 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Realgar (AS4S4) has been used in traditional medicines for malignancy, but the poor water solubility is still a major hindrance to its clinical use. Realgar quantum dots (RQDs) were therefore synthesized with improved water solubility and bioavailability. Human endometrial cancer JEC cells were exposed to various concentrations of RQDs to evaluate their anticancer effects and to explore mechanisms by the MTT assay, transmission electron microscopy (TEM), flow cytometry, real-time reverse transcriptase polymerase chain reaction (RT-PCR) and Western blot analysis. Results revealed that the highest photoluminescence quantum yield of the prepared RQDs was up to approximately 70%, with the average size of 5.48 nm. RQDs induced antipro-liferative activity against JEC cells in a concentration-dependent manner. In light microscopy and TEM examinations, RQDs induced vacuolization and endoplasmic reticulum (ER) dilation in JEC cells in a concentration-dependent manner. ER stress by RQDs were further confirmed by increased expression of GADD153 and GRP78 at both mRNA and protein levels. ER stress further led to JEC cell apoptosis and necrosis, as evidenced by flow cytometry and mitochondrial membrane potential detection. Our findings demonstrated that the newly synthesized RQDs were effective against human endometrial cancer cells. The underlying mechanism appears to be, at least partly, due to ER stress leading to apoptotic cell death and necrosis.
Introduction
Realgar (AS4S4) has been used in traditional medicines since ancient times. In ancient China, realgar (called Xiong-Huang) was applied in the treatment of several diseases including headache, vertigo, sore throat, mouth ulcers, tongue ulcers, etc.Citation1,Citation2 In recent years, the effects of realgar on the proliferation and apoptosis of human ovarian and cervical cancer cells,Citation3 human chronic myelogenous leukemia K562 cells,Citation4 and human promyelocytic leukemia HL-60 cellsCitation5 have been reported. However, poor water solubility and the resultant poor bioavailability greatly hamper the clinical applications of realgar.Citation6 Realgar nanoparticles were thus produced in an attempt to overcome such a hindrance.Citation2,Citation7
Compared with the crude realgar powders, the smaller realgar nanoparticles did improve the bioavailability, as manifested by the increased cytotoxicity toward tumor cells and increased urinary excretion of total arsenic in rats.Citation8 In general, realgar nanoparticles were more effective than crude realgar in antitumor studies.Citation1,Citation9 Recently, we have developed realgar quantum dots (RQDs) of smaller sizes, namely 5.48 nm.Citation10 The cytotoxic effects of realgar nanoparticles and RQDs on human gynecological cell lines (C180-13S, OVCAR, OVCAR-3, HeLa) were comparable to those of arsenic trioxide,Citation2,Citation8,Citation9 but less toxic against normal human lung fibroblast cells (MRC-5) and colonic fibroblast cells (CCD-18Co).Citation9 Having recently found that RQDs are also effective against cervical U14 tumor growth in U14 tumor-bearing mice,Citation10 we speculate that RQDs may demonstrate better activity and bioavailability in gynecological cancer therapy.
In this study, we investigated the cytotoxicity of RQDs in the JEC cells, an endometrial cancer cell line,Citation11 and found that RQDs-induced endoplasmic reticulum (ER) stress is important in its antitumor effects.
Materials and methods
Instruments, chemicals, and cell line
Ultravoilet-visible (UV-vis) absorption spectrum was recorded on a Shimadzu UV 2450PC spectrophotometer (Shimadzu Corp., Kyoto, Japan). Photoluminescence (PL) spectrum was monitored on a Perkin Elmer LS 55 luminescence spectrometer (PerkinElmer Inc., Waltham, MA, USA). High-resolution (HR) transmission electron microscopy (TEM) images were obtained with an FEI Tecnai G2F30 microscope (FEI, Hillsboro, OR, USA). The as-prepared realgar QDs were appropriately diluted with Milli-Q water before instrumental analysis.
Methods
Synthesis of RQDs
Approximately 2.0 g of arsenic sulfide chemical was dispersed into 50 mL of ethanolamine under Ar gas bubbling and ultrasonication at 100 W for 15 minutes. This was followed by centrifugation at 1,500 rpm for 5 minutes to remove undissolved residue. The resulting supernatant was transferred to a 250 mL three-neck flask and heated to 80°C with constant stirring for 12 hours. Then, the saturated water solution of citric acid was added dropwise to the above hot synthetic solution till pH 8.0±0.2, which was kept at 80°C for another 36 hours. No size sorting was applied to any sample used for characterization in this work.
Determination of photoluminescence quantum yield
The PL quantum yield (PL QY) of the synthesized RQDs was estimated by using the comparative method proposed by Williams et alCitation12 after slight modification as follows:
Rhodamine B (Sigma-Aldrich Co., St Louis, MO, USA) is used as the standard for the PL QY calculation. To minimize reabsorption effects, the absorbance values of the standard and RQDs were controlled to be less than 0.05 at the respective excitation wavelengths. We chose 365 nm as the excitation wavelength for RQDs and 550 nm for Rhodamine B.
Characterization of RQDs
UV-vis absorption spectrum was recorded on a Shimadzu UV 2450PC spectrophotometer (Shimadzu Corp.). PL spectrum was monitored on a Perkin Elmer LS 55 luminescence spectrometer (PerkinElmer Inc.). Upconversion fluorescence spectrum was recorded by a Tsunami® femtosecond Ti:sapphire oscillator (Spectra-Physics, Santa Clara, CA, USA). HRTEM images were observed with a FEI Tecnai G2F30 microscope (FEI). The as-prepared RQDs were appropriately diluted with Milli-Q water before instrumental analysis. Scanning electron microscopy (SEM) images were taken by a Hitachi S-4300 microscope (Hitachi Ltd., Tokyo, Japan). To prepare the samples for SEM imaging, dried precipitates obtained by adding formic acid into the solution of synthesized RQDs were firstly scattered onto carbon conductor tape followed by coating with gold.
Cell culture
JEC cells were cultivated in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific, Waltham, MA, USA) (Hyclone) supplemented with 10% fetal calf serum (Gibco® = product line of Thermo Fisher Scientific, Waltham, MA, USA) and cultured in a humidified atmosphere of 5% CO2 at 37°C.
Determination of cell proliferation
The sensitivity of JEC cells to RQDs was determined using the MTT colorimetric assay. In brief, 10 µL MTT reagents was added into each well and incubated at 37°C for 4 hours. The supernatant was aspirated, and formazan crystals were dissolved in 100 µL dimethyl sulfoxide (DMSO) at room temperature (RT) for 10 minutes with gentle agitation. Absorbance of each sample was measured at 570 nm using a Thermo Multiskan Spectrum microplate reader (Thermo Scientific, Cleveland, OH, USA).
Transmission electron microscopy
For TEM analysis, cell pellets were fixed overnight in 3.5% glutaraldehyde (0.1 M, pH 7.4 in Sorensen’s buffer) at 4°C and subsequently processed for TEM observation. The cells were observed and photographed under a transmission electron microscope (Hitachi-7650, Hitachi Ltd.).
Annexin V/propidium iodide double staining for apoptosis
JEC cells were stained with both Annexin V-FITC (fluorescein isothiocyanate) and propidium iodide (PI) using the Annexin-V-FITC/PI staining kit (Zoman Biotechnology, Beijing, People’s Republic of China) in accordance with the manufacturer’s instructions. Briefly, the cells (3×105 cells/well) were seeded in six-well plates and cultured overnight, followed by treatment of RQDs. Thereafter, the cells were digested with 0.25% trypsin and washed twice with PBS. The cells were suspended in 500 µL binding buffer, 5 µL Annexin V-FITC was added, and the resulting mixture was carefully agitated. Then, 10 µL PI was added to the mixture. Following incubation for 20 minutes at RT, the ratio of apoptosis was detected using a FACSCalibur and CellQuest software (Beckman Coulter, Brea, CA, USA).
Determination of mitochondrial membrane potential (JC-1 staining) by fluorescence microscopy
JEC cells were incubated in a medium containing 500 µL JC-1 dye (Molecular probes, Fanbo Biochemicals, Beijing, People’s Republic of China) for 20 minutes at 37°C, and washed with 1× JC-1 buffer. Subsequently, the cells were excited at 488 nm, and the fluorescence emission was recorded at 530 nm and 590 nm, respectively. Images were captured using the Leica SP2 confocal microscope (Leica Microsystems, Wetzlar, Germany).
mRNA extraction and real-time PCR
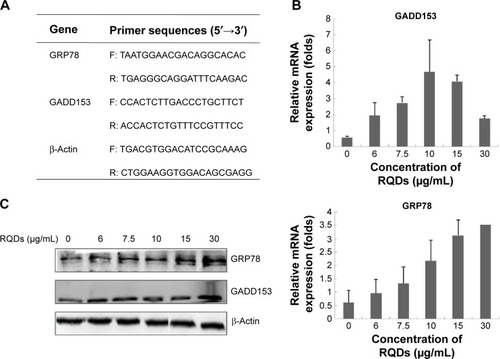
Total RNA was extracted using TRIquick Reagent (Solarbio, Beijing, People’s Republic of China), and reverse transcription was carried out with M-MuLV reverse transcriptase (Takara) according to the manufacturer’s protocol. For PCR amplification, the primer sequences for GADD153 and GRP78 (the target gene each) as well as β-actin (an internal control gene) are given in . Real-time PCR was done using the CFX96 Touch Detection System (Bio-Rad Laboratories Inc., Hercules, CA, USA) according to the manufacturer’s instructions. Finally, the comparative CT (2−ΔΔCT) method was used to determine the relative concentration of the amplified products.
Figure 1 The expression of ER stress marker in JEC cells treated with different concentrations of RQDs.
Notes: (A) Primer sequences for real-time PCR. (B) The expression of GRP78 and GADD153 mRNA. (C) The expression of GRP78 and GADD153 protein.
Abbreviations: RQDs, realgar quantum dots; ER, endoplasmic reticulum; PCR, polymerase chain reaction.
Western blot analysis
Western blot analysis was performed according to the standard procedure. Briefly, cell lysates with equal amounts of proteins were loaded, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels, and transferred onto the nitrocellulose membrane. The membrane was blocked with 5% BSA (bovine serum albumin) for 1 hour and was incubated with specific primary antibodies (CST, CA, USA) at 4°C overnight, followed by appropriate horseradish peroxidase-conjugated secondary antibodies at RT for 1 hour. The bands were detected with the use of the enhanced chemiluminescence system.
Statistical analysis
All the data were analyzed using the SPSS 7.5-Windows Student’s version software (SPSS Inc., Chicago, IL, USA). For all the measurements, one-way ANOVA followed by Tukey’s test was used to assess the statistical significance between groups. P≤0.05 was considered to be statistically significant.
Results
Using ethanolamine and citric acid to synthesize RQDs
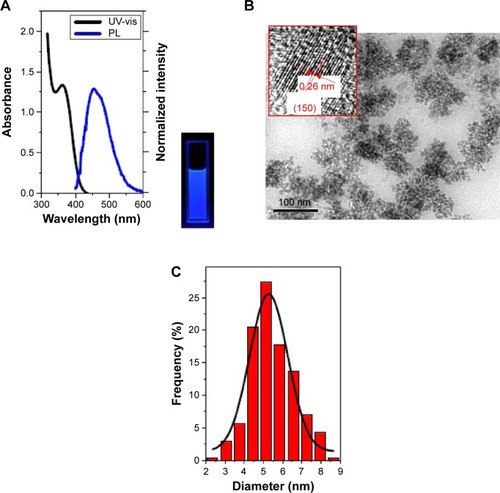
The synthesis was directly monitored by observing the fluorescence of the aliquots taken from the synthetic system under a 365 nm UV lamp. The as-prepared RQDs were characterized using ethanolamine and citric acid. shows UV-vis absorption and PL emission spectra of RQDs colloidal solution after appropriate dilution, demonstrating typical band-edge emission of QDs. The representative PL emission peak centered at 475 nm is well consistent with blue–cyan fluorescence observed under 365 nm UV light (). Single and symmetric PL emission peak shape suggests a narrow size distribution of the current RQDs (). Well-dispersed RQDs with an average size of 5.48±1.09 nm and a size distribution of ~20% were observed in the TEM images and the corresponding size histogram (), well consistent with the corresponding optical spectra findings. Clearly resolved lattice fringes observed in a representative HRTEM image reveal the highly crystalline nature of the synthesized RQDs, in which an interplanar spacing of ~0.26 nm matches the (150) facet of α-As4S4 crystal ( inset).
Figure 2 The characters of RQDs.
Notes: (A) UV-vis absorption and photoluminescence emission spectra of the as-prepared RQDs using ethanolamine and citric acid. Inset shows fluorescence photo under 365 nm UV light. (B) TEM and HRTEM images (inset) of the above RQDs, with the arrows emphasizing the 0.31 nm distance of parallel between them. (C) Gives the corresponding size histogram and curve.
Abbreviations: UV-vis, ultravoilet-visible emission spectra; PL, photoluminescence emission spectra; RQDs, realgar quantum dots; TEM, transmission electron microscopy; HRTEM, high-resolution TEM.
RQDs inhibit viability of human endometrial cancer
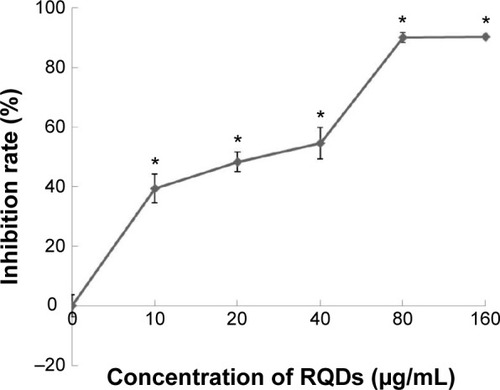
JEC cells and induce vacuolization and ER dilation JEC cells were treated with a range of concentrations of RQDs (10, 20, 40, 80, 160 µg/mL) for 48 hours. The percent cell viability compared with vehicle-treated controls was determined using the MTT assay. The cytotoxicity experiment () showed that RQDs reduced cell viability in a dose-dependent manner, with the highest decrease (approximately 90%) occurring with 80 µg/mL.
Figure 3 Concentration-dependent effects of RQDs on the cell viability of human endometrial cancer cells JEC.
Notes: After treatment of RQDs (10, 20, 40, 80, and 160 µg/mL) for 48 hours, the cell viability was analyzed using the MTT assay. The data are expressed as the mean ± SD of three experiments. *P<0.05 indicates statistically significant differences from the control group.
Abbreviations: RQDs, realgar quantum dots; SD, standard deviation.
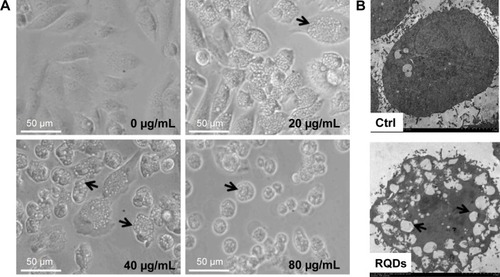
When we examined the cellular morphologies following treatment of RQDs, widespread vacuolization of JEC cells was observed after 24 hours treatment (), and it was more serious with increasing concentration of RQDs. The gross morphological changes were further confirmed by electron microscopy. The treated cells displayed distension of the ER ().
Figure 4 Morphological change of JEC cells treated with RQDs.
Notes: (A) Vacuolization induced by RQDs, with the arrows indicating the vacuolization and ER dilation. (B) Ultrastructural analysis of JEC cells by ultrathin section transmission electron microscopy.
Abbreviations: Ctrl, control; RQDs, realgar quantum dots.
RQDs induce JEC cells apoptosis
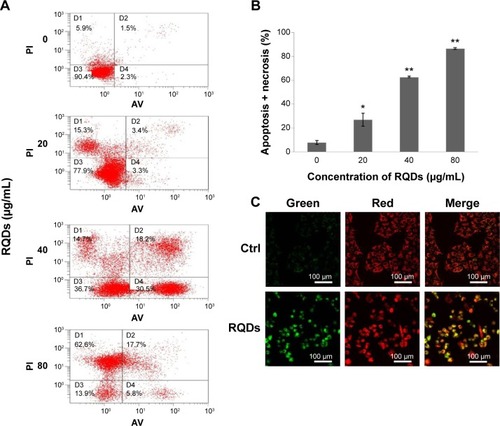
Annexin V-FITC/PI staining analysis demonstrated that treatment of RQDs resulted in a significant increase in apoptosis and necrosis in JEC cells (). In the 80 µg/mL group, the rate of apoptosis and necrosis was significantly increased to more than 80% after 24 hours treatment, higher than the other groups (P<0.01) (). Measurement of mitochondrial membrane potential (Δψm) is one of the methods used for studying the initiation of apoptosis. To explore the effects of RQDs on the mitochondrial membrane potential, JEC cells were cultured in RQDs at 15 µg/mL concentration for 24 hours and then processed for confocal microscopy analysis after being stained with fluorescent probe JC-1. After treatment with RQDs, the cells show increased green fluorescence (). This suggests that RQDs disrupt the mitochondrial membrane potential, resulting in the cytosolic accumulation of monomeric JC-1 as an indicator of apoptosis through the intrinsic pathway. Taken together, these data suggest that RQDs induce apoptosis and necrosis of JEC cells by activation of the mitochondrial pathway.
Figure 5 Induction of apoptosis in JEC cells following the RQDs treatment.
Notes: (A) Cells were treated with different concentrations of RQDs for 24 hours, and stained with Annexin V-FITC and PI, and then apoptotic cells were quantified by flow cytometry. In order to quantify the apoptotic rate, different subpopulations were distinguishable: D1, Annexin V-negative but PI-positive, ie, necrotic cells; D2, Annexin V/PI-double positive, ie, late apoptotic cells; D3, Annexin V/PI-double negative, ie, live cells; D4, Annexin V-positive but PI-negative, ie, early apoptotic cells. (B) The apoptotic rate was determined as the percentage of D2 + D4.Citation28 (C) Mitochondrial membrane potential (ΔΨm) was analyzed using JC-1 mitochondrial membrane dye. JEC cells were treated with DMSO (control) or RQDs (15 µg/mL) for 24 hour. The drug caused an increase in the green (JC-1 monomers) and decrease in the red fluorescence (JC-1 aggregates), indicative of loss of ΔΨm. *P<0.05, **P<0.01.
Abbreviations: RQDs, realgar quantum dots; PI, propidium iodide; DMSO, dimethyl sulfoxide; FITC, fluorescein isothiocyanate; ctrl, control; AV, annexin V.
Impact of RQDs on the ER stress signaling pathway in JEC cells
To further assess ER stress involved in RQDs-induced apoptosis of JEC cells, the expression of the genes of ER stress, GRP78 (BIP) and GADD153 (CHOP), were detected by real-time reverse transcriptase polymerase chain reaction (RT-PCR) and Western blots. The results showed that RQDs induced a concentration-dependent GRP78 activation (). The mRNA expression level of GRP78 was increased 5.77-fold in the 30 µg/mL group. The expression level of GADD153 was increased with the concentration of RQDs, the mRNA expression level of GADD153 was increased 7.98-fold in the 10 µg/mL group, while it slightly decreased if the concentration of RQDs was more than 10 µg/mL, probably due to cell death. The Western blot further confirmed the induction of GRP78 and GADD153 at the protein levels (). These results clearly indicate that RQDs induced ER stress in the JEC cells.
Discussion
Realgar (As4S4) presents promising anticancer effects.Citation2 However, realgar is insoluble in water, resulting in poor bioavailability. RQDs are ideal to solve the problem.
To our knowledge, only one research group has addressed synthesis of realgar nanoparticles by a chemical method involving top–down and bottom–up procedures.Citation9,Citation13 They used ethylenediamine as a coordinating solvent to dissolve the coarse realgar powder in the first step and, in the following step, added acrylic acid as a protic agent to trigger the growth of RQDs. Encouraged by the previous reports, we chose less toxic ethanolamine and citric acid to synthesize RQDs via a similar method after modification. The highest PL QY of the as-prepared RQDs using ethanolamine and citric acid was up to the yield of ~70%, much better than the reported RQDs using ethylenediamine and acrylic acid with 15% PL QY.Citation9 The RQDs are effective against human gynecological cell lines (C180-13S, OVCAR, OVCAR-3, HeLa)Citation2,Citation9 and against cervical tumor growth in mice without producing overt toxicity.Citation10 In this study, we further demonstrated that RQDs are effective against human endometrial cancer (JEC) cells, suggesting that the smaller size of realgar in the form of RQD is promising for gynecological malignancy tumors.
Endometrial cancer is one of the most common gynecological malignancy tumors. Primary treatments consist of surgery, radiotherapy, and chemotherapy in most cases.Citation14 Newly effective chemotherapeutic drugs are still greatly desired.Citation15 The current study using endometrial cancer JEC cells clearly demonstrated that RQDs are effective in killing gynecological cancer cells, and that this effect is mediated through the ER stress signaling pathway.
Arsenic is known to induce stress responses.Citation16 ER stress is initially shaped to reestablish ER homeostasis through the activation of an integrated intracellular signal transduction pathway termed as unfolded protein response (UPR). However, when ER stress is too severe or prolonged, the UPR turns from a prosurvival signal to a prodeath response, which is predominantly executed by mitochondrial apoptosis.Citation17 ER stress was suggested to be associated with arsenic effects in cells such as CD4+ T lymphocytesCitation18 chronic myeloid leukemia cell line K562,Citation19 laryngeal squamous cell line Hep-2 cells,Citation20 and in intact animals such as in mouse cerebrumCitation21 and in liver.Citation16 The current study clearly demonstrated that RQDs can also produce ER stress in JEC cells, as evidenced by widespread vacuolization with light microscopy and TEM. ER vacuolization is a characteristic of RQDs-induced morphological changes associated with the distension of ER. GRP78 and CHOP/GADD153 are typical ER stress protein genes.Citation22,Citation23 Our study revealed that RQDs-induced ER stress (as evidenced by morphology and increased GRP78 and GADD153) is an important mechanism for its anticancer effects.
Mitochondria represent key organelles for cell survival. JC-1 is a probe for the analysis of mitochondrial transmembrane potential changes occurring very early in apoptosis.Citation24 The results of JC-1 staining observed in this study indicate that RQDs are capable of decreasing the mitochondrial Δψm and thereby inducing apoptosis in JEC cells.
Targeting apoptosis pathways is an important strategy in cancer therapy. As a programmed cell death, apoptosis occurs through two main pathways, extrinsic (Fas) and intrinsic (mitochondria). Both pathways converge to final targets caspases and lead to cell death.Citation25 In this study, we found that RQDs induced JEC cell apoptosis and necrosis, as evidenced by flow cytometry. The RQDs-induced apoptosis is clearly associated with mitochondrial membrane loss through ER stress, and it has been reported that when an ER stress is prolonged, it promotes the apoptotic process.Citation26 Recently, the molecular mechanisms of realgar nanoparticle-mediated melanoma cell killing were described, including autophagy and apoptosis, induction, as well as modulation of NF-kB, Akt, and MAP kinase signaling pathways.Citation27 In our other work, we also found that RQDs are effective in induction of apoptosis protein Bax and suppression of antiapoptotic protein Bcl-2 in HepG2 cells.
Conclusion
In conclusion, the PL QY of RQD using ethanolamine and citric acid is up tô70%, and the newly synthetic RQDs are a promising candidate anticancer agent against endometrial cancer cells by inducing apoptosis and necrosis. RQDs-induced ER stress and the loss of membrane potential appear to be important for RQDs to kill tumor cells.
Acknowledgments
This study was supported by the Science and Technology Foundation of Guizhou (CK-856, 2013-03) and the Key Lab Construction Project of the Educational Department of Guizhou (KY[2014]212), postdoctoral fel lowship, Harbin Institute of Technology (HIT) (AUGA4110005410), the Fundamental Research Funds for the Central Universities (HIT. IBRSEM. 201331), and Program for Innovation Research of Science in Harbin Institute of Technology (PIRS of HIT 201411).
Disclosure
The authors report no conflicts of interest in this work.
References
- AnYLNieFWangZYZhangDSPreparation and characterization of realgar nanoparticles and their inhibitory effect on rat glioma cellsInt J Nanomedicine201163187319422238507
- WuJZShaoYBLiuJLChenGHoPCThe medicinal use of realgar (As4S4) and its recent development as an anticancer agentJ Ethnopharmacol201113559560221497649
- DuYHHoPCArsenic compounds induce cytotoxicity and apoptosis in cisplatin-sensitive and -resistant gynecological cancer cell linesCancer Chemother Pharmacol200147648149011459200
- LiJEWuWLWangZYSunGLApoptotic effect of As2S2 on K562 cells and its mechanismActa Pharmacol Sin2002231199199612421474
- YeHQGanLYangXLXuHBMembrane-associated cytotoxicity induced by realgar in promyelocytic leukemia HL-60 cellsJ Ethnopharmacol2006103336637116174554
- ZhaoWLuXYuanYEffect of size and processing method on the cytotoxicity of realgar nanoparticles in cancer cell linesInt J Nanomedicine201161569157721845047
- TianYWangXXiREnhanced antitumor activity of realgar mediated by milling it to nanosizeInt J Nanomedicine2014974575724516332
- WuJZHoPCEvaluation of the in vitro activity and in vivo bioavailability of realgar nanoparticles prepared by cryogrindingEur J Pharm Sci2006291354416824739
- WangJLinMZhangTArsenic(II) sulfide quantum dots prepared by a wet process from its bulkJ Am Chem Soc200813035115961159718693730
- QinYJinFJinHWuJZLiuJAnti-tumor effects of realgar quantum dots and Liu-Shen-Wan in tumor-bearing miceChin J New Drug Clin Rem
- WuZMLiuZLLiWCEstablishment of the cell line JEC-9 of a human endometrial adenocarcinomaActa Acad Med Zunyi19911414447 Chinese
- WilliamsATRWinfieldSAMillerJNRelative fluorescence quantum yields using a computer-controlled luminescence spectrometerAnalyst198310810671071
- WangJZLohKPWangZFluorescent nanogel of arsenic sulfide nanoclustersAngew Chem Int Ed2009483462826285
- GalaalKAI MoundhriMBryantALopesADLawrieTAAdjuvant chemotherapy for advanced endometrial cancerCochrane Database Syst Rev20145CD01068124832785
- WrightJDBarrena MedelNISehouliJFujiwaraKHerzogTJContemporary management of endometrial cancerLancet201237998231352136022444602
- LiuJKadiiskaMBLiuYLuTQuWWaalkesMPStress-related gene expression in mice treated with inorganic arsenicalsToxicol Sci200161231432011353140
- VerfaillieTGargADAgostinisPTargeting ER stress induced apoptosis and inflammation in cancerCancer Lett2013332224926420732741
- LiKZhangLXiangXArsenic trioxide alleviates airway hyperresponsiveness and promotes apoptosis of CD4+ T lymphocytes: evidence for involvement of the ER stress-CHOP pathwayIr Med Sci20131824573583
- DuYWangKFangHCoordination of intrinsic, extrinsic, and endoplasmic reticulum-mediated apoptosis by imatinib mesylate combined with arsenic trioxide in chronic myeloid leukemiaBlood200610741582159016249384
- YangXAnLLiXArsenic trioxide induced endoplasmic reticulum stress in laryngeal squamous cell line Hep-2 cellsAuris Nasus Larynx2014411818323880367
- YenCCHoTJWuCCInorganic arsenic causes cell apoptosis in mouse cerebrum through an oxidative stress-regulated signaling pathwayArch Toxicol201185656557521533816
- LakshmananAPThandavarayanRAPalaniyandiSSModulation of AT-1R/CHOP-JNK-Capase12 pathway by olmesartan treatment attenuates ER stress-induced renal apoptosis in streptozotocin-induced diabetic miceEur J Pharm Sci201144562763422033153
- WuTDongZGengJValsartan protects against ER stress-induced myocardial apoptosis via CHOP/Puma signaling pathway in streptozotocin-induced diabetic ratsEur J Pharm Sci20114249650221345370
- AnsilPNWillsPJVarunRLathaMSCytotoxic and apoptotic activitiesxs of Amorphophallus campanulatus (Roxb.) Bl. tuber extracts against human colon carcinoma cell line HCT-15Saudi J Biol Sci201421652453125473360
- GhobrialIMWitzigTEAdjeiAATargeting apoptosis pathways in cancer therapyCA Cancer J Clin200555317819415890640
- ChenLXuSWenXXuYChenJTengJCab45S inhibits the ER stress-induced IRE1-JNK pathway and apoptosis via GRP78/BiPCell Death Dis20145e121924810055
- PastorekMGronesovaPCholujovaDRealgar (As4S4) nanoparticles and arsenic trioxide (As2O3) induced autophagy and apoptosis in human melanoma cells in vitroNeoplasma201461670070925150315
- LiuXGaoRChenXPossible roles of mmu-miR-141 in the endometrium of mice in early pregnancy following embryo implantationPLoS One201386e6738223825654
