Abstract
Background
Silver nanoparticles (Ag-NPs) can enter the brain and induce neurotoxicity. However, the toxicity of Ag-NPs on the blood–brain barrier (BBB) and the underlying mechanism(s) of action on the BBB and the brain are not well understood.
Method
To investigate Ag-NP suspension (Ag-NPS)-induced toxicity, a triple coculture BBB model of rat brain microvascular endothelial cells, pericytes, and astrocytes was established. The BBB permeability and tight junction protein expression in response to Ag-NPS, NP-released Ag ions, and polystyrene-NP exposure were investigated. Ultrastructural changes of the microvascular endothelial cells, pericytes, and astrocytes were observed using transmission electron microscopy (TEM). Global gene expression of astrocytes was measured using a DNA microarray.
Results
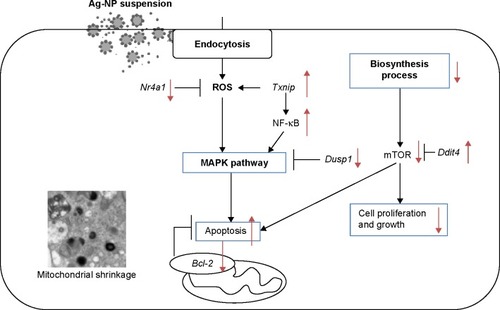
A triple coculture BBB model of primary rat brain microvascular endothelial cells, pericytes, and astrocytes was established, with the transendothelial electrical resistance values >200 Ω·cm2. After Ag-NPS exposure for 24 hours, the BBB permeability was significantly increased and expression of the tight junction (TJ) protein ZO-1 was decreased. Discontinuous TJs were also observed between microvascular endothelial cells. After Ag-NPS exposure, severe mitochondrial shrinkage, vacuolations, endoplasmic reticulum expansion, and Ag-NPs were observed in astrocytes by TEM. Global gene expression analysis showed that three genes were upregulated and 20 genes were downregulated in astrocytes treated with Ag-NPS. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis showed that the 23 genes were associated with metabolic processes, biosynthetic processes, response to stimuli, cell death, the MAPK pathway, and so on. No GO term and KEGG pathways were changed in the released-ion or polystyrene-NP groups. Ag-NPS inhibited the antioxidant defense of the astrocytes by increasing thioredoxin interacting protein, which inhibits the Trx system, and decreasing Nr4a1 and Dusp1. Meanwhile, Ag-NPS induced inflammation and apoptosis through modulation of the MAPK pathway or B-cell lymphoma-2 expression or mTOR activity in astrocytes.
Conclusion
These results draw our attention to the importance of Ag-NP-induced toxicity on the neurovascular unit and provide a better understanding of its toxicological mechanisms on astrocytes.
Introduction
Silver nanoparticles (Ag-NPs) are 1 to 100 nm-sized metallic colloidal particles widely used in engineering, manufacturing, and biomedicine. Compared to bulk silver metal, Ag-NPs showed greater efficacy against bacteria due to their relatively large surface area and high fraction of surface atoms.Citation1 Currently, Ag-NPs are the most commonly used nanomaterials, being used as antibiotic agents in textiles, medical devices (wound dressings),Citation2 and appliances such as refrigerators and washing machines.Citation3 However, understanding of Ag-NPs toxicity lags behind the applications for this technology. Inadequate identification of the potential hazards and the management of risks from exposures could lead to serious human health problems.Citation4
Brain capillary endothelial cells cooperate with pericytes and astrocytes to generate the unique barrier properties of the blood–brain barrier (BBB), which is part of the neurovascular unit that plays a crucial role in safeguarding the brain from potentially harmful endogenous and exogenous substances circulating in the blood. The BBB, with its tight junctions (TJs) and efflux transporters, restricts the entry of most therapeutic agents.Citation5 A promising approach to overcome limited flux into the central nervous system (CNS) is the use of nanoparticles.Citation6 However, the risk from nanoparticles for the BBB and CNS still needs more investigations. In recent years, how Ag-NPs induce BBB dysfunction and neurotoxicity has been of particular interest, because Ag-NPs have been shown to enter the brain by crossing the BBB in vitro and in vivo.Citation7–Citation10 Previous studies reported that intravenous or subcutaneous injection of Ag-NPs can induce BBB dysfunction, astrocyte swelling, and neuronal degeneration in rats.Citation7–Citation9 The BBB permeability mechanisms of Ag-NPs were further investigated using an in vitro BBB model (cocultures of rat brain microvascular endothelial cells with astrocytes). The results showed that Ag-NPs crossed the BBB mainly by transcytosis rather than through the paracellular pathway.Citation10 Another study showed that Ag-NPs can increase BBB permeability and interact with the cerebral microvascular unit, producing a proinflammatory cascade that may further induce brain inflammation and neurotoxicity.Citation11 Most of the studies of Ag-NP-induced BBB dysfunction only focused on permeability changes and/or toxicity to the endothelial cells. However, brain microvascular endothelial cells cooperate with pericytes and astrocytes to generate the unique barrier properties of the BBB. The BBB is part of the neurovascular unit that plays a crucial role in safeguarding the brain from potentially harmful endogenous and exogenous compounds circulating in the blood.Citation5 A comprehensive understanding of how Ag-NPs induce BBB dysfunction and cause further toxicity and the mechanisms of action in brain endothelial cells, pericytes, and astrocytes remain largely unknown.
Ag-NPs encounter astrocytes immediately after crossing the BBB, because these brain cells almost completely cover the brain capillaries with their end feet.Citation12 Astrocytes are the most abundant cell type in the brain. They provide metabolic nutrients to neurons and protect the brain against oxidative stress and metal toxicity.Citation13,Citation14 It is critical to understand how Ag-NPs affect astrocyte functions and induce potential toxicity. Recent studies showed that Ag-NPs accumulated in cultured astrocytes.Citation15–Citation17 The accumulated Ag-NPs did not compromise the viability and the basal metabolism of cultured primary astrocytes. Ag-NPs induced upregulation of metallothioneins and heme oxygenase 1 to prevent silver-mediated toxicity, which could be induced by Ag-NP-derived Ag ions.Citation16 However, another study, using a mixed primary cell model consisting mainly of neurons and astrocytes and a minor proportion of oligodendrocytes, demonstrated that Ag-NPs induced oxidative stress and acute calcium responses. The Ag-NPs were mainly taken up by astrocytes, which changed astrocyte morphology, but not by neurons.Citation17 These contradicting results demonstrated that the effects of Ag-NPs on astrocytes remain uncertain. More studies are needed to comprehensively investigate the effects and toxicological mechanism(s) of Ag-NPs on astrocytes after they cross the BBB.
In this study, a triple coculture BBB model comprising microvascular endothelial cells, pericytes, and astrocytes was used to mimic the in vivo anatomical situation. The effects of Ag-NP suspension (Ag-NPS, containing silver nanoparticles and released silver ions) on BBB permeability and toxicity were studied. Polystyrene-NPs (PS-NPs) and Ag-NP-released Ag ions were studied to compare the toxic effects with respect to different raw chemical composition and/or form of silver. BBB permeability was studied after exposure to Ag-NPS and PS-NP. Ultrastructural changes of microvascular endothelial cells, pericytes, and astrocytes were observed by transmission electron micro scopy (TEM). Then, DNA microarray was used to detect gene expression profiling of primary rat astrocytes in the triple coculture BBB model after exposure to Ag-NPS, the released Ag ions, and PS-NP. The mechanisms of Ag-NPS-induced toxicity on astrocytes were then evaluated using bioinformatics analyses, combined with observation of cellular morphological changes. Expressions of specific candidate genes were determined using real-time polymerase chain reaction (PCR). The results of this study provide comprehensive insight about Ag-NPS-induced toxicity on the biomimic BBB and the mechanism(s) of Ag-NPS-induced neurotoxicity on astrocytes, which will help us to have a better understanding of the importance of Ag-NPS-induced toxicity on astrocytes in the CNS.
Materials and methods
Characterization of materials
A Ag-NPS (2,000 μg/mL) was purchased from Nanux (SL1105001, Korea). PS-NP powder was purchased from Base Line Chrom Tech Research Centre (6-1-0005, People’s Republic of China). The PS-NP powder was dispersed in sterile deionized water (2,000 μg/mL). The Ag-NP or PS-NP suspensions were then diluted with cell culture medium to the required final concentrations. The suspensions were sonicated (300 W, 42 kHz) in an icewater bath for 5 minutes before use. The Ag ion solution used in this study was prepared by incubating the Ag-NPS (10 μg/mL) for 24 hours at 37°C and then centrifuging it at 20,000 rpm for 2 hours. The total Ag contents before centrifugation and Ag ions contained in the supernatant after centrifugation were determined using atomic absorption spectrometry. This supernatant contained only the released Ag ion portion of the incubated Ag-NPS, which was used as the Ag ion solution treatment. The morphology, characteristics, surface charge, and crystallinity of Ag-NPS were observed by using TEM (HITACHI, Ibaraki, Japan), energy-dispersive X-ray spectroscopy (EDX; EDAX, USA), X-ray photoelectron spectrometry (XPS; ESCAL-AB250Xi, USA), and X-ray diffraction (XRD; D/MAX-TTRIII; CBO, Japan), respectively. The size distribution of Ag-NPs and PS-NPs suspended in deionized water and the zeta-potential were determined using a Malvern Zeta Sizer Nano ZS (Malvern Instruments, Malvern, UK).
In vitro BBB model establishment
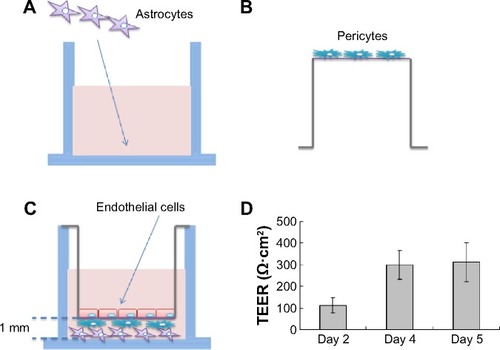
The primary rat microvascular endothelial cells, pericytes, and astrocytes were isolated and the in vitro biomimetic BBB was established as described in a previous study.Citation18 The animal work was approved by the Nagasaki University Institutional Animal Care and Use Committee. The primary rat cells were collected according to the guidelines in the use of animals in Japan. The details for cell isolation, cell culture, and characterization of cell purity are described in a previous study.Citation18 Briefly, pericytes were seeded in Transwell® membrane inverted cell culture inserts (1.12 cm2 for a 12-well polycarbonate plate; Corning Incorporated, NY, USA). After 4 hours, primary brain endothelial cells were added to the luminal compartment of the inserts at a density of 2×106 cells/cm2 having pericytes on the other side and positioned in the 12-well plates containing the astrocytes. The microvascular endothelial cells, pericytes, and astrocytes were cocultured in Dulbecco’s Modified Eagle’s Medium F-12 (with 10% fetal bovine plasma derived serum). From Day 2, cells were grown in culture media containing 500 nM hydrocortisone. Experiments were performed on Day 5. Transendothelial electrical resistance (TEER) was measured at days 2, 4, and 5 using a Millicell-ERS2 (Millipore, Billerica, MA, USA). Experiments were performed when TEER was >200 Ω·cm2. Previous research demonstrated that in vitro BBB models with TEER >150 Ω·cm2 give reasonable drug permeability results.Citation19–Citation21
Cell treatments
Ag-NPS (1 μg/mL and 10 μg/mL) and PS-NPs (100 μg/mL) were introduced into the Transwell® lumen at Day 5 of BBB model culture. After 24-hour treatment, TEER was measured using a Millicell-ERS2, and the Transwell® membrane (with microvascular endothelial cells and pericytes) and astrocytes were washed three times and cells were collected for confocal microscopy or TEM, respectively. These experiments were performed in triplicate wells.
For the DNA microarray experiment, Ag-NPS (10 μg/mL), Ag ions released from 10 μg/mL of Ag-NPS, and PS-NPs (100 μg/mL) were introduced into the Transwell® lumen. After 24 hours, astrocytes were collected for DNA microarray analysis. Briefly, astrocytes were washed three times using PBS, then digested with trypsin, and collected in Trizol solution for RNA extraction.
TEM and immunostaining
The Transwell® BBB model was exposed to Ag-NPS and PS-NPs for the concentrations and time durations indicated earlier. The Transwell® membrane and astrocytes were collected for TEM and immunostaining, respectively. The details of TEM were described in our previous study.Citation22 A certified pathologist identified different cell organelles and Ag-NPs were identified according to their high density under TEM. For Confocal laser scanning microscopy of ZO-1 immunostaining, the secondary antibodies Alexa Fluor 488 conjugated anti-mouse immunoglobulins (Invitrogen, CA, USA) were used in a dilution 1:1000. To counter stain cell nuclei TO-PRO-3 Iodide (Invitrogen Corporation, CA, USA) was used in a dilution of 1:300. The immunostaining of the TJ protein ZO-1 was examined by a LSM5 PASCAL Zeiss confocal laser scanning microscope (Leica, Japan).
Total RNA isolation and DNA microarray
Total RNA was extracted from the collected astrocytes using the RNeasy reagent and the mini protocol for isolation of total RNA from animal cells (QIAGEN, Tokyo, Japan) according to the instructions provided by the manufacturer. The experimental details were described in our previous study.Citation22 The DNA microarray experiment was conducted using Agilent G4130F Whole Rat Genome Microarray 4×44 K (Agilent, CA, US). To determine biologically relevant gene ontology (GO) terms (provided by the National Center for Biotechnology Information) of differentially expressed genes, the software “PANTHER” was used. It provides gene expression data analysis/comparison of gene lists (http://www.pantherdb.org/tools/genexAnalysis.jsp). The analysis was performed using Unigene ID as the identifier for biological process categories.
Quantitative real-time PCR
Quantitative real-time PCR (SYBR Green method) for five selected genes (“test” genes) was performed to determine their gene expression levels and to verify the reliability of the microarray data. Primers used in this study are listed in . The details of the method were described in our previous study.Citation22
Table 1 Primers used in the present study for real-time polymerase chain reaction
Results
Ag-NP characterization
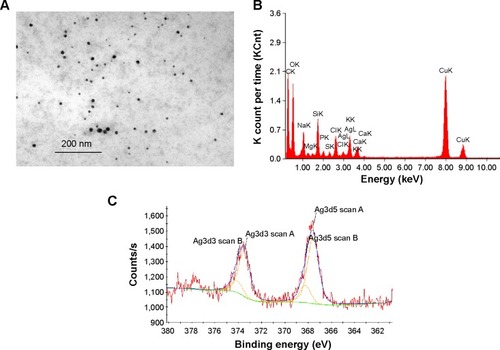
The characterization of Ag-NPs used in this study was described in our previous study.Citation23 The morphology of Ag-NPs in distilled water was observed using TEM (). The particle size distribution in distilled water was 7±2 nm. The zeta potential was −37.8±3.2 mV in water. In cell culture medium, the particle size distribution was 101±12 nm and the zeta potential was −7.9±0.6 mV. The XRD pattern of the Ag-NP showed the typical nature of the Ag-NP (data not shown). EDX results confirmed the presence of silver in cell culture medium () and XPS results showed both Ag0 (81%) and Ag+ (19%) in the deposits of Ag-NPS diluted with cell culture media (), indicating that both Ag-NPs and Ag ions exist in the Ag-NPS. The silver ion content contained in 10 μg/mL of Ag-NPS (incubated for 24 hours, at 37°C) occupied 1.1% of the total silver contents. The particle size distributions of PS-NPs in water and cell culture medium were 33±8 nm and 35±8 nm, respectively. The zeta potentials of PS-NPs in water and cell culture medium were −29.6±2.6 mV and −8.93±0.16 mV, respectively.
Figure 1 Characterization of Ag-NP.
Notes: (A) TEM image of Ag-NPs in distilled water. (B) EDX of Ag-NPS in cell culture media. (C) XPS analysis of Ag-NPS in cell culture media. The experiments were performed by using the deposits of Ag-NP/cell culture medium suspension.
Abbreviations: Ag-NP, silver nanoparticle; Ag-NPS, Ag-NP suspension; EDX, energy-dispersive X-ray spectroscopy; TEM, transmission electron microscopy; XPS, X-ray photoelectron spectrometry.
Characterization of isolated primary cells
Primarily isolated rat brain microvascular endothelial cells grew into continuous monolayers and showed tightly arranged, nonoverlapping, elongated, and fusiform morphology. Microvascular endothelial cells were strongly positively immunostained for Factor VIII, an endothelial marker, indicating high purity of endothelial cells. Expression of TJ proteins, such as ZO-1 and Claudin-5, was observed by confocal microscopy, indicating the properties of BBB cells (). Astrocytes were polygonal with long cell processes, resembling astrocyte end feet, and strongly positive for glial fibrillary acidic protein staining (). Pericytes in culture spread large with irregular projections. The cells described herein were used to establish the in vitro BBB model. A schematic drawing of the in vitro BBB model is shown in . Experiments were carried out on Day 5 when the TEER values of the BBB model were >200 Ω·cm2.
Figure 2 Characterization of primary rat brain microvascular endothelial cells.
Notes: (A) Primary rat brain microvascular endothelial cells form a pure, near-confluent monolayer by light microscopy (upper left panel). Factor VIII (a marker for endothelium; red), ZO-1, and Claudin-5 (tight junction proteins; red) expressions are positive in primary rat brain microvascular endothelial cells by confocal microscopy. (B) Characterization of primary astrocyte cultures in different confluence culture condition by light microscopy (upper left panel) and confocal microscopy. Astrocytes are positive for GFAP.
Abbreviation: GFAP, glial fibrillary acidic protein.
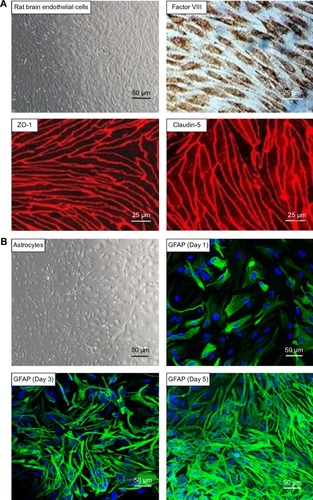
Figure 3 Schematic drawing of the preparation of the in vitro blood–brain barrier model.
Notes: (A) Rat astrocytes were seeded at the bottom of 12-well plates, while (B) rat brain pericytes were seeded at the against-lumen side of Transwell® membranes of inverted cell culture inserts. After 4 hours, (C) endothelial cells were seeded into the luminal compartment of the inserts having pericytes on the other side and positioned into the 12-well plates containing the astrocytes. (D) TEER (expressed as Ω·cm2) of the blood–brain barrier model, measured on different days. Data are presented as mean ± SD (n=3).
Abbreviations: SD, standard deviation; TEER, transendothelial electrical resistance.
Effect of Ag-NPS and PS-NPs on the TJ of the BBB
The TEER values of the BBB in vitro model were all >200 Ω·cm2 before the experiment (). After 24-hour treatments, the PS-NP and Ag-NPS (1 μg/mL) groups did not change their TEER values significantly compared to control. However, the 10 μg/mL Ag-NPS group showed significantly decreased TEER values compared to control (). The immunostaining of the TJ protein ZO-1 of microvascular endothelial cells in the coculture BBB model showed a continuous, smooth, pericellular, belt-like pattern in the control and PS-NP groups. However, ZO-1 expression was apparently decreased in the 10 μg/mL Ag-NPS group compared to control (), which was consistent with the TEER results.
Figure 4 The effect of Ag-NPS and PS-NP on BBB permeability measured by TEER.
Notes: TEER (expressed as Ω·cm2) of the BBB model (A) before and (B) after 24 hours’ treatment in control, PS-NP (100 μg/mL), and Ag-NPS (1 μg/mL and 10 μg/mL) groups. All data are presented as mean ± SD. *Significant difference compared to the control (P<0.05).
Abbreviations: Ag-NPS, silver nanoparticle suspension; BBB, blood–brain barrier; PS-NP, polystyrene nanoparticle; SD, standard deviation; TEER, transendothelial electrical resistance.
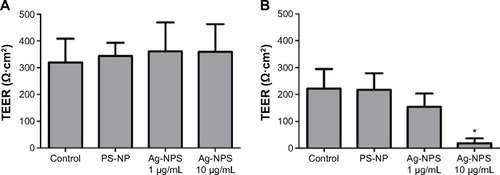
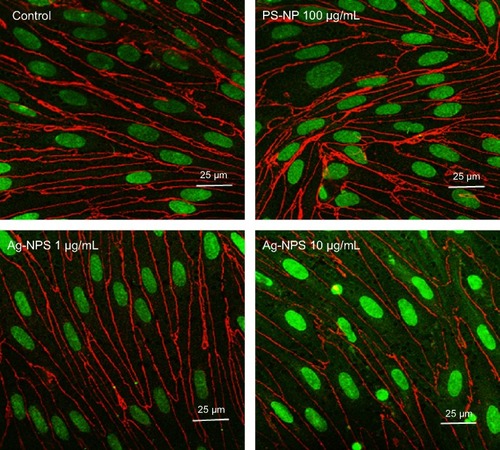
Figure 5 Immunofluorescent staining of tight junction protein ZO-1 (red) in confluent brain endothelial cell in triple coculture.
Notes: The ZO-1 immunostaining of endothelial cells in coculture shows a continuous, smooth, pericellular, belt-like pattern in control and PS-NP groups. However, ZO-1 immunostaining was significantly decreased in the 10 μg/mL Ag-NPS group compared to the control.
Abbreviations: Ag-NPS, silver nanoparticle suspension; PS-NP, polystyrene nanoparticle.
Effects of Ag-NPS and PS-NPs on the ultrastructural changes of the BBB
TEM images showed that microvascular endothelial cells formed the TJs very well in the in vitro BBB model (), and pericytes spread large with irregular projections on the other sides of the Transwell® membrane in the control group (). In PS-NP group, no significant TJ disruptions were observed between microvascular endothelial cells (). No ultrastructural changes were observed in the pericytes (). In the 1 μg/mL Ag-NPS group, discontinuous TJs were not observed. Vacuo lations and endoplasmic reticulum (ER) expansion were occasionally observed in the microvascular endothelial cells (). Vacuolations were also occasionally observed in pericytes (). In the 10 μg/mL Ag-NPS group, Ag-NP-like nanoparticles were observed inside the endothelial cells and pericytes. Apart from discontinuous TJ, ER expansion, vacuolations, and mitochondrial shrinkage were also observed (). Vacuolations were observed in pericytes in the 10 μg/mL Ag-NPS group ().
Figure 6 TEM images of primary rat brain (A) microvascular endothelial cells, (B) pericytes, and (C) astrocytes after 24-hour treatments.
Notes: (A) In the control group, endothelial cells form TJs (a, white dash box). In the PS-NP group, a TJ (b, white dash box) between endothelial cells was not disrupted. In the 1 μg/mL Ag-NPS group, the endothelial cells grew nicely on the membrane (c, white arrow) and discontinuous TJs were not observed. Vacuolations (d, white arrow) and ER expansion (d, dark arrow) were observed in the endothelial cells. In the 10 μg/mL Ag-NPS group, mitochondrial shrinkage (e, dark arrow) and ER expansion (e, white arrow) appeared; Discontinuous TJs (f, white dash box) and Ag-NP-like particles as showed in the insert for (f) (f, dark arrows) in endothelial cells were observed. (B) Pericytes grew nicely on the other side of the membrane (white arrow) in the control group (a) and PS-NP group (b). In the 1 μg/mL Ag-NPS group, vacuolations (white arrows) were observed occasionally in pericytes (c). In the 10 μg/mL Ag-NPS group, vacuolations (white arrows) were observed in pericytes (d). (C) In the control group, astrocytes had normal cell morphology (a). In the PS-NP group, mitochondrial shrinkage was observed occasionally (white arrow) (b). In the 1 μg/mL Ag-NPS group, mitochondrial shrinkage was occasionally observed (white arrows) (c). In the 10 μg/mL Ag-NPS group, nuclear atypia (black arrows) and severe mitochondrial shrinkage (white arrows) were observed (d and e); Ag-NP-like particles (showed in the insert for f) were also observed in astrocytes (f, black arrows), as well as in the enlarged picture.
Abbreviations: Ag-NPS, silver nanoparticle suspension; ER, endoplasmic reticulum; PS-NP, polystyrene nanoparticle; TEM, transmission electron microscopy; TJ, tight junction.
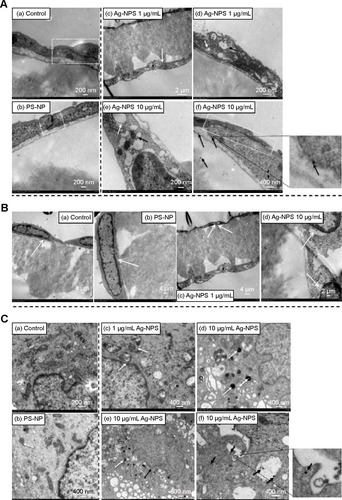
Effects of Ag-NPS on the ultrastructural changes of astrocytes
Astrocytes did not show significant cytotoxicity, such as morphological changes and damages in all treatment groups compared to the control, as observed by light microscopy (data not shown). TEM images showed that astrocytes grew with normal morphology in the control group (). In the PS-NP and 1 μg/mL Ag-NPS groups, no ultrastructural changes were observed in astrocytes in most images, and mitochondrial shrinkage was observed occasionally (). In the 10 μg/mL Ag-NPS group, severe mitochondrial shrinkage was observed () and nuclear atypia was observed occasionally (). Meanwhile, Ag-NP-like particles appeared inside astrocytes ().
Global gene expression changes
Filtering of the array results was done for astrocytes as follows: genes were considered to be upregulated or down-regulated when the relative expression levels [Log2(gene expression ratio of treated/control)] differed by >1 or less than −1 in treated groups (10 μg/mL Ag-NPS or released Ag ion or 100 μg/mL PS-NP) compared to control. We observed that 23 genes in total were differentially expressed in the 10 μg/mL Ag-NPS-treated group compared to control. Of the 23 genes, there were 20 downregulated genes and 3 upregulated genes in astrocytes (Table S1). Sixteen genes in total were differentially expressed in the released Ag ion-treated group compared to control, and there were 15 downregulated genes and 1 upregulated gene (Table S2).
By comparing the gene expression profiles of astrocytes exposed to Ag-NPS or their released Ag ions (Tables S1A, S1B, S2A, and S2B), we observed a total of nine genes (eight genes downregulated and one gene upregulated) that were changed in common, suggesting that changes in these genes are attributable to the released Ag ions. This implies that 2 genes from the total of 3 upregulated genes and 12 genes from the total of 28 downregulated genes were uniquely induced by Ag-NPs, not released Ag ions. Furthermore, the upregulated genes in common showed a higher expression level in the Ag-NPS treatment group than in the released Ag ion group. The downregulated genes (nuclear receptor subfamily 4, group A, member 1 [Nr4a1], early growth response 2 [Egr2], protein kinase KID2 [kid2], nuclear receptor subfamily 4, group A, member 3 [Nr4a3], and activity-regulated cytoskeleton-associated protein [Arc]) showed a greater extent of decreases in the Ag-NPS group compared with the levels in the released Ag ion group. The other three downregulated genes (protein sprouty homolog 1 [spry1], dual specificity phosphatase 5 [Dusp5], and chemokine (C–X–C motif) ligand 1 [Cxcl1]) have similar changes in both Ag-NPS and Ag ion groups. These results indicate that the toxic responses observed in Ag-NPS-exposed astrocytes are attributable to both Ag-NPs and their released Ag ions. Ag-NPS caused higher gene expression changes compared with their released Ag ion group, suggesting a synergistic toxic effect induced by Ag-NPS and their released Ag ions.
Pathway analysis
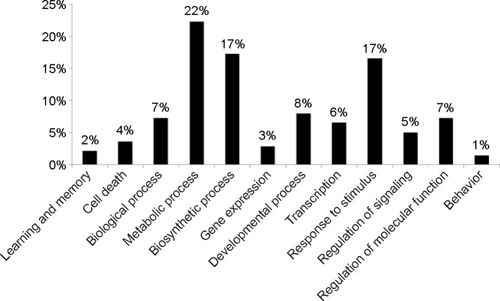
To further investigate the molecular mechanism of Ag-NPS-induced cellular responses, GO and Kyoto Encyclopedia of Genes and Genomes (KEGG) molecular pathway analyses were performed. In the 10 μg/mL Ag-NPS-treated group, 139 biological functions were changed based on GO (Table S3), which were associated with metabolic processes, biosynthetic processes, response to stimuli stimulus, developmental processes, regulation of molecular function, biological processes, transcription, regulation of signaling, cell death, gene expression, learning, memory, and behavior (). The MAPK signaling pathway was significantly changed in KEGG pathway analysis, which was linked to four genes (Dusp1, Dusp5, FBJ osteosarcoma oncogene [Fos], and Nr4a1). However, no GO and KEGG pathways were changed in the released Ag ion-treated group and PS-NP-treated group.
Figure 7 Functional classification (GO terms) of differentially expressed genes based on GO.
Notes: The data were collected from astrocytes exposed to 10 μg/mL of Ag-NPS for 24 hours. The percentage of related GO terms for each category is indicated. Percentage (%) = (related GO terms for each category/related GO terms for all categories) ×100%.
Abbreviations: Ag-NPS, silver nanoparticle suspension; GO, gene ontology.
Quantitative real-time PCR determination
To determine the gene expression level and verify the reliability of differentially expressed genes identified by the DNA microarray, five genes selected from the groups of up- and downregulated genes after exposure to Ag-NPS and released Ag ions were further examined using quantitative real-time PCR. Thioredoxin interacting protein-encoding gene (Txnip) and DNA damage inducible transcript 4 (Ddit4) were upregulated, while Nr4a1, Egr2, and Fos were downregulated in both reverse transcriptase-PCR and DNA microarray ().
Table 2 RT-PCR validation of selected genes from microarray data
Discussion
Nanoparticles have been increasingly used in medicine, cosmetics, electronics, and food additives. However, the influence of nanoparticles on human health and brain has not been studied well.Citation24 Several studies have demonstrated that Ag-NPs can enter the CNSCitation25 and induce brain edema and neurotoxicity.Citation10,Citation11,Citation26–Citation29 Our previous studies showed that Ag-NPS and/or released Ag ions crossed the BBB and subsequently caused damage to astrocytes and neurons.Citation23 Cadherin and claudin expression were slightly changed in the Ag-NPS-exposure group, and astrocyte swelling was the most significant change after a 2-week gastrointestinal exposure to 1 mg/kg or 10 mg/kg of Ag-NPS in rats. Furthermore, Ag-NPs interacting with the cerebral microvasculature can induce formation of reactive oxygen species (ROS) and proinflammatory mediators, which can increase BBB permeability.Citation7–Citation9,Citation11 However, the biological effects of Ag-NPs on the BBB and brain are still unclear. It is critical to further understand the toxicity of Ag-NPS using a biomimic BBB model. In the present study, we demonstrated for the first time the toxic responses and mechanisms of Ag-NPS by observing the ultrastructure and gene expression profile changes using a biomimetic BBB model (microvascular endothelial cells/pericytes/astrocytes).
In the current study, we established a primary BBB triple coculture model. The model used primary rat brain microvascular endothelial cells, pericytes, and astrocytes corresponding to the anatomical situation in brain capillaries. Previous research showed that pericytes contributed to the maturation and maintenance of BBB propertiesCitation30 and there was a clear correlation between a higher ratio of pericytes versus endothelial cells in blood vessels and the tightness of the endothelial barrier.Citation31 Furthermore, astrocytes can decrease the paracellular permeability of immortalized rat brain endothelial cells.Citation32 In the present study, the high expression of TJ proteins and the high TEER demonstrated that the triple coculture model is among the best primary BBB models to mimic the BBB in vivo.Citation33 This primary BBB model provides a very useful in vitro model to evaluate the effect of vasculotoxic or vasculoprotective agents on the BBB. In this study, this triple coculture model was used to investigate the changes to BBB integrity and the molecular mechanisms in vitro following exposure to Ag-NPS.
As mentioned herein, several studies showed that Ag-NPs can disrupt the BBB and cause CNS toxicity. However, the toxicological effects of Ag-NPs on the typical biomimetic BBB are still uncertain. In the present study, 10 μg/mL Ag-NPS showed significant effects on BBB permeability and TJ protein expression, whereas 1 μg/mL Ag-NPS did not. Vacuolations were also observed by TEM in pericytes after Ag-NPS exposure. Previous studies demonstrated that pericyte deficiency or dysfunction leads to chronic BBB damage and contributes to neurodegeneration during Alzheimer’s disease pathogenesis.Citation34–Citation36 Further studies are needed to investigate whether Ag-NPS can cause pericyte dysfunction and potential neuronal injury. We further observed a large amount of mitochondrial shrinkage and ER extension, as well as Ag-NP-like particles, in astrocytes by TEM, which demonstrated that Ag-NPs entered astrocytes and induced toxicity after crossing the endothelial cells and pericytes.
To identify the molecular mechanisms of multiple genes working together following exposure to Ag-NPS, released Ag ions, and PS-NPs, microarray assays of astrocytes were performed to establish a global gene expression profile. The functional classification of these genes was analyzed using GO. Our results demonstrated that the toxicological effects on astrocytes were significantly different between the Ag-NPS and the released Ag ion or PS-NP group. In contrast with the Ag-NPS-treated group that showed induction of 139 GO and 1 KEGG pathway genes, no GO term and KEGG pathway gene was changed in the released-ion or PS-NP exposure group. The expression levels of 16 genes were changed after exposure to released Ag ions, but the fold changes were less in general compared with the changes in the Ag-NPS treatment group (Table S3). The results were consistent with our previous research, which showed that Ag-NPS induced greater toxicity compared with the released Ag ions. The greater toxicity was attributed to the Ag-NPS. Ag-NPs, as heavy metal nanoparticles, but not the characteristics of a nano-sized particle themselves (as do PS-NP), caused severe toxicity in astrocytes.
Ag-NPs have been demonstrated to induce oxidative stress and apoptosis in the brain.Citation37 The effects of 25 nm Ag-NPs on gene expression were evaluated in different regions of the mouse brain. Ag-NPs produced apoptosis and neurotoxicity by generating free radical-induced oxidative stress. The oxidative stress induced by Ag-NPs in the brain was also related to reduction of antioxidant capacity. Ag-NPs altered expression of genes such as Txnip, which is a member of the α-arrestin family involved in redox sensing and metabolic control.Citation38 Our data in Tables S1 and S2 obtained from real-time PCR showed that Txnip was significantly upregulated 4.20-fold and 2.32-fold in the Ag-NPS and Ag ion treatment groups, respectively. Upregulation of Txnip modulates antioxidant defense by inhibiting the Trx system and increasing ROS and oxidative stress.Citation39 Txnip also upregulated the transcription factor NF-κB, which induces transcription of proinflammatory cytokines such as tumor necrosis factor-α and interleukin-1β.Citation40 These two pathways can further activate the apoptotic MAPK pathway, leading to cell death.Citation41 Ag-NPs also activated the p38 MAPK pathway, causing apoptosis of Jurkat T cells.Citation42 In the present study, Ag-NPS also induced Ddit4 expression. This was not produced by the released Ag ions. Ddit4 is known to inhibit mTOR activity, resulting in an increase in apoptosis in mouse embryonic fibroblasts.Citation43 Ddit4 can reduce cell proliferation, growth, and maturation by inhibiting mTOR-mediated synthesis of proteins.Citation44 Furthermore, in our study, five functional GO molecule annotations (nine genes) were involved in apoptosis/cell death only after Ag-NPS exposure. For example, Nr4a1, encoding an orphan nuclear receptor for neural apoptosis, was the most down-regulated gene in astrocytes. Nr4a1 has been implicated in apoptosis,Citation45 cell survival, and metabolism.Citation46 Nr4a1 can be induced after excitotoxic and oxidative stress in neurons, and it upregulates neuroprotective genes and increases neuronal survival. Nr4a1 also has early protective function in neural cells/tissues against various pathophysiological stresses.Citation47 In this study, Ag-NPS induced downregulation of Nr4a1 in the astrocyte, which could decrease the neuroprotective function against oxidative stress. Ag-NPS exposure also reduced B-cell lymphoma 2 (Bcl-2) expression, which is present in the inner mitochondrial membrane and plays an important role in regulation of ROS production and apoptosis. A previous study showed that oxidative stress reduced Bcl-2 expression, indicating onset of apoptosis in zebrafish brain.Citation48 Dusp1 can mediate MAPK signaling and inflammation.Citation49 The downregulation of Dusp1 in the Ag-NPS treatment group activates the MAPK signaling pathway, which induces inflammation and apoptosis. KEGG pathway analysis also showed that the MAPK signaling pathway was affected in astrocytes after exposure to Ag-NPS, but not after exposure to released Ag ions. In general, compared with released Ag ions, Ag-NPS induced more toxicity by generating ROS and significantly decreasing the expression of neuroprotective genes against ROS in astrocytes, weakening astrocytes’ ROS protective ability, and subsequently inducing inflammation and apoptosis. Taken together, our results were consistent with previous research,Citation50 which showed that ROS generation, inflammatory reactions, and apoptosis are the primary mechanisms of Ag-NP induced toxicity.Citation19,Citation48
Previous research has demonstrated that astrocytes play an important role in providing polyunsaturated fatty acids to the BBB and brain.Citation51 Polyunsaturated fatty acids are ROS targets in the brain due to their high metabolic rate and their rich composition in the brain,Citation52 which make astrocytes more sensitive to Ag-NP-induced ROS and more susceptible to losing their defense ability. In the present study, antioxidant defense was inhibited in astrocytes following Ag-NPS exposure. Meanwhile, Ag-NPS also induced inflammation and apoptosis through the MAPK pathway, Bcl-2 expression, or mTOR activity in astrocytes. The results raise concerns regarding Ag-NP toxicity in astrocytes. More studies are needed to understand how the toxicity effects induced by Ag-NPs in astrocytes influence neuron functions.
In the present study, 3 learning/memory/cognition processes, 11 developmental processes, 31 metabolic processes, and 24 biosynthetic processes were affected in astrocytes after Ag-NPS exposure (Table S3). Our results are consistent with previous studies that demonstrated that Ag-NPs can reduce learning and cognition of ratsCitation53 and can produce developmental neurotoxicity and behavioral effects.Citation54 Furthermore, the metabolic relationship between astrocytes and neurons is critical for energy metabolism and the synthesis of neurotransmitters.Citation55 We speculate that induction of a large amount of changes in metabolic processes of astrocytes by Ag-NPS could significantly influence neuronal functions. Astrocytes also release factors that sustain neuronal function and viability. Astrocytes synthesize and secrete a wide range of neurotrophic and growth factors, cytokines, extracellular matrix proteins, proteoglycans, and cholesterol, which are involved in neuronal survival, proliferation, differentiation, and synaptogenesis.Citation56 Ag-NPS exposure significantly inhibited biosynthetic processes in astrocytes and may decrease the secretion of important nutrients and signal factors. Further studies are needed to investigate how the toxicity effects induced by Ag-NPs in astrocytes influence neuronal survival, proliferation, and differentiation to better understand the mechanisms of Ag-NP-induced neurotoxicity.
Conclusion
We established a BBB model using microvascular endothelial cells, pericytes, and astrocytes, which enhanced levels of TJ proteins (claudin-5 and ZO-1), with TEER >200 Ω·cm2 on Day 5. After 10 μg/mL Ag-NPS exposure for 24 hours, the BBB permeability was significantly increased and ZO-1 expression was significantly decreased compared to control. Severe mitochondrial shrinkage, ER expansion, and nuclear atypia were observed in astrocytes. Global gene expression analysis showed 23 significantly changed genes that were associated with several biological processes. As illustrated in , Ag-NPS significantly increased Txnip expression, which modulated antioxidant defense. On the other hand, Ag-NPS significantly decreased Nr4a1 and Dusp1, which protect cells from oxidative stress, inflammation, and apoptosis. Ag-NPS induced toxicity by decreasing antioxidant defense in astrocytes. Meanwhile, Ag-NPS also induced ROS, inflammation, and apoptosis through modulation of the MAPK pathway or Bcl-2 expression or mTOR activity in astrocytes. Ag-NPS also inhibited eleven developmental processes and caused learning and cognition reduction. Furthermore, Ag-NPS significantly suppressed 31 metabolic and 24 biosynthesis processes in astrocytes, which may influence the main function of astrocytes in the CNS and increase the risk of Ag-NP-induced neurotoxicity.
Acknowledgments
The authors gratefully acknowledge Dr Chunying Chen and Dr Ru Bai (National Center for Nanoscience and Nanotechnology, People’s Republic of China), for their technical support for EDX and XPS characterization of silver nanoparticles, and Dr Chang Shi (Beijing Institute of Pharmacology and Toxicology, National Beijing Center for Drug Safety Evaluation and Research, People’s Republic of China) for her feedback.
This study was financially supported by the Beijing Natural Science Foundation of China (#3112024); National Natural Science Foundation of China (#81401517); National Key Technology Research and Development Program of the Ministry of Science and Technology of China (#2012BAK26B00); Principal Scientists Training Fund, National Institutes for Food and Drug Control (#2012X2). No writing assistance by persons other than the authors was used in the production of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- LaraHHGarza-TrevinoENIxtepan-TurrentLSinghDKSilver nanoparticles are broad-spectrum bactericidal and virucidal compoundsJ Nanobiotechnology201193021812950
- ChenXSchluesenerHJNanosilver: a nanoproduct in medical applicationToxicol Lett2008176111218022772
- TolaymatTMEl BadawyAMGenaidyAScheckelKGLuxtonTPSuidanMAn evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer-reviewed scientific papersSci Total Environ20104085999100619945151
- StensbergMCWeiQMcLamoreESPorterfieldDMWeiASepulvedaMSToxicological studies on silver nanoparticles: challenges and opportunities in assessment, monitoring and imagingNanomedicine (Lond)20116587989821793678
- BernackiJDobrowolskaANierwinskaKMaleckiAPhysiology and pharmacological role of the blood-brain barrierPharmacol Rep200860560062219066407
- AgarwalALariyaNSaraogiGDubeyNAgrawalHAgrawalGPNanoparticles as novel carrier for brain delivery: a reviewCurr Pharm2009158917925
- TangJXiongLWangSDistribution, translocation and accumulation of silver nanoparticles in ratsJ Nanosci Nanotechnol2009984924493219928170
- SharmaHSAliSFHussainSMSchlagerJJSharmaAInfluence of engineered nanoparticles from metals on the blood-brain barrier permeability, cerebral blood flow, brain edema and neurotoxicity. An experimental study in the rat and mice using biochemical and morphological approachesJ Nanosci Nanotechnol2009985055507219928185
- SharmaHSAliSFTianZRChronic treatment with nanoparticles exacerbate hyperthermia induced blood-brain barrier breakdown, cognitive dysfunction and brain pathology in the rat. Neuroprotective effects of nanowired-antioxidant compound H-290/51J Nanosci Nanotechnol2009985073509019928186
- TangJXiongLZhouGSilver nanoparticles crossing through and distribution in the blood-brain barrier in vitroJ Nanosci Nanotechnol201010106313631721137724
- TricklerWJLantzSMMurdockRCSilver nanoparticle induced blood-brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cellsToxicol Sci2010118116017020713472
- MathiisenTMLehreKPDanboltNCOttersenOPThe perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstructionGlia20105891094110320468051
- HirrlingerJDringenRThe cytosolic redox state of astrocytes: maintenance, regulation and functional implications for metabolite traffickingBrain Res Rev2010631–217718819883686
- ScheiberIFDringenRAstrocyte functions in the copper homeostasis of the brainNeurochem Int201362555656522982300
- HohnholtMCGeppertMLutherEMPettersCBulckeFDringenRHandling of iron oxide and silver nanoparticles by astrocytesNeurochem Res201338222723923224777
- LutherEMSchmidtMMDiendorfJEppleMDringenRUpregulation of metallothioneins after exposure of cultured primary astrocytes to silver nanoparticlesNeurochem Res20123781639164822476984
- HaaseARottSMantionAEffects of silver nanoparticles on primary mixed neural cell cultures: uptake, oxidative stress and acute calcium responsesToxicol Sci2012126245746822240980
- NakagawaSDeliMAKawaguchiHA new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytesNeurochem Int2009543–425326319111869
- GumbletonMAudusKLProgress and limitations in the use of in vitro cell cultures to serve as a permeability screen for the blood-brain barrierJ Pharm Sci200190111681169811745727
- ReichelABegleyDJAbbottNJAn overview of in vitro techniques for blood-brain barrier studiesMethods Mol Med20038930732412958429
- GaillardPJde BoerAGRelationship between permeability status of the blood-brain barrier and in vitro permeability coefficient of a drugEur J Pharm Sci20001229510211102736
- XuLLiXTakemuraTHanagataNWuGChouLLGenotoxicity and molecular response of silver nanoparticle (NP)-based hydrogelJ Nanobiotechnology2012101622548743
- XuLShaoAZhaoYNeurotoxicity of silver nanoparticles in rat brain after intragastric exposureJ Nanosci Nanotechnol2014141924730249
- HanadaSFujiokaKInoueYKanayaFManomeYYamamotoKCell-based in vitro blood–brain barrier model can rapidly evaluate nanoparticles’ Brain permeability in association with particle size and surface modificationInt J Mol Sci20141521812182524469316
- PanyalaNRPena-MendezEMHavelJSilver or silver nanoparticles: a hazardous threat to the environment and human health?J Appl Biomed20086117129
- YangZLiuZWAllakerRPA review of nanoparticle functionality and toxicity on the central nervous systemJ R Soc Interface20107suppl 4S411S42220519209
- KimSHKoJWKohSKSilver nanoparticles induce apoptotic cell death in cultured cerebral cortical neuronsMol Cell Toxicol2014102173179
- CramerSTackeSBornhorstJKlingaufJSchwerdtleTGallaHJThe influence of silver nanoparticles on the blood-brain and the blood-cerebrospinal fluid barrier in vitroJ Nanomed Nanotechnol20145225
- SharmaAMuresanuDFPatnaikRSharmaHSSize- and age-dependent neurotoxicity of engineered metal nanoparticles in ratsMol Neurobiol201348238639623821031
- LaiCHKuoKHThe critical component to establish in vitro BBB model: pericyteBrain Res Brain Res Rev200550225826516199092
- AlltGLawrensonJGPericytes: cell biology and pathologyCells Tissues Organs2001169111111340256
- SchieraGSalaSGalloAPermeability properties of a three-cell type in vitro model of blood-brain barrierJ Cell Mol Med20059237337915963256
- DeliMAAbrahamCSKataokaYNiwaMPermeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacologyCell Mol Neurobiol20052515912715962509
- SengilloJDWinklerEAWalkerCTSullivanJSJohnsonMZlokovicBVDeficiency in mural vascular cells coincides with blood–brain barrier disruption in Alzheimer’s diseaseBrain Pathol201323330331023126372
- BellRDWinklerEASinghIApolipoprotein E controls cerebrovascular integrity via cyclophilin ANature2012485739951251622622580
- SagareAPBellRDZhaoZPericyte loss influences Alzheimer-like neurodegeneration in miceNat Commun20134293224336108
- YinNLiuQLiuJSilver nanoparticle exposure attenuates the viability of rat cerebellum granule cells through apoptosis coupled to oxidative stressSmall201399–101831184123427069
- RahmanMFWangJPattersonTAExpression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticlesToxicol Lett20091871152119429238
- JunnEHanSHImJYVitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin functionJ Immunol2000164126287629510843682
- PerroneLDeviTSHosoyaK-ITerasakiTSinghLPThioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditionsJ Cell Physiol2009221126227219562690
- ChenC-LLinC-FChangW-THuangW-CTengC-FLinY-SCeramide induces p38 MAPK and JNK activation through a mechanism involving a thioredoxin-interacting protein-mediated pathwayBlood200811184365437418270325
- EomH-JChoiJp38 MAPK activation, DNA damage, cell cycle arrest and apoptosis as mechanisms of toxicity of silver nanoparticles in jurkat T cellsEnviron Sci Technol201044218337834220932003
- CorradettiMNInokiKGuanKLThe stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathwayJ Biol Chem2005280119769977215632201
- LisseTSLiuTIrmlerMGene targeting by the vitamin D response element binding protein reveals a role for vitamin D in osteoblast mTOR signalingFASEB J201125393794721123297
- LiQXKeNSundaramRWong-StaalFNR4A1, 2, 3 – an orphan nuclear hormone receptor family involved in cell apoptosis and carcinogenesisHistol Histopathol200621553354016493583
- FuYLuoLLuoNZhuXGarveyWTNR4A orphan nuclear receptors modulate insulin action and the glucose transport system: potential role in insulin resistanceJ Biol Chem200728243315253153317785466
- VolakakisNKadkhodaeiBJoodmardiENR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotectionProc Natl Acad Sci U S A201010727123171232220566846
- SarkarSMukherjeeSChattopadhyayABhattacharyaSLow dose of arsenic trioxide triggers oxidative stress in zebrafish brain: expression of antioxidant genesEcotoxicol Environ Saf2014107C1824905690
- MooreEDKooshkiMMetheny-BarlowLJGallagherPERobbinsMEAngiotensin-(1–7) prevents radiation-induced inflammation in rat primary astrocytes through regulation of MAP kinase signalingFree Radic Biol Med2013651060106824012919
- ChairuangkittiPLawanprasertSRoytrakulSSilver nanoparticles induce toxicity in A549 cells via ROS-dependent and ROS-independent pathwaysToxicol In Vitro201327133033822940466
- BernoudNFenartLBénistantCAstrocytes are mainly responsible for the polyunsaturated fatty acid enrichment in blood-brain barrier endothelial cells in vitroJ Lipid Res1998399181618249741694
- OzmenINazirogluMAliciHASahinFCengizMErenISpinal morphine administration reduces the fatty acid contents in spinal cord and brain by increasing oxidative stressNeurochem Res2007321192517151918
- LiuYGuanWRenGYangZThe possible mechanism of silver nanoparticle impact on hippocampal synaptic plasticity and spatial cognition in ratsToxicol Lett2012209322723122245254
- PowersCMSlotkinTASeidlerFJBadireddyARPadillaSSilver nanoparticles alter zebrafish development and larval behavior: distinct roles for particle size, coating and compositionNeurotoxicol Teratol201133670871421315816
- BakLKSchousboeAWaagepetersenHSThe glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transferJ Neurochem200698364165316787421
- FarinaCAloisiFMeinlEAstrocytes are active players in cerebral innate immunityTrends Immunol200728313814517276138