-MontealvoMGPlanchotVBranching features of amylopectins and glycogen determined by asymmetrical flow field flow fractionation coupled with multiangle laser light scatteringBiomacromolecules2007882520253217645307
- de MirandaJACacitaNOkanoLTEvaluation of amylopectin clusters and their interaction with nonionic surfactantsColloids Surf B Biointerfaces2007601192717601711
- ZhouYYangBRenXHyperbranched cationic amylopectin derivatives for gene deliveryBiomaterials201233184731474022445252
- LiangXRenXLiuZAn efficient nonviral gene-delivery vector based on hyperbranched cationic glycogen derivativesInt J Nanomedicine2014941943524520193
- XiongXBUludağHLavasanifarABiodegradable amphiphilic poly(ethylene oxide)-block-polyesters with grafted polyamines as supramolecular nanocarriers for efficient siRNA deliveryBiomaterials200930224225318838158
- LiXTZhangYChenGQNanofibrous polyhydroxyalkanoate matrices as cell growth supporting materialsBiomaterials200829273720372818585779
- CaoNChengDZouSAiHGaoJShuaiXThe synergistic effect of hierarchical assemblies of siRNA and chemotherapeutic drugs co-delivered into hepatic cancer cellsBiomaterials20113282222223221186059
- LiuYReinekeTMHydroxyl stereochemistry and amine number within poly(glycoamidoamine)s affect intracellular DNA deliveryJ Am Chem Soc200512793004301515740138
- LianxuCHongtiJChanglongYNF-kappaB p65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced chondrocytesOsteoarthritis Cartilage200614436737616376111
