Abstract
Cardiovascular disease is the leading cause of death across the globe. The use of synthetic materials is indispensable in the treatment of cardiovascular disease. Major drawbacks related to the use of biomaterials are their mechanical properties and biocompatibility, and these have to be circumvented before promoting the material to the market or clinical setting. Revolutionary advancements in nanotechnology have introduced a novel class of materials called nanocomposites which have superior properties for biomedical applications. Recently, there has been a widespread recognition of the nanocomposites utilizing polyhedral oligomeric silsesquioxane, bacterial cellulose, silk fibroin, iron oxide magnetic nanoparticles, and carbon nanotubes in cardiovascular grafts and stents. The unique characteristics of these nanocomposites have led to the development of a wide range of nanostructured copolymers with appreciably enhanced properties, such as improved mechanical, chemical, and physical characteristics suitable for cardiovascular implants. The incorporation of advanced nanocomposite materials in cardiovascular grafts and stents improves hemocompatibility, enhances antithrombogenicity, improves mechanical and surface properties, and decreases the microbial response to the cardiovascular implants. A thorough attempt is made to summarize the various applications of nanocomposites for cardiovascular graft and stent applications. This review will highlight the recent advances in nanocomposites and also address the need of future research in promoting nanocomposites as plausible candidates in a campaign against cardiovascular disease.
Introduction
Every year nearly 720,000 Americans suffer from a heart attack, and among these patients 515,000 have a heart attack for the first time.Citation1 Health data compiled from more than 190 countries indicates that heart disease remains the number one cause of death in the world, with 17.3 million deaths every year according to the 2015 update report. This count is expected to increase by more than 23.6 million by 2030.Citation2 Hence, the demand for addressing cardiovascular health care is increasing drastically day by day. This has led to the permanent growing demand for cardiovascular biomaterials to be used as effective implants and grafts. The statistics by Markets and Markets indicates that the orthopedic biomaterial market previously had the highest market share and this is gradually shifting toward cardiovascular biomaterials due to the increasing number of cardiac patients across the globe.Citation3 The world biomaterial market is anticipated to reach $88.4 billion by 2017 from $44 billion in 2012, where 34.5% will be cardiovascular biomaterials.Citation4 The application of cardiovascular biomaterials ranges from heart valve prostheses and vascular prostheses to indwelling catheters. The formation of blood clots and the initiation of thrombotic events once the biomaterial comes in contact with blood, still remain as great challenges for researchers to decipher. The deterrence of thrombotic deposition and occlusion, induced by the activation of the coagulation cascade and platelets, is an obligatory property required for a hemocompatible cardiovascular biomaterial. Thus, the search for a putative cardiovascular biomaterial is still continuing throughout the globe. The advent of the latest technology has paved the way to the discovery of novel materials known as nanocomposites. Nanocomposites have revolutionized biomedical engineering research in recent years and they typically find application in the biomedical engineering field, especially for cardiovascular applications.
Nanocomposites can be defined as materials which have components mixed at the nanometer scale. Nanocomposites are made up of at least two constituent materials which are a matrix or host along with a strengthening constituent called a nanofiller or guest.Citation5 It is common knowledge that the property of a material changes appreciably when the size is substantially small, as in the range of 1–100 nm. Since nanocomposites are in quantum scale sizes, these materials can function as a connecting bridge between molecules in the polymer. This has enabled the nanocomposite to demonstrate a different set of properties from regular microcomposites.
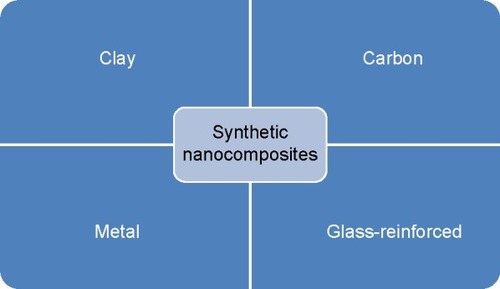
Nanocomposites materials are, at times, considered to be either organic, inorganic or hybrid in their constitution.Citation6–Citation11 The various examples for nanocomposites available in nature are bone, abalone shells, and teeth. Motivated by the excellent properties of natural nanocomposites, various studies have been conducted for creating synthetic nanocomposites which are expected to possess similar optimum properties as natural nanocomposites.Citation12,Citation13 The method by which the nanocomposite is created is a determining factor for the performance of the synthetic nanocomposite material. For example, parameters such as production technique, nature of the nanofiller, process utilized for nano-reinforcement, and types of reactions between polymeric and strengthening components regulate the performance of the nanocomposite material.Citation14 There are few types of synthetic nanocomposites and they can be divided into four categories which are clay-, carbon-, metal-, and glass-reinforced ().Citation15 Silicon and metal oxides (eg, ZnO and TiO2) are often used as nanofillers owing to their superior mechanical properties.Citation16 As the physical, chemical, and mechanical properties of the nanomaterial are superior to those of conventional biomaterials, they become a better choice for cardiovascular applications.
Cardiovascular implants such as bypass grafts and stents are susceptible to thrombogenesis. This happens due to lack of blood compatibility of the used biomaterial thereby leading to severe medical complications. In order to circumvent the problems associated with the blood compatibility of bypass grafts, as well as stents and their failure due to lack of mechanical strength and infection, various studies were done incorporating nanocomposites.Citation17–Citation23 Despite many studies having been done using nanocomposites for cardiovascular implants, all the works need to be reviewed extensively for the development of a viable heart implant. The prime motive of this review work is to summarize various studies done on nanocomposite cardiovascular applications especially for bypass grafts and stents.
Cardiovascular grafts
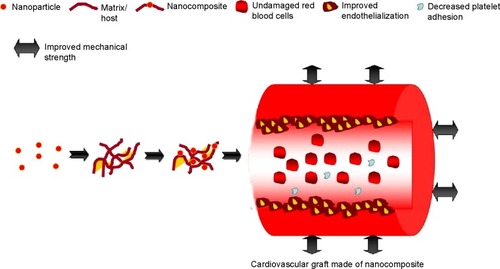
The cardiovascular graft should possess the behavior of withstanding high shear stresses, possess same bulk viscoelasticity as that of the native blood vessels it is anastomosed to, and, most importantly, should have antithrombogenic properties to serve as a perfect replacement conduit. When the vessel diameter decreases at low-flow states, the interactions between these factors are notably amplified.Citation24 Whilst results with poly(tetrafluoroethylene) (PTFE) and poly(ethylene terephthalate) (Dacron) are acceptable in bigger vessels, the patency rate for smaller diameter grafts is very low.Citation25,Citation26 Moreover, Dacron has high platelet adhesion which is a major setback. Microvessels (<1 mm diameter) of animal models which were made of PTFE had 20%–25% patency rate in rat femoral vessels but the control graft was found to be obstructed.Citation27 This is due to the fact that the polymers will adsorb a certain amount of fibrinogen and it will trigger the thrombus formation on its lumen.Citation28 This will lead to the formation of intimal hyperplasia (IH).Citation29–Citation31 Thrombosis and IH are different processes; however few studies reported the existence of a relationship between thrombosis and the occurrence of IH, particularly after bypass surgery or angioplasty.Citation32,Citation33 Thrombin is one of the vital proteins involved in the coagulation pathway: it converts fibrinogen into activated fibrin which ultimately leads to formation of a thrombus. In addition, thrombin also has the ability to activate platelets and induce the release of mitogenic factors from vascular smooth muscle cells (SMC).Citation33 These reactions are highly implicated in migration and proliferation of SMC which eventually results in occurrence of IH and restenosis. So, a plausible implant should possess capabilities to inhibit thrombosis formation which prevents IH by ensuring better patency of the implant. In order to circumvent all these issues, nanocomposites exert their role by improving the hemocompatiblity, endothelialization, and mechanical strength.
Nanocomposites to improve hemocompatibility of graft
The hemocompatibility of vascular grafts can be attained by inhibiting three main processes which are platelet adhesion, platelet activation and aggregation, and the formation of thrombin. Bacterial cellulose (BC) is a promising, sustainable and biodegradable nanofibrous material. BC fibers have a diameter of 40–70 nm and display different unique properties such as high purity, better degree of polymerization, and superior crystallinity thereby giving them high strength and modulus.Citation34–Citation36 The presence of a smaller fiber diameter offers BC a higher surface area, and its porous structure enables it to retain a large amount of water. In addition, BC also has good thermal stability, outstanding environmental biodegradability, and excellent biocompatibility which makes it a plausible candidate for vascular grafts.Citation34–Citation36 Hence, BC has been recommended as a plausible biomaterial for developing cardiovascular grafts. The BC can be combined with polyvinyl alcohol (PVA) to produce nanocomposites with superior properties. However, only a few studies have been done on the BC–blood interaction. Leitao et alCitation37 performed a detailed blood compatibility study of a BC-PVA nanocomposite by calculating whole blood clotting time, plasma recalcification, factor XII activation, platelet adhesion, and hemolytic index. The thrombogenicity and procoagulative activity of BC and BC-PVA were studied by determining whole blood clotting time by using expanded PTFE (ePTFE) as a control and polystyrene as a negative control. There was no significant difference between BC and the nanocomposite, but both exhibited better results than both controls. Similarly, the plasma recalcification analysis also showed no significant improvement caused by the addition of PVA to BC; however both samples showed superior properties to the controls. However, factor XII activation test (intrinsic pathway) and complement system activation tests demonstrated the improved activity of the BC-PVA nanocomposite when compared with both pristine BC and controls. Interestingly the extent of platelet adhesion was observed to be a time-dependent activity. Initially after 10 minutes, ePTFE (control) showed traces of platelet adhesion whereas BC and BC-PVA displayed zero percentage adhesion, but after 50 minutes, BC and BC-PVA showed the same amount of platelet adherence as ePTFE at that time. It clearly shows in both samples the platelet was activated only after 50 minutes, and it was meticulously analyzed by monitoring the signature of activated markers of adhered platelets. Further, the blood hemolysis index following contact with BC, BC-PVA, and ePTFE was found to be 1.85%, 1.94%, and 2.42%, respectively. This indicates that BC and BC-PVA performed better than the control, thereby elucidating its versatility for cardiovascular applications such as grafts.
The carbon nanotube (CNT) is a tube-shaped material which consists of a graphite sheet, having a diameter in the nanometer range. Depending on the number of graphite sheet layers, the CNTs can be divided into single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs).Citation38,Citation39 SWCNT is a single molecular nanomaterial made of only one layer of graphite sheet to form a molecular cylinder. It has diameter and length of 0.75–3 and 1–50 nm respectively. The MWCNT is made of more than two layers of curly graphite sheet with diameter ranges from 2 to 30 nm, and some may even exceed 100 nm. The distance between each layer of graphite sheet in MWCNT is approximately 0.42 nm. CNTs are used in various biomedical applications like biosensors, diagnostic agents to visualize cancer cells, etc, and recentlyCitation40,Citation41 Meng et alCitation42 prepared a multiwalled carbon nanotube–polyurethane nanocomposite (MWCNT-PU) via controlled coprecipitation. Platelet adhesion was determined using SEM, and platelet activation caused by PU and MWCNT-PU was investigated through flow cytometric analysis. The disruption of red blood cells by PU and MWCNT-PU was studied by measuring the absorbance of free hemoglobin. It was found that the MWCNTs with an oxygen-containing functional group are well dispersed in polyurethane matrix. The number of platelets adhered to the MWCNT-PU nanocomposite surface was remarkably reduced in comparison to that of the PU surface. Investigation of platelet activation was done by the analysis of conformational changes in glycoprotein measured via flow cytometry. The GPIIb/IIIa complex is a type of glycoprotein present on the membrane of resting platelets. Platelet activation would trigger a conformational change in GPIIb/IIIa thereby exposing a ligand-binding site for fibrinogen. It is common knowledge that binding of fibrinogen to the GPIIb/IIIa complex is the final common pathway for platelet aggregation.Citation43 It was observed that the percentage of positive GPIIb/IIIa complex induced by MWCNT-PU was reduced to a great extent compared to PU. Results obtained for the hemolysis index (HI) triggered by PU and MWCNT-PU materials shows that the HI decreased in case of MWCNT-PU compared to PU. Moreover, this also indicates that the interaction of MWNTs with adsorbed plasma proteins results in hemolysis resistance of the MWCNT-PU nanocomposite. Hence, the MWCNT-PU nanocomposite demonstrated an appreciably improved anticoagulant function indicating promising prospects of CNT-based materials for the cardiovascular graft application.
In a recent work, de Mel et alCitation20 worked on platelet interactions and thrombus development when the polymer comes in contact with blood, for investigating the antiplatelet or antithrobogenic property of polyhedral oligomeric silsesquioxane/poly(carbonate-urea)urethane impregnated with nanosilver (POSS-PCU-NS). The study showed that POSS increased the antithrombogenicity of PU along with a novel discovery that the inclusion of NS in POSS-PCU maintained or improved the antithrombogenic properties of POSS-PCU, with an additional property of enhanced antibacterial activity. To ascertain the platelet adhesion and activation, they performed the following assays using POSS-PCU-NS. Platelet adhesion studies to provide a better insight about the biocompatible nature of a material. The extent of platelet adhesion can be meticulously studied by performing the alamar blue (AB) assay. Platelets are added in test samples and stained using AB. The optical density (OD) reading obtained correlates with the cell viability since the living cells will have the ability to convert resazurin to resorufin which produces a bright red fluorescence.Citation44 Collagen and PTFE were utilized to serve as a positive control for significant platelet adhesion, which was dictated by the high OD reading. However, POSS-PCU surfaces displayed decreased platelet adherence in comparison to the commonly used materials for grafts, like collagen and PTFE. This was represented by a decrease in the OD of POSS-PCU and POSS-PCU-NS based AB assay, suggesting improved hemocompatibility. Similarly, platelet activation was determined by estimating the PF4 levels. PF4 is a CXC chemokine which is generally released from α granules of activated platelets. Chemokines belong to the family of small cytokines, or signaling proteins, released by cells. Chemokines have been divided into following subfamilies namely CXC, CC, CX3C, and XC. Among them, the CXC chemokine consists of two N-terminal cysteines of CXC chemokines (or α-chemokines) with one amino acid in between, denoted by an “X”. CXC chemokine release is commonly known to antagonize the effect of heparin-like molecules which inhibit antithrombin III activity.Citation45 PF4 is a unique marker for platelet activation, and it was demonstrated to have a prothrombotic as well as an antithrombotic role.Citation46,Citation47 Hence, CXC chemokine is considered vital to improve the hemocompatibility of cardiovascular grafts. Depending upon the percentage of NS integrated with POSS-PCU, there was a concomitant reduction in platelet activation.
In the same study, they also performed wettability studies on POSS-PCU and POSS-PCU-NS surfaces.Citation20 The contact angle of the surface determines its wettability, and it also plays a significant role on platelet adhesion as well as overall thrombogenicity. The water contact angle of the surface dictates the hydrophilic behavior of the material, and it may have a relationship with the surface charges of the silver nanoparticles present on the polymer surface. The more hydrophilic the surface, the smaller the contact angle would be, and the surface energy would be higher than that of the surface tension of the liquid.Citation48 Hydrophilic and hydrophobic surfaces selectively adsorb proteins at different rates rapidly, and this has an impact on the overall coagulation process. It was found that fibrin surfaces promote thrombosis whereas surfaces which promote albumin adhesion prevent platelet adhesion.Citation20 Even though there is no prominent correlation between the degrees of surface wettability, hydrophilic polymers are found to assist protein adsorption, thereby making it more hemocompatible.Citation49 On the other hand, it was found that PTFE which is highly hydrophobic has a high count of adhered platelets, which was the same as the effect on the collagen surface of the material which was used as a control. It was found that the increase in the concentration of the NS on the POSS-PCU surface resulted in a decrease in the water contact angle.Citation20 When the contact angle was minimized, a concentration-dependent reduction in platelet activation and platelet adhesion was observed. However, coagulation and thrombosis can be initiated through multiple factors besides surface hydrophilicity/hydrophobicity, such as size and the charge of any interacting molecules or particles.
Nanocomposites to improve endothelialization of graft
Endothelial cell layer formation on the surface increases the blood and biocompatibility of devices. When the endothelialization is implemented on the luminal surface of bypass grafts, it improves the lifetime and clinical results of the grafts.Citation19 The clinical studies done by researchers depict the benefits of lining PTFE vascular grafts with endothelial cells, specifically in lower limb and arterial bypass grafts, instead of uncoated polyethylene terephthalate grafts.Citation50 In a work done by Solouk et alCitation51 an in vitro study was carried out on human umbilical vein endothelial cells (HUVECs). The nanocomposite efficacy, safety, and compatibility with in vitro cell cultures was investigated. Such works serve as a reference for further extension of the development of biohybrid vascular grafts.
Generally nanocages are nanomaterials with porous walls and hollow interiors that can be utilized for the purpose of loading and release of drugs. The nanocage size can be manipulated from 20 to 500 nm which allows the optimized biodistribution as well as particle permeation via tissues. They possess excellent mechanical flexibility and stability, enabling them to survive in an intricate in vivo environment.Citation52 One of the most viable nanomaterials for cardiovascular grafts application is POSS, which is a fascinating nanocage consisting of an inner inorganic framework of silicon and oxygen atoms and an outer shell of organic groups. It was found that the POSS-based nanocomposite enhanced the cell adhesion property of the grafts in comparison to the existing silicone copolymers.Citation50,Citation53,Citation54 This is because the nanocages occupy a very small volume inside the polymer thereby providing a larger surface area to PU, which results in enhanced endothelialization. In addition, this structural arrangement of the nanocage provides a greater degree of polarity to the polymer thus making it more hydrophobic.
The abovementioned experiments also signify that once the endothelial cells (ECs) adhere to the surface of the polymer, they are capable of multiplying, by proliferation, to form a confluent monolayer. The light microscopic analysis demonstrated how the ECs proliferated on the polymer and PicoGreen® (Life Technologies, Carlsbad, CA, USA) assay showed the excellent proliferating behavior of ECs on the polymer. Before attaining cellular confluence, the EC was aligned in a reticular manner. The prevailing areas were later filled in to attain cellular confluence.Citation55 In a work done by Vara et alCitation56 the exposure of the EC-seeded POSS-PCU conduit to low shear stress before exposing it to physiological stress was found to improve the cell adhesion to a greater extent. Thus, this finding endorsed the advantages of performing a preseeding technique, followed by low shear stress application on POSS-PCU for cardiovascular graft applications.
To further improve the endothelialization potential of POSS-PCU, it can be biofunctionalized. Biofunctionalization can be achieved through modification of the POSS-PCU surface using biofunctional peptides such as RGD (arginine–glycine–aspartic acid) which is an extracellular matrix component. A study was done to investigate cell proliferation and adhesion of the RGD-modified POSS-PCU by using peripheral blood mononuclear cells (PBMCs) containing endothelial progenitor cells (EPCs). The result of this analysis showed that the quality of cell adhesion and proliferation on POSS-PCU was outstanding.Citation19 Meanwhile, the qualitative analysis done through scanning electron microscopy (SEM) elucidated the optimum cell–polymer interactions with formation of more filopodia on its surface, flattened ECs, and absence of rounded cells. Flow cytometry results showed that 1%–2% of PBMC (1.5×107±7×105) were obtained from 20 mL of venous blood expressed CD34.Citation19 It was also observed that POSS-PCU and uncoated culture dishes produced significantly fewer colonies than POSS-PCU-RGD. These results help us to conclude that ECs are capable of morphogenesis and have the ability to proliferate well. In another work, RGD-altered POSS-PCU was used to develop a porous small-diameter bypass graft which was later examined at static and pulsatile conditions. PBMC containing EPCs obtained from healthy adult volunteers were grown on a nanocomposites graft. The characteristics such as cell attachment, growth, endothelialization, and immunostaining for endothelial cell markers, including CD34, CD31, and eNOS, were assessed. This work indicated that POSS-PCU possesses good biofunctionalization capacity, and is capable of providing a suitable environment for cell growth and cell viability thereby encouraging rapid endothelialization from peripheral blood cells.Citation57 Selective biofunctionalization by making use of specific growth factors in combination with other surface modification methods may improve the affinity of the surface to ECs whilst reducing probable attachment and proliferation of other cells like smooth muscle cells or fibroblasts.
In a recent work done by Solouk et alCitation51 central composite design (CCD) was utilized for developing predictive models. A CCD is an experimental design, mainly used in response surface methodology, for building a second order (quadratic) model and a response variable without requiring the use of a complete three-level factorial experiment. The developed predictive models were quadratic models where power and duration of plasma exposure were utilized to model the surface energy (γs). It was done with the purpose of optimizing the operating conditions of plasma surface modification. From this study, it was observed that among the two process variables (power and duration of plasma exposure), plasma exposure was found to appreciably affect the surface energy (γs), chemistry, and topography of POSS-PCU films.Citation51 Based upon the experimental data, CCD was implemented to model the γs. This was done by quadratic modeling of process variables for achieving optimum surface energy thereby enhancing the interaction between ECs. By using experimental variables such as power output of 30 W for 75 seconds, 90 W for 40 seconds, and 90 W for 55 seconds in oxygen, the required optimum water contact angle (θ) for EC adhesion and retention was achieved. On the basis of the literature, it was reported that the optimum θ is 55° (equivalent to γs =51 mN/m). The in vitro cell culture and metabolic activity analysis on POSS-PCU films subjected to this plasma treatment displayed an increased adhesion, coverage, and growth of HUVECs, and formed a confluent layer of cells within a short span of time (<24 hours) compared with controls. Thus, this method of surface treatment of the nanocomposites enhanced the EC response and promoted endothelialization on optimized nanocomposites, thereby proving its efficacy to be used for cardiovascular graft development.
The natural regeneration capability of our body can be exploited for triggering endothelialization of cardiovascular devices. In a work done recently, PBMCs were segregated by Ficoll-Paque centrifugation.Citation58 EPCs are a rare population of stem cells present in circulating blood which have the ability to differentiate into endothelial cells which form the inner layer of blood vessels. In this research, EPCs were harvested using magnetic-activated cell sorting (MACS) and purified through the microbeads. These harvested cells were added onto 96-well plates which were coated with POSS-PCU or GRGDG/GRGDG-LA (peptide consisting of glycine, arginine, glycine, aspartic acid, glycine and lauric acid) modified POSS-PCU and PCU polymer for a duration of 21 days. The cultured cells were examined using light, confocal, and scanning electron microscopes. Moreover, fluorescence-activated cell sorting (FACS) was implemented for investigating cell surface markers, including CD34, CD133, VEGFR2, CD144, CD31, and vWF, for identifying EPCs’ potential for proliferation and differentiation to become mature endothelial cells. Cell attachment and growth was found in all POSS-PCU samples, with appreciably higher activity compared to the control polymers (P<0.05). Microscopic analysis depicted clonal expansion and morphological changes in cells seeded on POSS-PCU, GRGDG, and GRGDG-LA modified polymers. The occurrence of triple-positive EPC (cells positive for CD34, CD133, and VEGFR2) incremented from the initial culture period thereby elucidating the proliferation capability of cultured EPCs. After a week, SEM observation showed that there was a mixed population of morphologically differentiated endothelial cells and EPCs in the sample. A greater number of mature endothelial cell markers (CD31, vWF, CD144, and VEGFR2) with a confluent layer of EC were observed on day 21. Thus, this result dictates the self-endothelialization potential of cardiovascular implants made with the POSS-PCU polymer as well as delineating the possibility for utilization of the natural regenerative capacity of EPCs on POSS nanocomposite materials.
Tissue engineering is a novel approach for creating new vascular grafts. In this method, cells are seeded or encapsulated within a scaffold fabricated from a biodegradable polymer. It is thought that cells produce extracellular matrix (ECM) whilst the polymer is degraded, thereby creating the intended tissue. Research has been conducted exhaustively on tissue-engineered vascular grafts (TEVGs) and as a result, tremendous progress has been made in terms of achieving the remodeling of the tissue in TEVG constructs with almost the same properties as the native blood vessels.Citation59,Citation60 CNT can be combined with polycaprolactone (PCL), a versatile polymer, to form a CNT-PCL nanocomposite with improved endothelialization properties which can be exploited for cardiovascular graft application. In a work done by Wickham et alCitation61 PCL was fabricated via the solvent casting method and also the fibrous meshes method by electrospinning. Thiophene-conjugated carbon nanotubes (T-CNTs) were later incorporated into PCL. It was found that freshly isolated cardiac progenitor cells (CPCs) from murine heart proliferated better on the TCNT-PCL meshes compared to PCL alone. Hence, this TCNT-PCL can be considered as a putative choice for enhancing the endothelialization of scaffolds developed, which in turn forms the vascular graft via tissue engineering for the cardiovascular graft application.
Polarity is essential in determining the wettability of the surface. Engineering the hydrophilicity/hydrophobicity is essential in many applications like geochemical, biological, and technological systems such as lab-on-chip.Citation62 It has been perceived that the liquid contact angle, a macroscopic property, is the decisive criterion widely utilized to quantify the hydrophobicity based on the polarity of the surface. Typically it has been postulated that polar surfaces are generally hydrophilic compared to the apolar surfaces which are deemed to be hydrophobic. But a recent revelation indicated that some polar surfaces can be hydrophobic, in contrast to the widespread notion that all polar surfaces are hydrophilic.Citation63 Similarly the extent of polarity and its influence on wettability is essential in cardiovascular devices like grafts. High wettability of the surface has been found to promote endothelialization. For example, a recent study utilizing the hydrophilic coating on the surface was found to possess better endothelialization and anticoagulation properties.Citation64 However, the hydrophobic surface may not be the cause of attenuation of endothelialization. This is because POSS-PCU with a superhydrophobic surface has been shown to promote endothelialization.Citation58 The argument for endothelialization promotion comes from a multitude of factors. The chemical environment surrounding the surface, including cytokines, chemokines, and growth factors, has a significant role in promoting endothelialization. Further, the shear stress induced by blood flow may also act as a conducive factor for endothelialization of implants.Citation65 Hence, it has been suggested that it is the interplay of both physical and chemical factors that determine the endothelialization of superhydrophobic surfaces.Citation66
Nanocomposites to improve mechanical strength of grafts
Nanocomposites have more mechanical strength compared to their constituent parts. Various studies show that the mechanical strength of the nanocomposite depends on the alignment of nanofillers or “mineral platelets” contained in a soft protein matrix. Whilst the nanofillers provide tensile strength to the composite, the interfacial shear attributable to the lubricating proteins permits effective load transfer due to the increased surface area/volume ratio of the composite. Previously it was assumed that the failure strength of a material was E/10, where E was the Young’s modulus of a material. Conversely, it was found that those materials often fail at 1,000 times less than the predicted value. GriffithCitation67 discovered many microscopic cracks in every material and hypothesized that these cracks decreased the overall strength of the material. In addition, the Griffith criterion ascertains that the characteristic of propagation of a crack occurs in an elliptical nature, by the consideration of energy involved. When the crack length becomes greater than critical Griffith crack length, then the crack becomes dangerous. Thus, according to the Griffith criterion, utilization of nanocomposites in cardiovascular grafts that have a filler size less than the critical length does not affect the strength of the composite even if it is cracked, by ensuring optimum strength and maximum tolerance of flaws.Citation68
In a recent work done by Stout et alCitation69 carbon nanofibers (CNF) were embedded in poly(lactic-co-glycolic-acid) (PLGA). This combination of nanocomposites with polymer has demonstrated the ability to promote cardiomyocyte growth in comparison to conventional polymer substrates and the mechanism involved was studied. It was found that the tensile strength of the polymer increased by the addition of CNF as follows: 0.15 MPa for 100% PLGA to 5.41 MPa for the 50:50 (PLGA to CNF [wt%:wt%]) ratio at 0.025 g/mL. The investigation using atomic force microscopy revealed that adding CNF to PLGA increased the material surface area from 10% (100:0 [PLGA to carbon nanofiber (wt%:wt%)]) to over 60% (50:50 [PLGA to carbon nanofibers (wt%:wt%)]) thereby improving its mechanical properties and making it a suitable material for cardiovascular graft application.
Compared to polymethylmethacrylate (PMMA), calcium phosphate cement (CPC) has several advantages such as biocompatibility and biodegradability.Citation70 However, it poses a threat where its decay properties may contribute to an increased risk of pulmonary embolism and subsequent cardiovascular complications. Animal studies had shed light on the fact that disintegration of CPC forms more emboli, particularly microemboli, leading to severe cardiovascular complications compared with PMMA.Citation71,Citation72 Studies were done incorporating organic proteins or polymers into CPC to enhance its robustness, through hydroxyapatite (HA) formation and fluid penetration reduction.Citation73,Citation74 In a work done by Ding et alCitation70 silk fibroin (SF) was studied for its ability to regulate the mineralization process and it joins with HA to form fibroin-HA nanocomposites with improved gelation properties. SF also has outstanding biomechanical, biocompatible, and biodegradable properties, and its production process is cost effective. Thus, SF is a nanocomposite which can be considered as a putative material for the cardiovascular graft application.
In a work done by Millon and Wan,Citation75 BC, which is a hydrophilic nanofiber with an elevated elastic modulus and a degree of crystallinity, was combined with PVA thereby resulting in a novel biocompatible nanocomposite. The high surface area of BC per unit mass enables it to extensively form hydrogen bonds with different hydrophilic polymers like PVA.Citation36,Citation76,Citation77 The interfaces formed in between BC and PVA makes the resultant nanocomposite robust. This became evident from the obtained result of greater slope for stress–strain curve of the PVA-BC compared to PVA alone. When the number of thermal cycles increased, it was observed that the PVA crystallinity increased, which in turn improved the strength of the composite. An SEM study of bacterial cellulose shows that the average diameter of the fiber is 50 nm.Citation75 The stress–strain curve of the 10% PVA cycle 6 was found to be in between cycles 2 and 3 of the 10% PVA with 0.61% BC composite. Thus, this indicates that at a strain of around 40%, the elastic modulus of the PVA-BC composite greatly improves and deviates from the curve of the PVA reference. Owing to the hydrophilic nature of BC and PVA, the crystalline regions of the BC fibers can aid in formation of additional nucleation sites for PVA crystallite formation. Additional crystallites were found to be formed around the BC nanofibers for each freeze/thaw cycle. Since the surface area of BC is limited, the strength of the nanocomposite remains the same even after thermal cycling beyond cycle 3 or 4.Citation75 Likewise, Luprano et alCitation78 demonstrated that the mechanical properties of some hydrogel mixtures, like PVA–hyaluronic acid (HA), did not increase after the third cycle while using a high HA (1,000,000 MW) content.
As the heart beats at a rate of 1 Hz, it is necessary to investigate the dynamic response of the nanocomposite material before using it for cardiovascular grafts. Soft tissues are viscoelastic by nature. The viscoelastic behavior of a material can be accessed through stress decays with respect to time and it is regarded as a magnitude of the viscous flow in solid matter, or the capacity of the material to relax at a condition of applied stress. In a work done by Hernandez et alCitation79 viscoelastic properties of PVA hydrogels and ferrogels were characterized following thermal cycling with shear plates during varying sets of experimental conditions. Similarly, Stammen et alCitation80 demonstrated the relaxation response of a greater concentration PVA (20%–25%) during compressed conditions, and it displayed a long relaxation time. The result of the above study shows that faster relaxation rates and a lower relaxed stress of nanocomposites will pave the way toward a quicker recovery during the cardiac cycle in comparison to natural tissues.Citation75 Hence, this result further dictates that nanocomposites are a putative choice for aortic tissue replacement.
Continuous-space Monte Carlo simulations on POSS network structures depict the fact that when the linker length is increased, there will be a decrease in the reactiveness of their tethers. This in turn will increase the intercubic pore sizes within the nanocomposite, resulting in an even distribution of the nanocages. Hence, this phenomenon barricades the development of large mesopore and bulk cavities. On the other hand, this effect is countered if their tethers are rigid as the degree of cross-linking is decreased.Citation81 Work done by some researchers show that the POSS nanofillers improve the glass transition temperatures (Tg) at higher concentrations by decreasing the distances between these nanofillers by aggregating them. Molecular or segment rotation with reference to the polymer is prevented, thereby resulting in the decrease in dipole interaction potential and ultimately making the polymer stronger for cardiovascular graft application.Citation82
Zhang et alCitation83 used MWCNTs to reinforce alginate vascular conduits. In this study, alginate vascular conduits were fabricated utilizing an extrusion based deposition system. Lower fabrication time span, the capability to fabricate vascular conduits at any length, and good biocompatibility are the advantages of this vascular conduit fabrication method in comparison to other available methods of vascular conduit fabrication.Citation84,Citation85 Mechanical tests were done on vascular conduits in the presence and absence of MWCNT reinforcement. To determine their tensile strength, burst pressure of vascular conduits was examined. It was found that the tensile strengths for the control group, the 0.5% MWCNT reinforcement group and the 1% MWCNT reinforcement group were 382±19, 420±22, and 422±23 kPa, respectively. It was observed that different MWCNT concentrations could alter the vascular conduits’ mechanical properties. This variation in tensile strength could be caused due to the distribution, dispersion, and alignment of the MWCNT in alginate, which may affect the physical as well as mechanical properties and the orientation of the nanomaterial in the vascular conduits. The burst pressure for the control group, 0.5% (w/v) MWCNT reinforced group, and 1% MWCNT reinforced group was 208.14, 215.89, and 221.65 mmHg respectively. The 0.5% and 1% (w/v) MWCNT reinforcement improved the burst pressure by 3.7% and 6.5%, respectively. The enhancement in tensile strength dictates that the stress was transferred between the alginate matrix, and MWCNTs reinforced the vascular conduit’s composites. Moreover, the external stress was efficiently transferred from the alginate matrix to the reinforced MWCNTs via the better bonded interfaces in alginate-reinforced MWCNT composites. However, when compared to the natural vascular system, which has a burst pressure around 3,561 mmHg, further improvement is indispensable for transplantation purposes.Citation86 Nevertheless, the proposed reinforced vascular conduits would be capable of providing enough oxygenation for in vitro scale-up tissue and organ model fabrication. Thus, the MWCNT can be used as a plausible candidate for enhancing the mechanical strength of vascular grafts.
Work done by Mattioli-Belmonte et alCitation87 ascertains the mechanical, thermal, and biological characterization of a solid free-form microfabricated carbon nanotube–polycaprolactone composite. In this study, both the quantity of nanotubes in the matrix and the scaffold design were varied for tuning the mechanical properties of the material. The result obtained indicated that the elastic modulus improved initially with the inclusion of CNT (concentration up to 12.5 mg/mL) in PCL. However, after this concentration the elastic modulus was found to plummet, reaching values close to that of pure PCL. This behavior could be caused by the electrostatic nature of CNT. When the CNT concentrations are too high, the repulsive forces present on their surface could result in inhomogeneous dispersion of composite materials, ultimately leading to an abrupt decrease of elastic modulus and increase in fragility of the material.
Apart from that, in the same work by Wickham et alCitation61 which was discussed previously, the incorporation of T-CNT into PCL resulted in a significant increase in mechanical strength, but no morphological changes to the meshes was observed. It was found that there were significant differences in the mechanical properties of PCL meshes following the inclusion of T-CNT to the PCL. The inclusion of the T-CNT improved the tensile strength from 0.9 to 1.3 MPa, and elongation at the break by 2.5-fold, from 48% elongation at the break to 131%.
Chakoli et alCitation88 performed the synthesis of poly(l-lactide-co-ε-caprolactone)-functionalized multiwalled carbon nanotubes (MWCNT-OH-g-PCLA)s via grafting from approach and in situ ring-opening polymerization of l-lactide (LA) and ε-caprolactone (CL) using stannous octanoate as well as MWCNT-OH as initiating system. The surface of the prepared materials was studied using SEM. To demonstrate the effect of functionalization of MWCNTs and grafting of polymers on functionalized MWCNTs, transmission electron microscopy (TEM) analysis was carried out. The functional group present on MWCNTs was monitored with the help of Fourier transform infrared spectroscopy (FTIR). The glass transition, melting point, and thermal degradation of synthesized materials were studied by using differential scanning calorimetry (DSC) and thermo gravimetric analysis (TGA). To determine the mechanical reinforcement of PCLA using MWCNT-OH-g-PCLA, MWCNT-OH-g-PCLA/PCLA and pristine MWCNT/PCLA nanocomposites were prepared and compared. The tensile modulus, tensile strength, and elongation at failure of pure PCLA, MWCNT/PCLA, and MWCNT-OH-g-PCLA/PCLA composites were studied using a tensile test instrument. The result of the study shows that the nanocomposites based on functionalized MWCNTs demonstrate higher mechanical strength compared to controls, and this indicates that stress transfers from the matrix to the functionalized MWCNTs.
In a work done by Lee et alCitation89 an effective method was developed to produce homogeneous CNT-PCL nanocomposites with various controllable CNT concentrations using an ionically-modified MWCNT. The modified MWCNTs were found to be capable of dispersing homogeneously in tetrahydrofuran (THF). This was followed by the dissolving of PCL in the homogeneous MWCNT-CI (controllable CNT concentrations using an ionically-modified multi-walled CNT)/THF solution without agglomeration of MWCNT-PCL. The mechanical properties of PCL and MWCNT-PCL were studied by applying a tensile stress at room temperature. It was found that the presence of MWCNT in PCL resulted in increased tensile strength and elastic modulus of the samples. Moreover, even in presence of a lower level of nanotubes (0.25–1.0 wt%), the strength was improved by about 13%. The elastic modulus of MWCNT-PCL was enhanced by 55.5%–71.8% due to the inclusion of 0.25–0.5 wt% of the carbon nanotube. This study shows that a nanotube content of 0.25–0.5 wt% is the most effective in improving the mechanical strength of PCL. This evident improvement in mechanical strength by inclusion of a low nanotube content ascertains the homogeneous dispersion of MWCNT within PCL matrix. Hence, the MWCNT-PCL nanocomposite can be considered as a plausible candidate for the development of cardiovascular grafts. The properties which are enhanced by the use of nanocomposites for cardiovascular grafts are represented in .
Cardiovascular stents
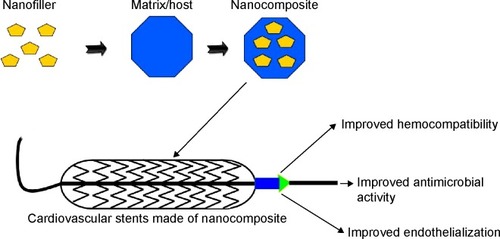
Atherosclerosis is a type of arteriosclerosis in which the artery wall thickens due to the accumulation of fatty deposits. If atherosclerosis occurs in coronary arteries and is left untreated, these blocked arteries may lead to myocardial infarction (heart attack) and even death.Citation90 There are two distinct types of stents that are widely used for treating the blocked coronary arteries, namely, bare-metal stents (BMS) and drug-eluting stents (DES). In-stent restenosis (ISR) caused by intimal hyperplasia occurs frequently in BMS whilst late stent thrombosis (ST) is quite common in DES.Citation91,Citation92 The ISR happens due to immunological responses mounted against the metal struts, whilst late ST originates from drug–polymer matrix hypersensitivity and impaired re-endothelialization.Citation93 In order to circumvent these issues, novel methods such as the development of a polymer–matrix platform and surface immobilized biomolecules are under intense investigation. Enhancement of hemocompatibility, antimicrobial activity, and endothelialization properties of nanocomposites and their ability to improve cardiovascular stents is discussed later in this review.
Nanocomposites to improve hemocompatibility of stent
Nowadays, angioplasty has been utilized commonly for atherosclerosis treatment. The use of a stent which is coated with antithrombogenic agents at the infected lesion site has been studied to regulate platelet aggregation.Citation94 Long-term stent implantation triggers smooth muscle cell migration and rupture of the atheroma thin layer, resulting in thrombosis and restenosis.Citation95 Hence, there is still an unmet demand for development of novel materials to antagonize in-stent restenosis and thrombosis.Citation95–Citation98 Thus, the nanocomposites which hold a great potential to improve stent hemocompatibility can be considered as a putative candidate for developing cardiovascular stents.
Tan et alCitation99 demonstrated the preparation as well as characterization of a POSS-PCU nanocomposite polymer with covalently attached anti-CD34 antibodies for improving the capture of circulating EPC. This can be utilized as a novel coating material for bare metal stents. This is done with the expectation that it may improve the re-endothelialization of bare metal stents used following balloon angioplasty. Biophysical characterization methods were implemented to investigate POSS-PCU and its subsequent functionalization with anti-CD34 antibodies. Water contact angle of the material determines the hydrophobicity/hydrophilicity nature of a material. POSS-PCU displayed a certain degree of hydrophobicity due to a higher contact angle and, following conjugation with anti-CD34 antibodies, the hydrophobicity of POSS-PCU decreased due to the decrease in its contact angle. Teflon® (DuPont, Hertfordshire, UK), which is an exceedingly hydrophobic material owing to its greater contact angle, was utilized as a positive control, and Acuvue® (Johnson & Johnson Medical Ltd, Berkshire, UK), an exceedingly hydrophilic material due to its very smaller contact angle, was utilized as a negative control. Tests were statistically significant (P<0.05) in relation to controls. The water contact angle results showed that the surface of POSS-PCU became more hydrophilic following the antibody attachment on the surface of the sample. This is because the polar groups on antibodies provide a higher surface energy, thereby lowering the surface’s water contact angle. Likewise, similar work done by other researchers also demonstrated a reduction in water contact angle after attachment of antibody onto polymer surfaces.Citation100
The thromboelastography (TEG) test results showed that POSS-PCU-coated TEG cups did not diverge much from controls, indicating that POSS-PCU does not have an inhibitory effect on clotting kinetics of human blood.Citation17 CD34 antibody-attached POSS-PCU (CD34-POSS-PCU) was coated on TEG cups; k-values, MA values, r-values, and α-angles were not statistically significant from uncoated TEG cups, thereby showing the hemocompatibility in terms of blood coagulation kinetics. The r-time denotes the reaction time, which is the time taken for the formation of the clot. It was observed that the uncoated TEG r-time was greater than the POSS-PCU coated cups r-time and less than CD34-POSS-PCU coated cups r-time. Similarly, k-time is the measure of speed at which the blood clot formed attains a size of 20 mm. It was found that uncoated TEG cups k-time was greater than POSS-PCU coated cups k-time but less than CD34-POSS-PCU k-time. Maximum amplitude (MA) is the breadth of the curve at a point where it provides us information on clot strength. TEG cups which were not coated had an MA greater than cups coated with POSS-PCU and CD34-POSS-PCU had the greatest MA. The α-angle measures the rate of increase of elastic shear modulus of the sample. TEG cups which were not coated had the highest α-angle followed by POSS-PCU coated cups and, finally, CD34-POSS-PCU coated cups. Tests were not statistically significant for all the above obtained values of r-time, k-time, MA, and α-angle from control (P>0.05). Hence, the above obtained result shows that POSS-PCU and CD34-POSS-PCU coated cups did not deviate away from uncoated TEG cups, thus proving their negligible effect on coagulation kinetics and their high degree of blood compatibility.
It is a well-known fact that the cell affinity toward biomaterials can be improved by immobilization of ECM proteins on the surface of the biomaterial. However, the properties of the POSS-PCU nanocomposite surface make it impossible to immobilize such biomolecules via this technique. In order to circumvent this issue, work was done by Solouk et alCitation101 to develop a predictive model for optimizing the operating conditions for grafting controlled amounts of carboxylic functional groups onto the surface of POSS-PCU. This can be followed by the coupling of ECM proteins on to the surface of POSS-PCU. Grafting of poly(acrylic acid) was done using a two-step plasma treatment, where grafted films were characterized via ATR-FTIR spectroscopy, SEM, and water contact angle measurements. The existence of the grafted layers was demonstrated by the appearance of a broad peak of the hydroxyl groups in ATR-FTIR spectrum, reduction in water contact angle, and morphological alterations identified by SEM micrographs. The effect of pretreatment and copolymerization time on the grafting density was studied, and it was optimized with the help of central composite design. Precision of the model was demonstrated, and it was shown to be high. In the case of process optimization, it was found that the maximum value of grafting density of 13.9±28 μ/cm2 can be achieved when the pretreatment time was 75 seconds and copolymerization time was 120 seconds. Thus, the investigators concluded that grafted POSS-PCU can serve as a good biomaterial with improved hemocompatibility for cardiovascular stent application.
Nanocomposites to improve antimicrobial activity of stent
In addition to biocompatibility, implants should also possess commendable antimicrobial properties to survive the attacks of pathogens. Apart from graft rejection, microbial activity can also lead to a platform for the pathogens to infect nearby tissues and organs. So, the presence of appreciable antimicrobial property is always wanted for a plausible stent material.Citation102,Citation103 Magnetic nanoparticles have diverse applications in biomedicine owing to their controllable size of <100 nm which confers on them the ability to attach to microbial cells. Magnetic biomaterials provide the versatility to be directed and concentrated within the target tissue via an external magnetic field and be removed when the therapy is completed.Citation104 The drug-loaded magnetic nanoparticles facilitate the controlled release of the drug, thereby reducing adverse effects due to the lower dosage. Furthermore, it also helps to minimize or prevent drug degradation and also provides a multitude of advantages like small particle size, larger surface area, magnetic response, biocompatibility, and nontoxicity.Citation105
In a recent work, iron oxide magnetic nanoparticles (MNPs) were prepared by the coprecipitation of iron salts in sodium hydroxide. Later, MNP was coated separately with chitosan (CS) and polyethylene glycol (PEG) to form CS-MNP and PEG-MNP nanoparticles followed by loading them with kojic acid (KA). KA is a pharmacologically bioactive compound, and when it is loaded with the abovementioned components, it will form KA-CS-MNP and KA-PEG-MNP nanocomposites.Citation106 The MNPs and their nanocomposites were investigated using powder X-ray diffraction, FTIR, thermogravimetric analysis, vibrating sample magnetometry, and SEM. The powder X-ray diffraction result of the sample showed that all formulations consisted of highly crystalline, pure magnetite Fe3O4. The FTIR spectroscopy and thermogravimetric study suggested that both polymers and KA were present in the nanocomposites. The magnetization curve indicated that both nanocomposites (KA-CS-MNPs and KA-PEG-MNPs) were superparamagnetic and possessed saturation magnetization of 8.1 and 26.4 emu/g, respectively. A loading of 12.2% and 8.3% was observed for the KA-CS-MNP and KA-PEG-MNP nanocomposites when KA drug loading was investigated using ultraviolet–visible spectroscopy. It was found that this release profile of KA followed pseudo second-order kinetic model. Powder X-ray diffraction patterns of the prepared MNPs, as well as KACS-MNP and KA-PEG-MNP nanocomposites, show six characteristic peaks for MNPs. The average size of the KA-CS-MNPs and KA-PEG-MNPs was determined to be 19.2 and 16.7 nm, respectively. FTIR spectra for MNPs, KA-CS-MNPs, KA-PEG-MNPs, and free KA drug were determined. The presence of KA bands in KA-CS-MNP and KA-PEG-MNP nanocomposites indicates the loading of KA on the CS-MNP and PEG-MNP nanoparticle surface since there is a peak specific to KA at 1,634 cm−1. Moreover, the band at 1,610 cm−1, which relates to the C=O of free KA was deviated to 1,563 and 1,568 cm−1 in KA-CS-MNP and KA-PEG-MNP nanocomposites. This shifting happens because of the loading of KA and binding with polymers by hydrogen bonds. The thermal behavior of MNPs before and after polymer coating as well as KA loading was studied using TGA. For MNPs, two important thermal events were observed. The first event was found to occur in the region of 30°C–220°C, which was caused due to the removal of surface hydroxyl groups as well as adsorbed water with a 3.9% weight loss. Later, the second stage event occurs at 220°C–850°C with a 1.9% weight loss.Citation107 SEM images of the pure MNPs and the KA-CS-MNP and KA-PEG-MNP nanocomposites demonstrate the presence of strong agglomeration of the synthesized MNPs owing to the van der Waals forces between the particles.
Further in the earlier study the antimicrobial activity of the synthesized KA-CS-MNP nanocomposite was investigated with an agar-based method and three bacterial strains.Citation106 The bacterial strains that were used are the strains that cause minor to life-threatening infections in the skin due to burns, and respiratory, urinary tract, gastrointestinal, or blood infections. The antimicrobial activity of MNPs separately and then with KA-loaded nanocomposites was tested. The result showed that MNPs had no antimicrobial effect on the microorganisms tested. On the other hand, the KA-CS-MNP and KA-PEG-MNP nanocomposites displayed minimal to no antimicrobial activity against the three microorganisms under the test conditions. In the case of Bacillus subtilis, the inhibition zone was identified to be one-half for KA alone and one-tenth for KA-CS-MNPs in comparison to the control. Likewise, for the methicillin-resistant Staphylococcus aureus strain, the zone of inhibition was identified to be two-thirds for KA alone and one-tenth for KA-CS-MNPs in comparison to control. For Salmonella enterica, the inhibition zone was found to be one-half for KA alone and one-fifteenth for the KA-CS-MNPs when compared to controls. Hence, all work done demonstrates the efficiency of the nanocomposites for antibacterial activity, where it can be used for stent development. A thorough literature survey indicates that although there have been various studies done on the antimicrobial activity of KA and its derivatives against selective microorganisms, however negligible, no work has been done for KA nanocomposites.Citation108–Citation110 We envisage that incorporation of derivatives of KA which has higher antibacterial activity may further disclose more information for the understanding of antimicrobial activity of KA-polymer-nanocomposites. Hence, KA-CS-MNP composition can be used as a plausible candidate for stent development.
In another independent study, researchers investigated the role of nystatin (Nyst) in a Nyst-CS-MNP nanocomposite.Citation111 Nyst is a tetraene diene polyene antibiotic showing a wide range of antifungal activity. The nystatin nanocomposite (Nyst-CS-MNP) was prepared by loading Nyst on chitosan (CS) coated magnetic nanoparticles (MNPs). The XRD results showed that the MNPs and nanocomposite are pure magnetite. The FTIR analysis ascertained the binding of CS on the surface of the MNPs as well as the loading of Nyst in the nanocomposite. The Nyst drug loading was determined using Ultraviolet–Visible spectroscopy, where it showed a 14.9% loading in the nanocomposite. The TEM size image of the MNPs, CS-MNP, and Nyst-CS-MNP was 13, 11, and 8 nm, respectively. The release profile of the Nyst drug from the nanocomposite was found to be a pseudo second-order kinetic model. They evaluated the antimicrobial activity of Nyst and Nyst-CS-MNP nanocomposite utilizing an agar diffusion method. Results of the study showed improved antifungal activity against Candida albicans. Thus, this Nyst-CS-MNP may be considered for improving the antimicrobial activity of the cardiovascular stent using Nyst in MNP.
Nanocomposites to improve endothelialization of stent
Coronary artery stenting has been widely used in the treatment of coronary artery disease.Citation112,Citation113 Drug-eluting stents, which make use of drugs which are capable of efficiently minimizing the proliferation and migration of smooth muscle, like Cypher, Taxus, etc, were introduced and clinically proven.Citation113 However, late angiographic stent thrombosis (LAST) seems to be a major threat, which often results in the failure of stent implantation in a clinical setting. This occurs as a result of poor endothelium-paving, either by injury and loss of the endothelium or by retardation of endothelialization in/after implantation, which are the prime contributors to this type of thrombosis.Citation114–Citation116 From this viewpoint, by improving the re-endothelialization or relining the denudated regions after implantation of the stent can be arguably the most efficient technique to enhance the clinical patency rate, especially in the case of small- and medium-diameter vascular prostheses. To attain rapid re-endothelialization, various techniques have been developed in the past few years, such as active endothelial cell coating and also the use of nanocomposites which promote endothelialization.Citation117 Thus, the various intriguing properties of nanocomposites which can be exploited for improving the endothelialization of stents are discussed as follows.
In a recent study performed by Oh and Lee,Citation118 nanofibers were loaded with nanoparticles which contain β-estradiol and this was used to avoid stent-induced restenosis via regulation of reactive oxygen species (ROS). In this work, Eudragit S-100 (ES), a polymer selected for its versatility, was utilized as a nanoparticle (NP) base, and the combination of hexafluoro-2-propanol (HFIP), PLGA, and polylactic acid (PLA) at different ratios was utilized as a nanofiber base. β-Estradiol was incorporated as the prime compound for alleviating the ROS activity at the subcellular level. Nile-Red served as a visual marker for this study. The coating of the stent was done with nanofibers synthesized by an electrospinning technique, which consists of a two-step process. This was followed by the determination of their properties like the loading capacity of β-estradiol, the release profiles of β-estradiol, cell cytotoxicity, and antioxidant responses to ROS. The properties were characterized and compared. Among different composite nanofibers loaded with nanoparticles, NPCHB (ES-NP with a chitosan layer added in H2O, mixed with HFIP) demonstrated maximal yield and drug-loading. The nanofibers of NP-CHB coated on a metallic mandrel conferred highest sustained release profile of β-estradiol. A confocal microscopy study indicated that the NP-W (ES-NP were dispersed in H2O, which was mixed with HFIP (1:1 (v/v) and then added with 15% PLGA) demonstrated a low fluorescence intensity of Nile-Red in comparison to the NP-HW (ES-NP were dispersed in H2O, which was mixed with HFIP [1:1 (v/v)] already containing 15% PLGA), demonstrating the decrease in stability of nanoparticles as the percentage volume of the organic solvent increased. The treatment of cells with increased level of H2O2 (>1 mM: as the ROS source) was found to be nonviable (81.1%±12.4%, P<0.01), indicating that ROS induced cell apoptosis and triggered the rupture of the atheroma thin layer in a concentration dependent manner. Nanofibers which contain β-estradiol proficiently protected human primary coronary artery endothelial cells (hPCECs) from ROS-induced cytotoxicity. The quantity of NO produced in hPCECs in the presence of β-estradiol after 6 days of incubation was found to be more than that of the control without β-estradiol. Hence, nanofibers loaded with nanoparticles which contain β-estradiol can be used as a plausible candidate for surface coating of a cardiovascular stent, to achieve improved endothelialization at the implanted sites of blood vessels.
In a recent study by Stout et alCitation69 CNF were embedded in PLGA to encourage cardiomyocyte growth in comparison with conventional polymer substrates. The study was conducted to find out the basic mechanism involved behind the cell growth on these novel nanocomposites.Citation69 The CNF were added to biodegradable PLGA (50:50 PGA:PLA weight ratio) to improve the conductivity, mechanical and cytocompatibility of pure PLGA. For the investigation of the effect of CNF inclusion in PLGA, varying PLGA to CNF ratios and densities were utilized, and their compatibility with cardiomyocytes was investigated. The cytocompatibility experiment results demonstrated that the cardiomyocytes were able to grow and express important biomarkers, including cardiac troponin T, connexin-43, and alpha-sarcomeric actin (α-SCA). The adhesion and proliferation experiment result showed that a PLGA density of 0.025 g/mL with a PLGA to CNF ratio of 75:25 and 50:50 (wt%) improved the overall cell growth. Moreover, a 55% increase in cardiomyocyte density was observed after 120 hours in comparison to pure PLGA, and a surprising 75% increase in cardiomyocytes was observed compared to the control at the same time point for 50:50 (wt%). Apart from that, the CNF-embedded PLGA materials were found to be conductive, and their conductivity increased appreciably when the amount of CNF to pure PLGA is also increased. Hence, CNF:PLGA nanocomposites with enhanced endothelialization can be considered as a biomaterial for cardiovascular stent development.
It was found that EPCs adhere and differentiate on CD34-POSS-PCU polymer films. However, there was no EPC capture on pure POSS-PCU films and nonspecific IgG-POSS-PCU films. The obtained result indicates that only CD34-POSS-PCU is capable of capturing EPCs, apart from serving as a platform for growth and proliferation of EPC. When the polymer was cultured with HUVECs and then examined with confocal microscopy studies, it showed that the CD34-POSS-PCU film was capable of supporting growth and proliferation of HUVEC cells.Citation93 The agglomeration of cells which occurred to a great extent on POSS-PCU-CD34 panels may be caused as a result of protein multiplexing. The AlamarBlue® (Life Technologies) assay was used to investigate biocompatibility of the samples. The result shows that cells adhered to POSS-PCU and CD34-POSS-PCU displayed a favorable level of normalized metabolic activity in the same way as the positive controls. Cells grown on empty tissue culture plates devoid of polymer films were assigned as the positive control, whilst 100% ethanol was utilized as the negative control. The metabolic activity was normalized to the positive controls. This was followed by a metabolic activity investigation for EPCs as well as HUVECs. The normalized metabolic activity observed for EPCs and HUVECs on four different samples was, in ascending order, as follows: negative control, POSS-PCU, POSS-PCU-CD34, and finally positive control. Ultimately, it was observed that the normalized metabolic activities of cells cultured on POSS-PCU and POSS-PCU-CD34 were statistically significant from controls (P<0.05). Thus, this result shows that either pure POSS-PCU or POSS-PCU-CD34 are capable of supporting growth and proliferation of EPCs and HUVECs to the same extent as positive controls.
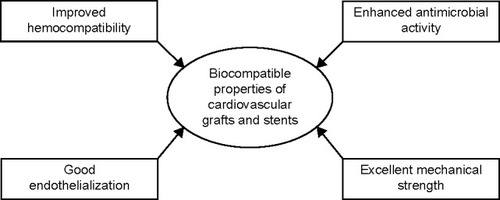
In order to assess the strength, viability, and stability of a covalently-linked antibody on the POSS-PCU surface, antibody-POSS-PCU-coated stents were kept in a custom-built flow circuit which mimics the physiological flow conditions in human arteries.Citation93 After 28 days, the stents were taken out from the custom-built flow circuit and were investigated using confocal microscopy. The result obtained from the confocal microscopy showed that antibodies were still attached to the surface of the stent, thereby substantiating its stability even under flow conditions. Preliminary data suggests that it was possible to culture EPCs in a bioreactor subjected to flow conditions, but the levels of EPCs were too low. Hence, there is scope for optimization of this technique. However, researchers have succeeded in demonstrating the stability of immobilized anti-CD34 antibodies even in flow conditions using the above method. The properties improved by the utilization of nanocomposites for cardiovascular stents are depicted in .
Conclusion
In recent years, nanocomposites utilizing POSS, BC, SF, MNPs, and CNTs have been explored for cardiovascular graft and stent application. BC inclusion in a material was found to improve the mechanical strength and blood compatibility of the nanocomposite. In the case of SF, it was observed that it improves the mechanical strength of the host in which it is added. When MNPs are incorporated in a matrix material, they are found to improve the antibacterial activity of the host, thereby suggesting their potential use for cardiovascular stent application. Likewise, POSS or CNT inclusion in a matrix improves hemocompatibility, endothelialization, and mechanical strength of the material in which they are added. The vital properties improved by nanocomposites are represented in . The key properties of discussed nanocomposites exploited for cardiovascular graft and stent application are tabulated in and , respectively.
Table 1 Nanocomposites used for cardiovascular grafts and their key properties
Table 2 Nanocomposites used for cardiovascular stents application and their key properties
The properties of nanocomposites make them suitable for diverse applications including cardiovascular grafts and stents. The limiting point of nanocomposites is their toxicity, which has to be tackled before utilizing them for medical applications. Although five plausible nanocomposites are discussed in this work, their toxicity tendency in in vivo conditions is not well established. In-depth studies on the patency rate and studies describing the toxicity of the nanocomposite to the surrounding tissues need to be performed. Moreover, the prediscussed nanocomposites demonstrated desirable characteristics for cardiovascular graft and stent applications in different experiment settings. The question of the best nanocomposite for cardiovascular applications like stents and grafts is difficult to promulgate unless experiments are conducted in similar settings. Hence, it is time for researchers to conduct a comparison study using these nanocomposites which will yield better understanding to promote them as plausible candidates in spearheading the campaign against cardiovascular disease.
Acknowledgments
This work was supported partly by the Ministry of Higher Education Malaysia with the Grant Vot number: R.J130000. 7809.4F444.
Disclosure
The authors report no conflicts of interest in this work.
References
- GoASMozaffarianDRogerVLHeart disease and stroke statistics – 2014 update: a report from the American Heart AssociationCirculation20141293e28e29224352519
- American Heart Association Available from: http://blog.heart.org/american-heart-association-statistical-repo000000rt-tracks-global-figures-first-time/Accessed March 5, 2015
- Biomaterials Market worth $88.4 Billion – 2017Markets and Markets Available from: http://www.marketsandmarkets.com/PressReleases/global-biomaterials.aspAccessed March 20, 2015
- WangZGMassimoCGLiMDeployment of endograft in the ascending aorta to reverse type A aortic dissectionAsian J Surg200326211711912732497
- KomarneniSFeature article. NanocompositesJ Mater Chem199221212191230
- HeinemannSHeinemannCJagerMNeunzehnJWiesmannHPHankeTEffect of silica and hydroxyapatite mineralization on the mechanical properties and the biocompatibility of nanocomposite collagen scaffoldsACS Appl Mater Interfaces20113114323433121942510
- WuJKamalyNShiJDevelopment of multinuclear polymeric nanoparticles as robust protein nanocarriersAngew Chem Int Ed Engl201453348975897924990548
- BoccacciniARErolMStarkWJMohnDHongZManoJFPolymer/bioactive glass nanocomposites for biomedical applications: a reviewCompos Sci Technol2010701317641776
- CatherineMSJoeyMFaridAAlexLRigobertoCADeboraFRGraphene nanocomposite for biomedical applications: fabrication, antimicrobial and cytotoxic investigationsNanotechnology2012233939510122962260
- HuangHYuanQYangXMorphology study of gold–chitosan nanocompositesJ Colloid Interface Sci20052821263115576077
- BertrandNWuJXuXKamalyNFarokhzadOCCancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biologyAdv Drug Deliv Rev20146622524270007
- MengGCaoAChengJYAjayanPMCarbon nanotubes grafted on silicon oxide nanowiresJ Nanosci Nanotechnol20044771271515570949
- SuhrJKoratkarNKeblinskiPAjayanPViscoelasticity in carbon nanotube compositesNat Mater20054213413715640807
- LeeALichtenhanJDViscoelastic responses of polyhedral oligosilsesquioxane reinforced epoxy systemsMacromolecules19983115497049749680436
- LiuYLHsuCYSuYHLaiJYChitosan-silica complex membranes from sulfonic acid functionalized silica nanoparticles for pervaporation dehydration of ethanol-water solutionsBiomacromolecules20056136837315638541
- YangHGZengHCSynthetic architectures of TiO2/H2Ti5O11.H2O, ZnO/H2Ti5O11.H2O, ZnO/TiO2/H2Ti5O11.H2O, and ZnO/TiO2 nanocompositesJ Am Chem Soc2005127127027815631476
- TanAGohDFarhatniaYAn anti-CD34 antibody-functionalized clinical-grade POSS-PCU nanocomposite polymer for cardiovascular stent coating applications: a preliminary assessment of endothelial progenitor cell capture and hemocompatibilityPloS One2013810e7711224116210
- SeifalianAMde MelAGhanbariHAhmedMChaloupkaKDarbyshireADevelopment of cardiovascular implants using nanocomposite polymer and stem cell technology: from lab to commercialisationAdv Sci Technol201076207213
- AlobaidNSalacinskiHJSalesKMNanocomposite containing bioactive peptides promote endothelialisation by circulating progenitor cells: an in vitro evaluationEur J Vasc Endovasc Surg2006321768316466940
- de MelAChaloupkaKMalamYDarbyshireACousinsBSeifalianAMA silver nanocomposite biomaterial for blood-contacting implantsJ Biomed Mater Res A201210092348235722528182
- KannanRYSalacinskiHJDe GrootJThe antithrombogenic potential of a polyhedral oligomeric silsesquioxane (POSS) nanocompositeBiomacromolecules20067121522316398518
- AhmedMGhanbariHCousinsBGHamiltonGSeifalianAMSmall calibre polyhedral oligomeric silsesquioxane nanocomposite cardiovascular grafts: influence of porosity on the structure, haemocompatibility and mechanical propertiesActa Biomater20117113857386721763798
- AhmedMHamiltonGSeifalianAMViscoelastic behaviour of a small calibre vascular graft made from a POSS-nanocompositeConf Proc IEEE Eng Med Biol Soc2010201025125421096748
- ConteMSThe ideal small arterial substitute: a search for the Holy Grail?FASEB J199812143459438409
- KannanRYSalacinskiHJButlerPEHamiltonGSeifalianAMCurrent status of prosthetic bypass grafts: a reviewJ Biomed Mater Res B2005741570581
- DesaiNPHubbellJABiological responses to polyethylene oxide modified polyethylene terephthalate surfacesJ Biomed Mater Res19912578298431833405
- SchmedlenRHElbjeiramiWMGobinASWestJLTissue engineered small-diameter vascular graftsClin Plast Surg200330450751714621299
- BalasubramanianVGrusinNKBucherRWTurittoVTSlackSMResidence-time dependent changes in fibrinogen adsorbed to polymeric biomaterialsJ Biomed Mater Res199944325326010397927
- BallykPDWalshCButanyJOjhaMCompliance mismatch may promote graft-artery intimal hyperplasia by altering suture-line stressesJ Biomech19983132292379645537
- PerktoldKLeuprechtAProsiMFluid dynamics, wall mechanics, and oxygen transfer in peripheral bypass anastomosesAnn Biomed Eng200230444746012085997
- TaiNRSalacinskiHJEdwardsAHamiltonGSeifalianAMCompliance properties of conduits used in vascular reconstructionBr J Surg200087111516152411091239
- FagerGThrombin and proliferation of vascular smooth muscle cellsCirc Res19957746456507554108
- RoqueMReisEDFusterVInhibition of tissue factor reduces thrombus formation and intimal hyperplasia after porcine coronary angioplastyJ Am Coll Cardiol20003672303231011127477
- IguchiMYamanakaSBudhionoABacterial cellulose – a masterpiece of nature’s artsJ Mater Sci2000352261270
- BackdahlHHeleniusGBodinAMechanical properties of bacterial cellulose and interactions with smooth muscle cellsBiomaterials20062792141214916310848
- KlemmDSchumannDUdhardtUMarschSBacterial synthesized cellulose – artificial blood vessels for microsurgeryProg Polym Sci200126915611603
- LeitãoAFGuptaSSilvaJPReviakineIGamaMHemocompatibility study of a bacterial cellulose/polyvinyl alcohol nanocompositeColloids Surf B Biointerfaces201311149350223880088
- KalbacovaMKalbacMDunschLKatauraHHempelUThe study of the interaction of human mesenchymal stem cells and monocytes/macrophages with single-walled carbon nanotube filmsPhys Status Solidi (b)20062431335143518
- LinYTaylorSLiHAdvances toward bioapplications of carbon nanotubesJ Mater Chem2004144527541
- YangNChenXRenTZhangPYangDCarbon nanotube based biosensorsSens Actuators B: Chem2015207Pt A690715
- BaslakCDemirel KarsMKaramanMKusMCengelogluYErsozMBiocompatible multi-walled carbon nanotube–CdTe quantum dot–polymer hybrids for medical applicationsJ Lumin2015160915
- MengJKongHXuHYSongLWangCYXieSSImproving the blood compatibility of polyurethane using carbon nanotubes as fillers and its implications to cardiovascular surgeryJ Biomed Mater Res A200574220821415962271
- ChouTCFuEWuCJYehJHChitosan enhances platelet adhesion and aggregationBiochem Biophys Res Commun2003302348048312615058
- SeifalianAMSalacinskiHJPunshonGKrijgsmanBHamiltonGA new technique for measuring the cell growth and metabolism of endothelial cells seeded on vascular prosthesesJ Biomed Mater Res200155463764411288093
- ShumanMALevineSPRelationship between secretion of platelet factor 4 and thrombin generation during in vitro blood clottingJ Clin Invest19806523073136243308
- ThachilJThe prothrombotic potential of platelet factor 4Eur J Intern Med2010212798320206875
- PrechelMMJeskeWPWalengaJMPhysiological changes in membrane-expressed platelet factor 4: implications in heparin-induced thrombocytopeniaThromb Res20101254e143e14819931120
- GoodRJContact angle, wetting, and adhesion: a critical reviewJ Adhes Sci Technol199261212691302
- VieiraEPRochaSCarmo PereiraMMohwaldHCoelhoMAAdsorption and diffusion of plasma proteins on hydrophilic and hydrophobic surfaces: effect of trifluoroethanol on protein structureLangmuir200925179879988619705886
- ZillaPFasolRDeutschMEndothelial cell seeding of polytetrafluoroethylene vascular grafts in humans: a preliminary reportJ Vasc Surg1987665355413320387
- SoloukACousinsBGMirzadehHSolati-HashtjinMNajarianSSeifalianAMSurface modification of POSS-nanocomposite biomaterials using reactive oxygen plasma treatment for cardiovascular surgical implant applicationsBiotechnol Appl Biochem201158314716121679238
- XiaXXiaYGold nanocages as multifunctional materials for nanomedicineFront Phys201493378384
- AiHLvovYMMillsDKJenningsMAlexanderJSJonesSACoating and selective deposition of nanofilm on silicone rubber for cell adhesion and growthCell Biochem Biophys200338210311412777710
- HesseYKampmeierJLangGKBaldysiak-FigielALangGEAdherence and viability of porcine lens epithelial cells on three different IOL materials in vitroGraefes Arch Clin Exp Ophthalmol20032411082382612937993
- KannanRYSalacinskiHJSalesKMButlerPESeifalianAMThe endothelialization of polyhedral oligomeric silsesquioxane nanocomposites: an in vitro studyCell Biochem Biophys200645212913616757813
- VaraDSPunshonGSalesKMSarkarSHamiltonGSeifalianAMEndothelial cell retention on a viscoelastic nanocomposite vascular conduit is improved by exposure to shear stress preconditioning prior to physiological flowArtif Organs2008321297798119133028
- de MelAPunshonGRameshBIn situ endothelialization potential of a biofunctionalised nanocomposite biomaterial-based small diameter bypass graftBiomed Mater Eng2009194–531733120042799
- GhanbariHde MelASeifalianAMCardiovascular application of polyhedral oligomeric silsesquioxane nanomaterials: a glimpse into prospective horizonsInt J Nanomedicine2011677578621589645
- L’HeureuxNDusserreNKonigGHuman tissue-engineered blood vessels for adult arterial revascularizationNat Med200612336136516491087
- HeWNieponiceASolettiLPericyte-based human tissue engineered vascular graftsBiomaterials201031328235824420684982
- WickhamAMIslamMMMondalDPolycaprolactone-thiophene-conjugated carbon nanotube meshes as scaffolds for cardiac progenitor cellsJ Biomed Mater Res B Appl Biomater201410271553156124664884
- DawRFinkelsteinJLab on a chipNature20064427101367367
- GiovambattistaNDebenedettiPGRosskyPJEffect of surface polarity on water contact angle and interfacial hydration structureJ Phys Chem B2007111329581958717658789
- ZhangQShenYTangCWuXYuQWangGSurface modification of coronary stents with SiCOH plasma nanocoatings for improving endothelialization and anticoagulationJ Biomed Mater Res B Appl Biomater2015103246447224919787
- MelchiorriAJHibinoNFisherJPStrategies and techniques to enhance the in situ endothelialization of small-diameter biodegradable polymeric vascular graftsTissue Eng Part B Rev201319429230723252992
- AhmedFChoudhuryNRDuttaNKZannettinoAKnottRNear superhydrophobic fibrous scaffold for endothelialization: fabrication, characterization and cellular activitiesBiomacromolecules201314113850386024047190
- GriffithAAThe Phenomena of Rupture and Flow in SolidsPhilosophical Transactions A of the Royal Society. Mathematical, Physical and Engineering Sciences1921221582–593163198
- GaoHJiBJägerILArztEFratzlPMaterials become insensitive to flaws at nanoscale: Lessons from natureProc Natl Acad Sci U S A2003100105597560012732735
- StoutDAYooJSantiago-MirandaANWebsterTJMechanisms of greater cardiomyocyte functions on conductive nanoengineered composites for cardiovascular applicationsInt J Nanomedicine201275653566923180962
- DingTYangHMaltenfortMXieRSilk fibroin added to calcium phosphate cement to prevent severe cardiovascular complicationsMed Sci Monit2010169HY23HY2620802422
- KrebsJAebliNGossBGCardiovascular changes after pulmonary embolism from injecting calcium phosphate cementJ Biomed Mater Res B Appl Biomater20078252653217285605
- BernardsJMirzaSLethality of embolized norian bone cement varies with the time between mixing and embolizationTransactions of the 50th Annual Meeting of the Orthopaedic Research Society2004San Francisco, CA254
- KongXCuiFWangXSilk fibroin regulated mineralization of hydroxyapatite nanocrystalsJ Cryst Growth2004270197202
- WangLNingGSennaMMicrostructure and gelation behavior of hydroxyapatite-based nanocomposite sol containing chemically modified silk fibroinColloids Surf A Physicochem Eng Asp2005254159164
- MillonLEWanWKThe polyvinyl alcohol-bacterial cellulose system as a new nanocomposite for biomedical applicationsJ Biomed Mater Res Part B Appl Biomater200679224525316680717
- EichhornSJBaillieCAZafeiropoulosNReview: current international research into cellulosic fibres and compositesJ Mater Sci200136921072131
- GuhadosGWanWHutterJLMeasurement of the elastic modulus of single bacterial cellulose fibers using atomic force microscopyLangmuir200521146642664615982078
- LupranoVARamiresPAMontagnaGMilellaENon-destructive characterization of hydrogelsJ Mater Sci Mater Med19978317517815348771
- HernándezRSarafianALópezDMijangosCViscoelastic properties of poly(vinyl alcohol) hydrogels and ferrogels obtained through freezing–thawing cyclesPolymer2004451655435549
- StammenJAWilliamsSKuDNGuldbergREMechanical properties of a novel PVA hydrogel in shear and unconfined compressionBiomaterials200122879980611246948
- ShengYJLinWJChenWCNetwork structures of polyhedral oligomeric silsesquioxane based nanocomposites: a Monte Carlo studyJ Chem Phys2004121199693970115538893
- XuHKuoS-WLeeJ-SChangF-CPreparations, thermal properties, and Tg increase mechanism of inorganic/organic hybrid polymers based on polyhedral oligomeric silsesquioxanesMacromolecules2002352387888793
- ZhangYYuYDolatiFOzbolatITEffect of multiwall carbon nanotube reinforcement on coaxially extruded cellular vascular conduitsMater Sci Eng C Mater Biol Appl20143912613324863208
- ZhangYYuYChenHOzbolatITCharacterization of printable cellular micro-fluidic channels for tissue engineeringBiofabrication20135202500423458889
- YuYZhangYMartinJAOzbolatITEvaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channelsJ Biomech Eng201313599101123719889
- KonigGMcAllisterTNDusserreNMechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary arteryBiomaterials20093081542155019111338
- Mattioli-BelmonteMVozziGWhulanzaYTuning polycaprolactone–carbon nanotube composites for bone tissue engineering scaffoldsMater Sci Eng C Mater Biol Appl2012322152159
- ChakoliANWanJFengJTAmirianMSuiJHCaiWFunctionalization of multiwalled carbon nanotubes for reinforcing of poly (l-lactide-co-ε-caprolactone) biodegradable copolymersAppl Surf Sci20092561170177
- LeeH-HShinU-SJinG-ZKimH-WHighly homogeneous carbon nanotube-polycaprolactone composites with various and controllable concentrations of ionically-modified-MWCNTsBull Korean Chem Soc2011321157161
- HanssonGKLibbyPThe immune response in atherosclerosis: a double-edged swordNat Rev Immunol20066750851916778830
- StoneGWEllisSGCannonLComparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trialJAMA2005294101215122316160130
- DaemenJWenaweserPTsuchidaKEarly and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort studyLancet2007369956266767817321312
- NiccoliGMontoneRAFerranteGCreaFThe evolving role of inflammatory biomarkers in risk assessment after stent implantationJ Am Coll Cardiol201056221783179321087705
- ByrneRAJonerMTadaTKastratiARestenosis in bare metal and drug-eluting stents: distinct mechanistic insights from histopa-thology and optical intravascular imagingMinerva Cardioangiol201260547348923018428
- BonaventuraKLeberAWSohnsCCost-effectiveness of paclitaxel-coated balloon angioplasty and paclitaxel-eluting stent implantation for treatment of coronary in-stent restenosis in patients with stable coronary artery diseaseClin Res Cardiol2012101757358422350752
- VedanthamKChaterjiSKimSWParkKDevelopment of a probucol-releasing antithrombogenic drug eluting stentJ Biomed Mater Res B Appl Biomater201210041068107722331580
- ThierryBWinnikFMMerhiYSilverJTabrizianMBioactive coatings of endovascular stents based on polyelectrolyte multilayersBiomacromolecules2003461564157114606881
- LiistroFAngioliPPortoIPaclitaxel-eluting balloon vs standard angioplasty to reduce recurrent restenosis in diabetic patients with in-stent restenosis of the superficial femoral and proximal popliteal arteries: the DEBATE-ISR studyJ Endovasc Ther20142111824502477
- TanAFarhatniaYGohDSurface modification of a polyhedral oligomeric silsesquioxane poly(carbonate-urea) urethane (POSS-PCU) nanocomposite polymer as a stent coating for enhanced capture of endothelial progenitor cellsBiointerphases2013812324706135
- ChongMSTeohSHTeoEYBeyond cell capture: antibody conjugation improves hemocompatibility for vascular tissue engineering applicationsTissue Eng Part A20101682485249520214450
- SoloukASolati-HashjinMNajarianSMirzadehHSeifalianAMOptimization of acrylic acid grafting onto poss-pcu nanocomposite using response surface methodologyIran Polym J Engl Ed201120291107
- JordanJRBloomHPrevention of bacterial infections in patients with CIED: old traditions meet new technologyJ Innovations Cardiac Rhythm Manag2010201016371
- AlfonsoFMorenoRVergasJFatal infection after rapamycin eluting coronary stent implantationHeart2005916e5115894752
- ColomboMCarregal-RomeroSCasulaMFBiological applications of magnetic nanoparticlesChem Soc Rev201241114306433422481569
- ZhengY-HChengYBaoFWangY-SSynthesis and magnetic properties of Fe3O4 nanoparticlesMater Res Bull2006413525529
- Hussein-Al-AliSHEl ZowalatyMEHusseinMZIsmailMDornianiDWebsterTJNovel kojic acid-polymer-based magnetic nanocomposites for medical applicationsInt J Nanomedicine2014935136224453486
- AlexiouCArnoldWHulinPMagnetic mitoxantrone nanoparticle detection by histology, X-ray and MRI after magnetic tumor targetingJ Magn Magn Mater20012251–2187193
- BrtkoJRondahlLFickovaMHudecovaDEyblVUherMKojic acid and its derivatives: history and present state of artCent Eur J Public Health200412 SupplS16S1815141965
- BalazSUherMBrtkoJRelationship between antifungal activity and hydrophobicity of kojic acid derivativesFolia Microbiol19933853873918262449
- AytemirMÖzçelikBSynthesis and biological activities of new Mannich bases of chlorokojic acid derivativesMed Chem Res2011204443452
- Hussein-Al-AliSHEl ZowalatyMEKuraAUAntimicrobial and controlled release studies of a novel nystatin conjugated iron oxide nanocompositeBiomed Res Int2014201413
- MauriLHsiehWHMassaroJMHoKKD’AgostinoRCutlipDEStent thrombosis in randomized clinical trials of drug-eluting stentsN Engl J Med2007356101020102917296821
- YinMYuanYLiuCWangJDevelopment of mussel adhesive polypeptide mimics coating for in-situ inducing re-endothelialization of intravascular stent devicesBiomaterials200930142764277319223071
- MengSLiuZShenLThe effect of a layer-by-layer chitosan-heparin coating on the endothelialization and coagulation properties of a coronary stent systemBiomaterials200930122276228319168214
- FinnAVKolodgieFDHarnekJDifferential response of delayed healing and persistent inflammation at sites of overlapping sirolimusor paclitaxel-eluting stentsCirculation2005112227027815998681
- LinQDingXQiuFSongXFuGJiJIn situ endothelialization of intravascular stents coated with an anti-CD34 antibody functionalized heparin-collagen multilayerBiomaterials201031144017402520149438
- Avci-AdaliMPaulAZiemerGWendelHPNew strategies for in vivo tissue engineering by mimicry of homing factors for self-endothelialisation of blood contacting materialsBiomaterials200829293936394518640715
- OhBLeeCHAdvanced cardiovascular stent coated with nanofiberMol Pharm201310124432444224050259