Abstract
Hemophilia A is a rare X-linked bleeding disorder caused by lack or dysfunction of coagulation factor VIII (FVIII). Hemophilia A is treated with replacement therapy, but frequent injections of the missing FVIII often lead to the formation of inhibitory antibodies. Patients who develop high levels of inhibitors must be treated with bypassing agents such as activated FVII (FVIIa). Both FVIII and FVIIa have short half-lives and require multiple injections. Long-acting forms of these proteins would therefore reduce the frequency of injections, improve patient compliance and reduce complications. In this article we present a new platform technology that produces long-acting forms of FVIII and FVIIa and improves the efficacy of hemophilia treatment. This technology is based on the binding of proteins/peptides to the outer surface of PEGylated liposomes (PEGLip). Binding is dependent on an amino acid consensus sequence within the proteins and is highly specific. At the same time, binding is non-covalent and does not require any modification of the therapeutic agent or its production process. Association of proteins with PEGLip results in substantial enhancements in their pharmacodynamic properties following administration. These improvements seem to arise from the association of formulated proteins with platelets prior to induction of coagulation.
Introduction
Hemophilia
Hemophilia is an inherited bleeding disorder caused by the lack or dysfunction of coagulation factors VIII (FVIII) or IX (FIX). The genes for FVIII and FIX are located on the X chromosome, and the vast majority of individuals with hemophilia are males. Hemophilia A accounts for approximately 75%–80% of all hemophilia cases with a prevalence of approximately 1/10,000 in the male population, whereas the prevalence of hemophilia B is 1/30,000.Citation1,Citation2 The severity of the bleeding tendency is determined by the residual clotting factor activity. Approximately 40% of hemophilia patients have a severe form of the disease, defined as having less than 1% (0.01 IU/ml) of normal factor activity.Citation3
Hemophilia patients experience spontaneous hemorrhages into the joints, particularly the weight bearing joints such as knees, ankles and hips. This bleeding is painful and leads to long term inflammation and deterioration of the joint, resulting in permanent deformities, misalignment and loss of mobility. This is the major complication of hemophilia.Citation4,Citation5 Bleeding may also occur into the muscles, soft tissues and other organs.
Hemophilia therapy
Hemophilia is treated by supplying patients with the missing coagulation factor (FVIII or FIX). Replacement therapy may be provided on-demand (to stop a bleed) or prophylactically (to prevent bleeds). When treatment is provided on-demand, injections must be initiated at the first onset of symptoms so as to limit both the amount of bleeding and the extent of the resulting tissue damage.Citation6 Prophylactic treatment has been shown to provide hemophilia patients with better overall care than on-demand treatment as it reduces the frequency of hemorrhages and slows the development of long term arthropathy.Citation7 However, prophylactic treatment is more expensive than on-demand treatment and is not accessible to many patients.Citation6
In this review, we focus on the treatment of hemophilia A, the most common form of the disease. The FVIII used to treat hemophilia A may be produced in mammalian cell lines genetically engineered to synthesize human FVIII (recombinant FVIII, rFVIII) or may be purified from normal pooled plasma (plasma-derived FVIII, pdFVIII).Citation8
FVIII is a large glycoprotein (2332 amino acids) that is synthesized as a 330 kDa precursor and undergoes multiple processing steps to yield a heterodimer composed of a light chain (80 kDa) and a heterogeneous heavy chain (90–210 kDa)Citation9 FVIII circulates in the plasma as an inactive precursor, tightly complexed with von Willebrand factor (vWF). Upon initiation of coagulation, FVIII is activated by thrombin. It dissociates from vWF, interacts with negatively charged phospholipids on the surface of activated platelets, and acts as a cofactor for factor IXa in the activation of factor X.Citation1 This in turn leads to the generation of substantial amounts of thrombin, the key enzyme in the coagulation cascade, and to the generation of a hemostatic plug.
The half-life of human FVIII is about 10–12 hours.Citation10,Citation11 Effective prophylactic therapy therefore requires three weekly infusions of 20–40 IU/kg to maintain FVIII above levels at which spontaneous bleeding occurs.Citation7 The need for frequent infusions reduces quality of life and may lead to problems with compliance and injection complications.Citation12 Central venous access devices are frequently required, especially in children, but these devices are plagued by recurrent infections and thrombosis.Citation13
Development of inhibitors and acquired hemophilia
Replacement treatment is complicated by the emergence of antibodies that inhibit the activity of the injected protein.Citation14 Approximately 20%–40% of hemophilia A patients and 2%–3% of hemophilia B patients develop inhibitors.Citation15,Citation16 As expected, the more profound mutations resulting in the lack of circulating FVIII or FIX are associated with higher risk of inhibitor development.Citation17 Neutralizing or inactivating autoantibodies to FVIII may also develop in patients without a history of coagulation factor deficiency. This condition, known as acquired hemophilia, affects 0.2–1 out of every million people per year.Citation18 Despite the low incidence of acquired hemophilia, the disorder is often devastating and the costs of treatment are immense. There is therefore considerable interest in improving and optimizing existing treatment regimens.Citation18,Citation19
Bypassing agents
Treatment of hemophilia patients with inhibitors is based primarily on controlling or preventing acute hemorrhages and their sometimes life threatening complications. Patients with low inhibitor titers may be given high doses of factor concentrate. While some of the factor is neutralized by the inhibitors, enough remains in the circulation to induce hemostasis. Patients with high inhibitor titers (≥5 Bethasda units, BU) cannot be treated by replacement therapy because even high doses of coagulation factor are rapidly inactivated by the circulating antibodies.Citation20 These patients must be treated with agents capable of inducing hemostasis independent of the presence of FVIII or FIX (bypassing agents). Two such agents are in widespread use: activated prothrombin complex concentrate (aPCC, also known as factor eight inhibitor bypass activity, FEIBA, Baxter AG, Vienna, Austria)Citation21 and recombinant activated factor VII (rFVIIa; NovoSeven, Novo Nordisk AS, Bagsværd, Denmark).Citation15,Citation22 rFVIIa, given at supraphysiological doses, has been approved in many countries for the treatment of bleeding episodes in patients with congenital hemophilia and inhibitors to FVIII or FIX and patients with acquired hemophilia.Citation23 The half-life of FVIIa in the circulation is approximately 2.3 hours.Citation24,Citation25 In most patients, bleeding episodes are therefore brought under control by administering two or three doses of 90 μg/kg given at two hour intervals.Citation26,Citation27
The need for a long-acting therapy for hemophilia
A long-acting form of FVIII would provide extended protection against bleeding and reduce gaps in protection caused by drops in FVIII levels between injections. Therefore, fewer injections would be required, resulting in fewer injection related complications, and better quality of life. Several attempts have been made to generate long-acting forms of FVIII.Citation28 These included mutagenesis of the FVIII molecule to increase its stabilityCitation29–Citation31 and direct PEGylation of the FVIII protein.Citation32–Citation35 Modifying a large and complex protein such as FVIII is a difficult undertaking, though. The integrity of multiple active sites must be maintained and protein conformation must be preserved. Development of modified forms of FVIII is still in the preclinical stage and the safety and efficacy of these approaches has yet to be demonstrated in humans.Citation28
There is also a clinical need for a more potent and long-acting form of FVIIa. As in the case of FVIII, long-acting FVIIa would require fewer injections, would reduce injection associated complications, and would provide patients with better control of bleeding episodes with fewer dips in FVIIa levels. Several approaches have been employed in attempts to improve FVIIa. These include substitution of one or more amino acids within the protein,Citation36 PEGylation,Citation37 and fusion of FVIIa to albumin.Citation38 These proteins showed promising results in-vitro and in-animal models, but as in the case of FVIII, the efficacy and safety of these approaches has yet to be demonstrated in humans.
PEGylated liposome (PEGLip) technology
Liposomes, artificial phospholipid vesicles, have proven to be useful in stabilizing drugs and improving their pharmacological properties. In most cases, liposomes are used to encapsulate the therapeutic agent (usually a small molecule drug).Citation39 Modifying the liposome surface with molecules such as polyethylene glycol prevents adsorption of plasma proteins to the liposome surface and interferes with recognition and uptake by the reticoloendothelial system (RES). This results in the generation of liposomes with a dramatically extended circulation time.Citation40,Citation41
PEGylated liposome (PEGLip) technology is a new approach to improving the pharmacodynamic properties of therapeutic proteins. Instead of encapsulating the drug, PEGylated liposomes are used as carriers with the protein bound non-covalently but with high specificity to the outer surface (). Unlike approaches such as mutagenesis, direct PEGylation, or fusion to carrier proteins, PEGLip technology does not involve changes to a protein’s amino acid sequence and does not involve covalent attachment of stabilizing agents.
Figure 1 A schematic diagram showing a PEGLip formulated coagulation factor VIII (FVIII) or activated factor VII (FVIIa). The protein is non-covalently bound to a polyethylene glycol moiety on the outer surface of a PEGylated liposome. Binding is mediated by an amino acid consensus sequence within the protein (boxed in red). The actual consensus sequences, locations within the proteins’ sequences and affinity constants (KD) for FVIII (two binding sites) and FVIIa are shown above.
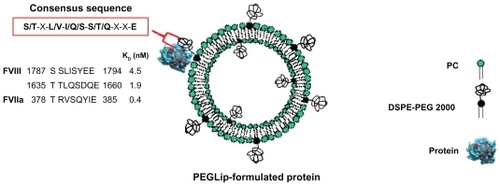
The PEGLip that we typically produce are composed of a 97:3 molar ratio of 1-palmitoyl-2-oleoyl phosphatidylcholine (POPC) to 1,2 distearoyl-sn-glycero-3-phosphatidylethanolamine-N-[methoxy(polyethyleneglycol)-2000] (DSPE-PEG 2000). The liposomes are prepared as follows. Lipids are dissolved in tert-butanol and lyophilized. The resulting dry lipid powder is resuspended to 110 mM lipid in 50 mM sodium citrate, pH 6.7 to form liposomes. The liposomes are then down-sized by extrusion through sequentially smaller polycarbonate filters until they reach a final diameter of 80–100 nm. The PEG molecules that extend outward from the liposome surface mediate binding of FVIII, FVIIa and other proteins to the liposome surface (). As mentioned above, the PEG molecules also limit uptake of the liposomes by the RES, thereby extending half-life in the circulation.Citation42–Citation44
The binding of PEGLip to FVIII and FVIIa was demonstrated in real time using surface plasmon resonance (SPR). The protein being assayed was bound to the surface of a chip and association of liposomes was measured as a liposome solution flowed over the chip. SPR measurements showed that PEGLip bind both recombinant and plasma-derived FVIIICitation45,Citation46 as well as FVIIa.Citation47 PEGLip also bind additional proteins such as recombinant human granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), and glucagon-like peptide 1 (GLP-1).Citation48 Affinity constants (KD) for all of these interactions were in the low nM range (0.4–12 nM). All of the proteins that bind PEGLip share a consensus sequence of 8 amino acids (S/T–X–L/V–I/Q/S–S/T/Q–X–X–E) (). A synthetic peptide derived from one of the two consensus sequences in FVIII (amino acids 1783–1796 of FVIII) also binds PEGLip with high affinity (KD of 2.3 nM). PEGLip do not bind several proteins that lack the consensus sequence, highlighting its significance for binding. These proteins include human serum albumin (HSA), human IgG, insulin, interferon alpha 2a, interferon alpha 2b, human growth hormone, and erythropoietin.Citation48 Together, our data show that the consensus sequence is sufficient to mediate the binding of the proteins/peptides to PEGLip.
We also sought to identify the molecules or structure on PEGLip responsible for protein/peptide binding. We found that non-PEGylated POPC liposomes do not bind FVIII,Citation45,Citation46 FVIIa,Citation47 or the FVIII-derived synthetic peptide.Citation48 Analyses performed with liposomes composed of various types of lipids and lipid polymers revealed that the interaction between PEGLip and proteins is mediated primarily by the PEG molecule and the carbamate group adjacent to the PEG molecule within DSPE-PEG 2000.Citation48 When PEG molecules were not present on the liposome surface (as in the case of POPC liposomes) no binding occurred. Likewise, when proteins lacking the consensus sequence were assayed, no binding was observed. Liposome-protein binding is therefore highly specific.
Formulation of FVIII and FVIIa with PEGLip
Formulation of a protein with PEGylated liposomes is very straightforward. The lyophilized protein powder of FVIII or FVIIa is simply reconstituted in liposome solution and allowed to fully dissolve. Even large and sensitive proteins such as FVIII may be formulated without any change in the production process or the purification procedure.Citation45–Citation47,Citation49 Formulation is very gentle and it does not involve any covalent modification of the protein. The protein’s native structure is not changed, and the likelihood of antibody production does not increase. The protein is fully active immediately after formulationCitation45–Citation47 and it is free to interact with its normal binding partners.Citation45 Administration to patients remains for the most part unchanged, though dosage and frequency of injections may need to be adjusted to account for extended therapeutic activity.
PEGLip-formulated FVIII
FVIII binds PEGLip specifically and with high affinity (two binding sites, KD of 4.5 and 1.9 nM), but this interaction does not alter FVIII activity, as shown by maintenance of full activity after formulation.Citation45,Citation46 The association of FVIII with PEGLip does not affect the FVIII protein’s structure. This was demonstrated by binding of several anti-FVIII antibodies to FVIII following PEGLip formulationCitation45 Moreover, PEGLip formulation allows FVIII to interact with its natural binding partners. This was shown by in-vitro binding of PEGLip-formulated FVIII to vWF.Citation45 Thus binding of PEGLip to FVIII does not change the protein’s biological properties.
PEGLip formulation of both recombinant and plasma–derived forms of FVIII extends hemostatic efficacy in vivo. This was demonstrated by better survival of hemophilic mice following tail-vein transection. Standard FVIII, PEGLip-FVIII, or saline were administered to hemophilic mice 24 hours prior to transection of the left lateral tail vein. Mice that received PEGLip-FVIII bled less and survived significantly longer (P < 0.05) than mice that received standard FVIII or saline ().Citation45,Citation46 This significantly increased survival of tail-vein transected hemophilic mice following the injection of PEGip formulated recombinant FVIII was also demonstrated by others.Citation50 The increased survival rate was dependent on the pre-formation of a complex of FVIII and PEGLip. Clotting times of whole blood samples from hemophilic mice injected with PEGLip-FVIII were much shorter than the clotting times of blood samples from mice injected with free FVIII. This faster clotting was detected shortly after injection and at various time points up to 72 hours post injection.Citation50
Figure 2 Efficacy of PEGLip-formulated FVIII in preclinical experiments and a clinical trial. A) Efficacy in an animal model. Hemophilic mice were injected into the tail vein with PEGLip-formulated FVIII, standard FVIII (both 0.1 IU/mouse), or saline. Twenty-four hours after injection, the left lateral tail vein of each mouse was cut and survival was scored. B) Efficacy in a clinical trial. Hemophilia A patients were given 25 IU/kg or 35 IU/kg of standard or PEGLip-formulated FVIII and the time between the prophylactic infusion and the next spontaneous bleed was recorded. The number of bleeding-free days following each treatment is shown. Results are average ± SEM.
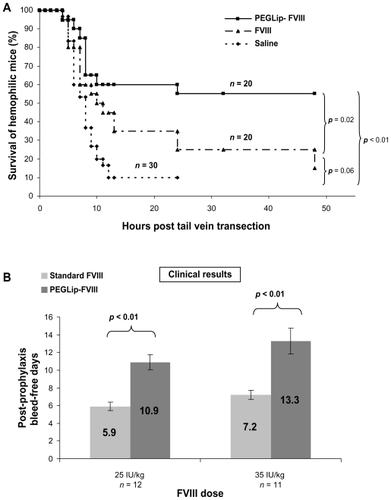
PEGLip-FVIII provides human subjects with extended protection from bleeding.Citation49 Several clinical trials tested the safety and the efficacy of PEGLip rFVIII (also referred to as BAY 79-4980).Citation51 A single arm, subject blinded clinical trial was performed to assess the efficacy and safety of two levels of FVIII (25 or 35 IU/kg) with a fixed dose of liposomes (22 mg lipids/kg). Twenty-three severe hemophilia A patients were treated in three study segments. Standard FVIII was administered in the first study segment whereas PEGLip-FVIII was provided in the second and third segments. Segments were separated by 4-day washout periods and each prophylactic infusion was administered while subjects were in a non-bleeding state. As a measure of efficacy, the time between each prophylactic infusion and the next spontaneous bleed was recorded. Prophylactic treatment with PEGLip-FVIII nearly doubled the length of time subjects were protected from spontaneous bleeding (). A single prophylactic injection of 25 IU/kg PEGLip-FVIII resulted in a mean bleed-free interval of 10.9 days compared to 5.9 days with standard FVIII. Similarly, injection of 35 IU/kg PEGLip-FVIII resulted in a mean bleed-free interval of 13.3 days compared to 7.2 days with standard FVIII. This difference was significant (P < 0.05) for both dose levels ().Citation49
A subsequent randomized, subject blinded, four-way crossover study involving 16 hemophilia A patients evaluated the efficacy and safety of prophylactic infusions of various PEGLip doses (4.2, 12.6, or 22.1 mg/kg) with a fixed FVIII dose (35 IU/kg). Mean number of bleeding-free days after each infusion increased from 7.8 days for 35 IU/ml of standard FVIII to 8.7, 10.8, and 10.9 days for 35 IU/ml of FVIII formulated in 4.2, 12.6, and 22.1 mg/kg of PEGLip, respectively.Citation52 The study showed a dose response to PEGLip that reached saturation at the highest dose level.
An additional study tested the safety of PEGLip-FVIII and compared its pharmacokinetic profile to that of standard FVIII.Citation53 In this randomized double-blind study, 26 severe hemophilia A patients received a single injection of standard FVIII (35 IU/kg) followed by 12 observation days and a 4-day Washout period. Patients then received a single injection of PEGLip-FVIII (35 IU/kg FVIII, 13 or 22 mg/kg PEGLip) followed once again by 12 days of observation. Pharmacokinetic (PK) analysis based on samples taken from patients during the trial showed no significant difference between standard FVIII and PEGLip-FVIII. This suggests that the increased protection from bleeding observed with PEGLip-FVIII did not result from a simple prolongation of FVIII half-life in the blood stream.
Safety and tolerability of PEGLip-FVIII were assessed in another clinical trial involving 18 severe hemophilia A patients. This study was directed primarily at determining the optimal infusion rate for PEGLip-FVIII. The study showed that PEGLip-FVIII may be administered at an infusion rate similar to that of standard FVIII.Citation54
In all four of the studies described above, no production of inhibitory antibodies was detected, and no serious adverse events were reported. However, a few subjects experienced increased breathing frequency and flushing.Citation53,Citation54 At the first sign of symptoms, infusions were stopped and the patients recovered fully without further medical intervention. This type of hypersensitivity reaction is known as complement activation related pseudoallergy (CARPA) and has been described following administration of radiocontrast media, liposomal drugs, and micellar solvents. Unlike IgE mediated reactions, CARPA reactions arise at first treatment and become milder or disappear upon repeated exposure.Citation54,Citation55
The combined results of the phase I and II clinical studies described above show that PEGLip-FVIII is well tolerated and provides extended protection from bleeding following prophylactic treatment.
PEGLip-formulated FVIIa
PEGLip formulation is desirable for FVIIa because this therapeutic protein has an extremely short half-life in the circulation (approximately 2.3 hours).Citation24,Citation25 In most patients treated with FVIIa, effective hemostasis is achieved only after two or three doses of 90 μg/kgCitation26,Citation27 given at two hour intervals.
SPR analyses showed that PEGLip bind specifically to FVIIa. As in the case of FVIII, binding of liposomes to FVIIa was dependant on the presence of the DSPE-PEG lipopolymer. POPC liposomes lacking DSPE-PEG bound FVIIa to a much lower level than PEGLip. Kinetic analysis of the PEGLip-FVIIa interaction indicated that the PEGLip bind to FVIIa with an affinity (KD) of 0.4 nM.Citation47 Formulation of FVIIa with PEGLip did not affect its in vitro activity.Citation47 Most importantly, formulation of FVIIa with PEGLip improved survival of hemophilic mice following tail vein transection. Hemophilic mice were injected with standard FVIIa, PEGLip-FVIIa, or saline. Fifteen minutes after injection, tail veins were transected and survival was scored up to 24 hours. We found that mice injected with PEGLip-FVIIa survived significantly longer (P < 0.05) than mice injected with standard FVIIa ().
Figure 3 Efficacy of PEGLip-formulated FVIIa in preclinical experiments and a clinical trial. A) Efficacy in an animal model. Hemophilic mice were injected with PEGLip-FVIIa, standard FVIIa (both 10 μg/mouse), or saline. The right and left lateral tail veins of each mouse were cut 15 min post injection and survival was scored. B) Efficacy in a clinical trial. Hemophilia patients with inhibitors were injected prophylactically with 90 μg/kg FVIIa (○) or 90 μg/kg PEGLip-FVIIa (●). Total clotting times at 0.5–5 hours post injection were analyzed by thrombelastography. No clotting (total clotting time >3600 sec) was detected in any of the subjects in the hour preceding infusions. The dashed lines compare clotting times induced by PEGLip-FVIIa to those induced by standard FVIIa. Results are average ± SEM (n = 6). *P < 0.05, **P = 0.08 (FVIIa vs PEGLip-FVIIa, paired t-test).
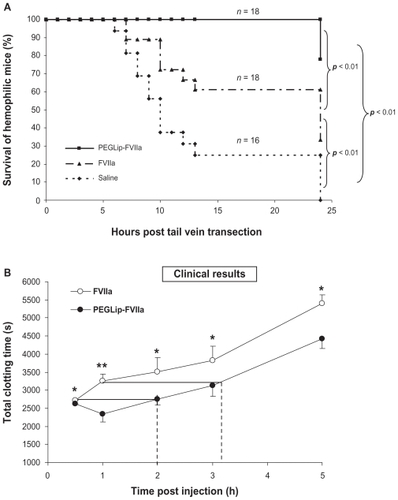
The safety and efficacy of PEGLip-FVIIa were tested in humans in an open label, exploratory, cross over, phase I/II clinical study in six adult subjects with severe hemophilia A and inhibitory FVIII antibodies.Citation56 Each subject received two infusions: one infusion of 90 μg/kg standard FVIIa and one infusion of 90 μg/kg PEGLip-formulated FVIIa. The two infusions were separated by a 10-day washout period. Injection volume was kept constant while injection order was randomized. Blood samples were collected from patients at various time points both before and after each infusion of FVIIa or PEGLip-FVIIa. Whole blood samples were then analyzed by rotational thrombelastography, a method recording the kinetics of clot formation and the firmness of the clots. PEGLip-formulated FVIIa produced significantly (P < 0.05) shorter clotting times and higher clot firmness than standard FVIIa up to 5 hours post injection. Clotting time of standard FVIIa 30 min post injection was the same as clotting time of PEGLip-FVIIa 2 hours post injection. Clotting time of standard FVIIa 1 hour post injection was the same as clotting time of PEGLip-FVIIa 3.2 hours post injection (). Maximal clot firmness induced by standard FVIIa 0.5 hour post injection was as high as that induced by PEGLip-FVIIa ~2.7 hours post injection. Thrombin generation assays showed that thrombin was produced faster and more efficiently following infusion of PEGLip-FVIIa than following infusion of standard FVIIa. No significant differences were detected between the PK of PEGLip-FVIIa and standard FVIIa.
One of the subjects in the trial experienced transient hyperemia, an increase in blood pressure and anxiety during the infusion of the first 1 mL of PEGLip-FVIIa. This non-IgE mediated reaction (CARPA) is associated with PEGLip.Citation55 and is not related to FVIIa. The reaction subsided within one hour. In all other subjects, PEGLip-FVIIa was well tolerated and there were no serious adverse events nor were there any significant changes in vital signs, clinical chemistry or hematological parameters. Measurements of coagulation parameters indicated that there was no increase in thrombotic risk.
An analysis of the results of the clinical trial indicates that PEGLip-FVIIa provides about two more “efficacy hours” than standard FVIIa. In treatment of bleeding episodes in hemophilia patients with inhibitors, one 90 μg/kg dose of PEGLip-FVIIa may be roughly equivalent to two infusions of standard FVIIa given at 2-hour intervals.
Mechanism of action
In preclinical models, formulation of FVIII and FVIIa with PEGLip improved pharmacokinetic properties in vivo and increased circulation half-life.Citation45–Citation47 However, phase I clinical experiments showed that there was no difference between the pharmacokinetic behavior of standard FVIII and PEGLip-formulated FVIIICitation53 and between standard FVIIa and PEGLip-formulated FVIIa.Citation56 This suggests that extension of circulation half-life is not the primary mechanism responsible for increased hemostatic efficacy.
Extensive in vitro experiments measuring clot formation and lysis by rotational thrombelastography, indicate that platelets must be present in order for PEGLip formulation to improve the hemostatic properties of FVIII and FVIIa.Citation47,Citation57 When PEGLip-FVIII and PEGLip-FVIIa were added to severe hemophilic whole blood or platelet rich plasma, clots formed much faster than when similar concentrations of standard FVIII and FVIIa were used. Clots were also firmer and more resistant to fibrinolysis. Similarly, ex-vivo rotational thrombelastometry experiments in whole blood drawn from hemophilic mice indicated that clotting times were much faster in mice injected with PEGLip-FVIII rather than with free FVIII.Citation50 Such improvements in kinetics, clot firmness, and resistance to fibrinolysis depended on platelets as they were not observed when experiments were performed in platelet poor plasma.Citation57
In order to gain a deeper understanding of the mechanisms responsible for these improvements in efficacy, we tested whether PEGLip themselves bind platelets. Flow cytometry analysis indicated that fluorescent PEGLip associate with platelets in-vitro in a dose related manner. This association was further confirmed in-vivo following the injection of fluorescent PEGLip into hemophilic mice.Citation57 Interactions between liposomes of various compositions, including PEG liposomes, and blood cells have been reported previously by others.Citation50,Citation58
Having shown that PEGLip bind FVIII and FVIIaCitation45,Citation47 and that PEGLip bind platelets, we next tested whether PEGLip are capable of delivering proteins to platelets. Fluorescently labeled FVIII and FVIIa were formulated with non-fluorescent PEGLip and shown by flow cytometry to bind human platelets in-vitro. These results were also found in-vivo as PEGLip-formulated fluorescent proteins interacted with the platelets of hemophilic mice following injection.Citation57 Fluorescently labeled human serum albumin (HSA), which does not bind to PEGLip, did not show increased binding to platelets following formulation with PEGLip. This indicates that PEGLip-mediated association of proteins with platelets is dependent on prior binding of the proteins to PEGLip.
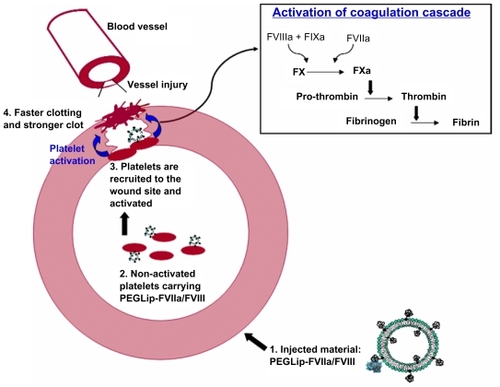
The combined results indicate the following mechanism (): Formulation of FVIII or FVIIa with PEGLip leads to non-covalent binding of the protein to the outer surface of the PEGylated liposomes. After injection of the formulated proteins to the bloodstream the liposomes associate with non-activated platelets and a complex of platelet-PEGLip-protein (FVIII/FVIIa) is formed in-vivo. Following injury, platelets are recruited to sites of injury where they adhere to the damaged vessel wall. Due to their prior association with PEGLip, they carry FVIII and FVIIa with them. Platelets are activated at the wound site and coagulation complexes form on the surface of the activated platelets. Since FVIII and FVIIa are already present on the platelets prior to activation, the coagulation cascade is more efficient. Clots form faster and the clots are more stable.
Figure 4 Mechanism of action of PEGLip-formulated FVIII and FVIIa.
1. Formulation of FVIII or FVIIa with PEGLip leads to non-covalent binding of the protein to the outer surface of the PEGylated liposomes.
2. The liposomes are then injected into the bloodstream where they associate with non-activated platelets.
3. When injury occurs, platelets are recruited to the wound where they adhere to the damaged vessel wall. They carry FVIII and FVIIa with them. Platelet activation and initiation of the coagulation cascade occur simultaneously.
4. Coagulation complexes form on the surface of the activated platelets. Since FVIII and FVIIa are already present on the platelets prior to activation, the coagulation cascade is more efficient. Clots form faster and the clots are more stable.
The association of PEGLip-FVIII or PEGLip-FVIIa with platelets may also lead to increased concentration of FVIII or FVIIa at the wound site, even when the overall concentration of FVIII or FVIIa in the circulation is low. Accordingly, expression of FVIII in platelets was shown to be effective at inducing hemostasis even when FVIII protein expression levels did not exceed 1% of normal levels. This was probably due to recruitment of platelets to the sites of injury and local release of FVIII from activated platelets at wound sites.Citation59
Summary
PEGLip technology improves the pharmacodynamic properties of both coagulation factors VIII and VIIa. This was demonstrated in preclinical experiments and in five different clinical trials with both FVIII and FVIIa. The combined results indicate that PEGLip-FVIII prevents bleeding for a significantly longer period than standard FVIII, and that one dose of PEGLip-FVIIa may be roughly equivalent to two infusions of standard FVIIa given at 2 hour intervals when treating a bleed in hemophilia patients with inhibitors.
Of the approaches currently available to generate long-acting forms of FVIII and FVIIa, PEGLip formulation has the advantage of being the only approach that has been tested extensively in clinical trials. PEGLip-FVIII and PEGLip-FVIIa have been shown to be safe in trials involving more than 90 hemophilia patients. In general, treatments with PEGLip-formulated FVIII and FVIIa were well tolerated.
One of the most problematic complications of hemophilia treatment is the induction of inhibitory antibodies. PEGLip formulation does not change the structure of FVIII and FVIIa. It thereby avoids one of the biggest obstacles to producing improved coagulation factors: changes in structure that induce antibody formation. Because of the large size and complexity of the FVIII protein and the sensitivity of hemophilia A patients to even small changes in the protein,Citation60,Citation61 FVIII can be considered a worst case scenario for increased immunogenicity. Extensive experience with PEGLip-FVIII in several clinical studies has shown no increase in antibody generation. PEGLip formulation thus avoids one of the major pitfalls of the drug development process.Citation49,Citation52,Citation53
PEGLip-FVIII/FVIIa are well tolerated and suitable for long-term treatment of hemophilia. This was demonstrated in toxicology studies, which included injecting high doses of PEGLip-FVIII and PEGip-FVIIa into rats and rabbits (acute toxicology) and repeated injections (up to nine months of weekly injections) of PEGLip into rats and rabbits.Citation45 PEGylated liposomes were also shown to be non-toxic in mice and dogs.Citation62
In the years to come, we expect PEGLip-FVIII and PEGLip-FVIIa to become an attractive treatment for hemophilia A patients and hemophilia patients with inhibitors.
Disclosure
The authors are employees of Omri Laboratories Ltd. The authors report no conflicts of interest in this work.
References
- DahlbackBBlood coagulation and its regulation by anticoagulant pathways: genetic pathogenesis of bleeding and thrombotic diseasesJ Intern Med200525720922315715678
- HednerUGinsburgDLusherJMHighKACongenital Hemorrhagic Disorders: New Insights into the Pathophysiology and Treatment of HemophiliaHematology Am Soc Hematol Educ Program200024126511701545
- SoucieJMEvattBJacksonDOccurrence of hemophilia in the United States. The Hemophilia Surveillance System Project InvestigatorsAm J Hematol1998592882949840909
- KaufmanRJAnthonorakisSEFayPJHemostasis and Thrombosis: Basic Principles and Clinical Practice4th edPhiladelphiaLippincott Williams and Wilkins2001
- RoosendaalGMauser-BunschotenEPDe KleijnPSynovium in haemophilic arthropathyHaemophilia199845025059873782
- ArunBKesslerCColmanWHirshJMarderVClowesAGeorgeJInherited hemorrhagic disordersHemostasis and thrombosis4th edPhiladelphiaLippincott Williams & Willkins2001815825
- HootsWKNugentDJEvidence for the benefits of prophylaxis in the management of hemophilia AThromb Haemost20069643344017003919
- KesslerCMNew perspectives in hemophilia treatmentHematology Am Soc Hematol Educ Program200542943516304415
- JankowskiMAPatelHRouseJCMarzilliLAWestonSBSharpePJDefining ‘full-length’ recombinant factor VIII: a comparative structural analysisHaemophilia200713303717212722
- FijnvandraatKBerntorpEten CateJWRecombinant, B-domain deleted factor VIII (r-VIII SQ): pharmacokinetics and initial safety aspects in hemophilia A patientsThromb Haemost1997772983029157585
- WeissHJSussmanIIHoyerLWStabilization of factor VIII in plasma by the von Willebrand factor. Studies on posttransfusion and dissociated factor VIII and in patients with von Willebrand’s diseaseJ Clin Invest19776039040417621
- SantagostinoEMancusoMEBarriers to primary prophylaxis in haemophilic children: the issue of the venous accessBlood Transfus20086S121619105504
- SaenkoELAnanyevaNMMoayeriMRamezaniAHawleyRGDevelopment of improved factor VIII molecules and new gene transfer approaches for hemophilia ACurr Gene Ther20033274112553533
- MathewPCurrent opinion on inhibitor treatment optionsSemin Hematol200643S81316690374
- LusherJMRobertsHRDavignonGA randomized, double-blind comparison of two dosage levels of recombinant factor VIIa in the treatment of joint, muscle and mucocutaneous haemorrhages in persons with haemophilia A and B, with and without inhibitors. rFVIIa Study GroupHaemophilia1998479079810028299
- ShapiroAInhibitor treatment: state of the artDis Mon200349223812525826
- RickMEWalshCEKeyNSCongenital bleeding disordersHematology Am Soc Hematol Educ Program200355957414633799
- ZeitlerHUlrich-MerzenichGHessLTreatment of acquired hemophilia by the Bonn-Malmo Protocol: documentation of an in vivo immunomodulating conceptBlood20051052287229315542586
- MaADCarrizosaDAcquired factor VIII inhibitors: pathophysiology and treatmentHematology Am Soc Hematol Educ Program200643243717124095
- KemptonCLWhiteGC2ndHow we treat a hemophilia A patient with a factor VIII inhibitorBlood2009113111718820129
- TjonnfjordGEHolmePAFactor eight inhibitor bypass activity (FEIBA) in the management of bleeds in hemophilia patients with high-titer inhibitorsVasc Health Risk Manag2007352753117969383
- HednerUGlazerSPingelKSuccessful use of recombinant factor VIIa in patient with severe haemophilia A during synovectomyLancet1988211932903400
- IngerslevJEfficacy and safety of recombinant factor VIIa in the prophylaxis of bleeding in various surgical procedures in hemophilic patients with factor VIII and factor IX inhibitorsSemin Thromb Hemost20002642543211092219
- FridbergMJHednerURobertsHRErhardtsenEA study of the pharmacokinetics and safety of recombinant activated factor VII in healthy Caucasian and Japanese subjectsBlood Coagul Fibrinolysis20051625926615870545
- LindleyCMSawyerWTMacikBGPharmacokinetics and pharmacodynamics of recombinant factor VIIaClin Pharmacol Ther1994556386488004880
- IngerslevJThykjaerHKudsk JensenOFredbergUHome treatment with recombinant activated factor VII: results from one centreBlood Coagul Fibrinolysis19989S1071109819039
- KeyNSAledortLMBeardsleyDHome treatment of mild to moderate bleeding episodes using recombinant factor VIIa (Novo-seven) in haemophiliacs with inhibitorsJ Thromb Haemost199880912918
- SaenkoELPipeSWStrategies towards a longer acting factor VIIIHaemophilia200612Suppl 3425116683996
- GaleAJPellequerJLAn engineered interdomain disulfide bond stabilizes human blood coagulation factor VIIIaJ Thromb Haemost200311966197112941038
- GaleAJRadtkeKPCunninghamMAChamberlainDPellequerJLGriffinJHIntrinsic stability and functional properties of disulfide bond-stabilized coagulation factor VIIIa variantsJ Thromb Haemost200641315213216706977
- PipeSWKaufmanRJCharacterization of a genetically engineered inactivation-resistant coagulation factor VIIIaProc Natl Acad Sci U S A19979411851118569342326
- MeiBPanCJiangHRational design of a fully active, long-acting PEGylated factor VIII for hemophilia A treatmentBlood
- MurphyJEPanCBarnettTSite-specific PEGylation of rFVIII results in prolonged in vivo efficacyJ Thromb Haemost20075P-T-022
- ReganLMJiangXRamseyPBiological activity of PEGylated factor VIIIJ Thromb Haemost20075P-T-026
- TangLPanCAtwalHPEGylation protects factor VIII from the inhibition of antibody inhibitorsJ Thromb Haemost20075P-T-036
- AllenGAPerssonECampbellRAEzbanMHednerUWolbergASA variant of recombinant factor VIIa with enhanced procoagulant and antifibrinolytic activities in an in vitro model of hemophiliaArterioscler Thromb Vasc Biol20072768368917204663
- StennickeHROstergaardHBayerRJGeneration and biochemical characterization of glycoPEGylated factor VIIa derivativesJ Thromb Haemost2008100920928
- SchulteSUse of albumin fusion technology to prolong the half-life of recombinant factor VIIaThromb Res2008122S141918929521
- ZamboniWCConcept and clinical evaluation of carrier-mediated anticancer agentsOncologist20081324826018378535
- ElbayoumiTATorchilinVPLiposomes for targeted delivery of antithrombotic drugsExpert Opin Drug Deliv200851185119818976130
- GabizonAShmeedaHBarenholzYPharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studiesClin Pharmacokinet20034241943612739982
- GabizonACataneRUzielyBProlonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomesCancer Res1994549879928313389
- GabizonAABarenholzYBialerMProlongation of the circulation time of doxorubicin encapsulated in liposomes containing a polyethylene glycol-derivatized phospholipid: pharmacokinetic studies in rodents and dogsPharm Res1993107037088321835
- KlibanovALMaruyamaKTorchilinVPHuangLAmphipathic polyethyleneglycols effectively prolong the circulation time of liposomesFEBS Lett19902682352372384160
- BaruMCarmel-GorenLBarenholzYFactor VIII efficient and specific non-covalent binding to PEGylated liposomes enables prolongation of its circulation time and haemostatic efficacyJ Thromb Haemost20059310611068
- DayanIRobinsonMBaruMEnhancement of haemostatic efficacy of plasma-derived FVIII by formulation with PEGylated liposomesHaemophilia2009151006101319486171
- YatuvRDayanICarmel-GorenLEnhancement of factor VIIa haemostatic efficacy by formulation with PEGylated liposomesHaemophilia20081447648318393980
- YatuvRCarmel-GorenLDayanIRobinsonMBaruMBinding of proteins to PEGylated liposomes and improvement of G-CSF efficacy in mobilization of hematopoietic stem cellsJ Control Release2009135445019135487
- SpiraJPlyushchOPAndreevaTAAndreevYProlonged bleeding-free period following prophylactic infusion of recombinant factor VIII reconstituted with pegylated liposomesBlood20061083668367316888098
- PanJLiuTKimJYEnhanced efficacy of recombinant FVIII in noncovalent complex with PEGylated liposome in hemophilia A miceBlood20091142802281119654409
- PowellJSLiposomal approach towards the development of a longer-acting factor VIIIHaemophilia200713232817685920
- SpiraJPlyushchOPAndreevaTAKhametovaRNEvaluation of liposomal dose in recombinant factor VIII reconstituted with pegylated liposomes for the treatment of patients with severe haemophilia AJ Thromb Haemost2008100429434
- PowellJSNugentDJHarrisonJASafety and pharmacokinetics of a recombinant factor VIII with pegylated liposomes in severe hemophilia AJ Thromb Haemost2008627728318039351
- MartinowitzULalezariSLuboshitzJLubetskyASpiraJInfusion rates of recombinant FVIII-FS with PEGylated liposomes in haemophilia AHaemophilia2008141122112418564190
- SzebeniJComplement activation-related pseudoallergy: a new class of drug-induced acute immune toxicityToxicology200521610612116140450
- SpiraJPlyushchOPZozulyaNSafety, pharmacokinetics and efficacy of factor VIIa formulated with PEGylated liposomes in haemophilia A patients with inhibitors to factor VIII. An open label, exploratory, cross over, phase I/II studyHaemophilia2010 In Press
- YatuvRRobinsonMDayanIBaruMEnhancement of the efficacy of therapeutic proteins by formulation with PEGylated liposomes; a case of FVIII, FVIIa and G-CSFExpert Opin Drug Deliv2010718720120095942
- ConstantinescuILevinEGyongyossy-IssaMLiposomes and blood cells: a flow cytometric studyArtif Cells Blood Substit Immobil Biotechnol20033139542414672416
- HighKAThe leak stops here: platelets as delivery vehicles for coagulation factorsJ Clin Invest20061161840184216823486
- LaubRDi GiambattistaMFonduPInhibitors in German hemophilia A patients treated with a double virus inactivated factor VIII concentrate bind to the C2 domain of FVIII light chainJ Thromb Haemost1999813944
- RautSDi GiambattistaMBevanSAHubbardARBarrowcliffeTWLaubRModification of factor VIII in therapeutic concentrates after virus inactivation by solvent-detergent and pasteurisationJ Thromb Haemost199880624631
- KanterPMBullardGAPilkiewiczFGMayerLDCullisPRPavelicZPPreclinical toxicology study of liposome encapsulated doxorubicin (TLC D-99): comparison with doxorubicin and empty liposomes in mice and dogsIn Vivo1993785958504212