Abstract
Nanotechnology has rapidly expanded into all areas of science; it offers significant alternative ways to solve scientific and medical questions and problems. In dentistry, nanotechnology has been exploited in the development of restorative materials with some significant success. This review discusses nanointerfaces that could compromise the longevity of dental restorations, and how nanotechnolgy has been employed to modify them for providing long-term successful restorations. It also focuses on some challenging areas in dentistry, eg, oral biofilm and cancers, and how nanotechnology overcomes these challenges. The recent advances in nanodentistry and innovations in oral health-related diagnostic, preventive, and therapeutic methods required to maintain and obtain perfect oral health, have been discussed. The recent advances in nanotechnology could hold promise in bringing a paradigm shift in dental field. Although there are numerous complex therapies being developed to treat many diseases, their clinical use requires careful consideration of the expense of synthesis and implementation.
Introduction
Nanotechnology as a discipline has rapidly expanded into all areas of science as it offers significant alternative ways to solve scientific and medical questions and problems. Nanotechnology is a branch of technology that works on the dimensions of less than 100 nm. It covers objects such as viruses at around 100 nm size, down to glucose molecules at around 1 nm. Hence, it is very much concerned with structures at the molecular and atomic scale.
Much of the works that have been carried out over the last 20–30 years (when we have seen an explosion in interest in nanotechnology), have been concerned with nanoparticles, and these exemplify very clearly why there is such an interest in nanotechnology and the properties of the materials at these length scales. For example, one can calculate the surface area of 1 g of powder at different spherical particle diameter sizes, and plotting these data shows that below ~100 nm, the surface area per gram rises exponentially. This leads to a step change in the properties of these materials as properties such as surface energy per gram of material will also change hugely. This massive increase in surface area can be exploited for a wide range of applications. One of the most simplistic exploitations of nanotechnology is in the pharmaceutical industry, where many drugs are poorly or sparingly soluble in aqueous media due to their hydrophobic nature. Reducing the pharmaceutical down to the nanoparticle size range can allow more of the drug to move into solution due to the massive increase in surface area.
One area of nanotechnology that has been extensively utilized in dentistry is that of nanoparticles in composites. The rationale for utilization of nanoparticles is two-fold: 1) to improve the esthetics by making the materials more translucent and 2) to improve (or at least modify) the wear properties. However, exploitation of nanoparticles in a composite (dental composites or composites more generally) does not come without its technical challenges. Nanoparticles, as already mentioned, can have extremely high surface charge per gram of powder, which can lead to severe and almost permanent agglomeration and which can give rise to rapid loss or non-attainment of expected properties. One good example of this is the processing of carbon nanotubes (CNTs) into polystyrene or perspex.Citation1 CNT incorporation into these polymers (as opposed to conventional glass or carbon fibers) can give much better mechanical properties (or similar properties to the conventional glass or carbon fiber composite), with little loss of the optical properties of the unfilled polymer, and can also impart the additional functionality of improved conductivity, which may be useful.Citation1 To achieve similar reinforcing effects, only 0.5–1 wt% of CNTs need to be added to the polymer to achieve similar mechanical properties to a conventional composite containing 20–30 wt% reinforcing fiber. But at these very low additions, dispersion must be absolute and complete to achieve the desired properties, and this can be extremely difficult and in some cases impossible to achieve in a cost-effective way as the surface energy keeps the particles adherent and leads to agglomeration in the composite. However, with a careful understanding of the physics and chemistry of the particles, optimal processing may be achieved and the desired properties attained.
Another area where nanotechnology has been exploited is in the use of submicron grain size ceramics for the production of all-ceramic restorations. The logic for using nanometer-sized powders for the production of ceramic monoliths is esthetics and wear properties and also for maximizing the strength of the ceramic. Processing of technical ceramics with such small grain sizes is technically challenging. Again surface energy plays a major role. During the thermal processing stage for a ceramic, surface energy drives the solid-state sintering process and can lead to sintering occurring easily and rapidly. The small grain size (as well as a non-uniform grain size distribution) can also lead to uncontrolled grain growth and catastrophic loss of mechanical properties. Thus, the synthesis and characterization of the starting material is paramount to achieving the properties required.
The aim of this review paper is not to cover all areas of nanotechnology as this would not be achievable within a single review, but to focus on some areas that have technical challenges (eg, oral biofilm and cancers), and to highlight how these challenges have been overcome. The recent advances in oral health-related preventive, diagnostic, and therapeutic methods that could hold promise in bringing a paradigm shift in dentistry are the main focus of this review. The clinical implementation of these advances requires careful consideration of the expense of synthesis and possible nanotoxicity. Since nanotoxicity is a general issue, it has been extensively reviewed elsewhere in literature.Citation2–Citation4
Bio-nanointerfaces of clinical significance
To restore partial (enamel and dentin) or complete tooth loss, dental composite or implants are normally used. The retention of resin composite or dental implants is obtained mainly through micromechanical retention. The interface between resin composites and dental tissues or between dental implants and bone is therefore important for the success of these restorations. Looking at the structure of enamel, dentin, and bone, they are composed of organic matrix, mainly collagen and noncollagenous proteins and hydroxyapatite (HA). The ratio of the organic to mineral phase varies according to the tissue. This section reviews the importance of resin composite–tooth as well as implant–bone interface and how nanotechnology has been employed to modify these interfaces to increase the longevity of resin composites and dental implants, respectively.
Adhesive resin–dentin interface
Bonding of dental composites to tooth structure is obtained through different adhesive resin systems that depend on separate or combined use of etchant, primer, and adhesive resin.Citation5 The etchant selectively removes the HA crystals, a natural shelter around collagen-rich demineralized dentin ().Citation6,Citation7 The unprotected collagen network is then more vulnerable to enzymatic degradation by endogenous matrix metalloproteinases (MMPs)Citation8 and cysteine cathepsinsCitation9,Citation10 that are released from demineralized collagen. The primer helps the subsequent penetration of the adhesive resin. After its polymerization, the entanglement of the adhesive resin into demineralized collagen network creates a transitional zone called hybrid layer or inter-diffusion zone of resin-reinforced collagen.Citation11 The polymerized adhesive resin helps to protect the collagen from enzymatic degradation; and the long-term success of resin composites depends on the integrity and durability of adhesive resin–dentin interface. The durability of resin–dentin interface can be jeopardized by: 1) degradation of collagen matrix; 2) incomplete penetration and polymerization of resin monomer; and c) hydrolysis of the resin monomer. Compromised interface integrity leads to nanoleakage, which occurs as spotted, reticular type and water trees at the hybrid or adhesive layer.Citation12,Citation13 The nanoleakge is always associated with pain, sensitivity, recurrent decay, and finally failure of restorations. Nanotechnology has been adopted to extend the longevity of resin–dentin bond via: 1) protection of exposed collagen through reinforcement, cross-linking, and biomimetic remineralization; 2) inhibiting the action of MMPs; and 3) modifying the adhesive resin monomer ().
Figure 1 (A) Optical image of a transverse section of a molar displaying an occlusal cavity, (B) reconstructed optical image of the demineralized region of the dentin in the cavity, and (C) AFM image (deflection) of a single dentin tubule from the demineralized region. Aligned collagen fibrils can be observed wrapping around the tubule. (D) Higher resolution AFM image of the exposed collagen in the vicinity of the dentin tubule; the D-banding periodicity of collage can be clearly resolved as presented in (E).
Abbreviation: AFM, atomic force microscopy.
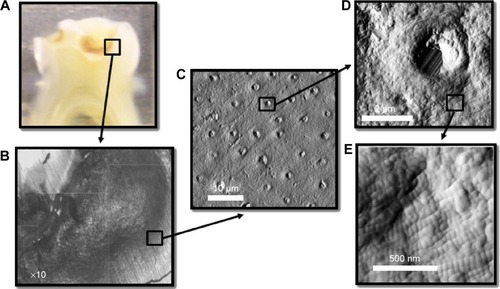
Figure 2 AFM image (deflection) of the interface between a dental composite and dentin.
Notes: The dental composite appears very homogeneous, whereas the cross-sectional cut of dentin can be seen throughout the dentin part. The interface shows no apparent gaps. Compromised interface integrity, however, leads to nanoleakage that is always associated with pain, sensitivity, recurrent decay, and finally, failure of restorations.
Abbreviation: AFM, atomic force microscopy.
Protection of exposed collagen networks by reinforcement with nanofillers or nanogels has been attempted. In addition to their protective action, these nanofillers or nanogels could help in mineral deposition and hence possible interference with MMPs.Citation14 Examples include zirconia (20–50 nm),Citation15 HA (20–70 nm),Citation16 colloidal silica (5–40 nm) or barium aluminosilicate nanofillers (400 nm),Citation17 reactive nanogels,Citation18 and bioactive calcium/sodium phosphosilicate.Citation14 Cross-linking could also improve the nanomechanical properties of the exposed collagen networkCitation19,Citation20 and hence reduce its degradation by MMPs.Citation20 Examples of cross-linking agents include tannic acid,Citation21 combinations of riboflavin and ultraviolet radiation,Citation19 riboflavin and/or chitosan,Citation22 glutaraldehyde,Citation20 grape seed extract,Citation20 and proanthocyanidins.Citation23 Biomimetic remineralization is a recent approach to protect resin-free, acid-etched dentin. This approach relies on the use of fluidic amorphous nanoprecursors (eg, Portland cement/simulated body fluid/polyacrylic acid/poly(vinylphosphonic acid). These nanoprecursors can be transformed into mesoscopic crystalline intermediates that eventually fuse to form single microscopic crystals, ie, a bottom-up nanotechnology approach. This technique proved to be successful in remineralizing both interfibrillar and intrafibrillar collagen after 2–4 months and maintaining the nanomechanical properties of normal dentin. It could also help in closing the porosity of the adhesive layer.Citation24,Citation25
Inhibition of MMPs action and hence collagen degradation has been also attempted to improve the longevity of the resin–dentin interface. The MMP inhibitor has been used as a separate dentin preconditioning step or included within the resin adhesive monomer. Examples of these inhibitors include benzalkonium chloride,Citation8 galardin,Citation26 BB94 and GM6001,Citation27 chlorhexidine digluconateCitation28,Citation29 and ZnO particles,Citation30 ethylenediaminetetraacetic acid,Citation31 sodium oxalate,Citation32 titanium tetrafluoride and caffeic acid phenethyl ether,Citation33 and phosphoric acid/sodium hypochlorite (NaOCl) mixture.Citation34
Self-healing adhesives, capable of closing micro- or nanocracks before destroying the integrity of resin–dentin bonds, have been also introduced.Citation35 They contain healing agent-filled nanocapsules; the rupture of the nanocapsules and hence the release of their content is usually initiated by the presence of a crack in the resin matrix.Citation36 Nanocontrolled molecular interaction between the resin monomer and HA remaining within the hybrid layer has also been employed for improving the longevity of the resin–dentin interface.Citation6
Dental implant–bone interface
The implant–tissue interface is a key for the success of dental implants. Peri-implant healing proceeds from recruitment and migration of bone cells to the implant surface through the action of persistent blood clot, ie, osteoconduction phase to de novo bone formation and finally bone remodeling. Both osteoconduction and de novo bone formation are essential for providing contact osteogenesis.Citation37 An intimate contact of the implant at its neck with the surrounding gingival tissue is essential for prevention of bacterial invasion and growth. Getting a fusion of the implant root with the surrounding bone at both structural and functional levels, ie, osseointegration, is crucial for the success of dental implant.Citation38 Implant osseointegration is first achieved through primary mechanical and then biological anchorage.Citation39 Microscale modification of topography and chemistry of the implant surface indirectly affected implant osseointegration, and it could inevitably induce changes at the nanoscale level.Citation40 Since cells can directly interact with nano-scale features, nanotechnology has been introduced to mediate bone formation and implant osseointegration.Citation41 Nanoscale modification of dental implant surfaces may include both surface topography as well as chemistry.
Regarding the surface topography of dental implants, several methods, eg, lithography, ionic implantation, anodization,Citation42 and radio-frequency plasma treatments, have been used to induce controlled nanosurface features (eg, tubes,Citation43,Citation44 dots,Citation45 and nodulesCitation46) on dental implants. These features can be arranged in an organized (isotropic) or random (anisotropic) manner to induce altered physicochemical behavior (eg, bone bonding) or specific biochemical events (eg, altered protein adsorption and cell behavior).Citation47 Controlling the dimension and spaces between the surface nanofeatures/patterns changed cell behavior since cells are able to sense the surface and adjust their response accordingly. Changing the diameter of nanotubes from 30 nm to 70–100 nm caused the transition of cell behavior from adhesion to differentiation.Citation43,Citation44 A spacing between the nanofeatures of 58 nm promoted the formation of a stable focal adhesion and hence persistence of cell spreading, while 108 nm spacing produced delayed cell spreading.Citation45 Regarding the bone–implant contact, higher density and curvature of 60 nm features demonstrated higher bone–implant contact than 120 nm features.Citation48
Since dental implants contact both hard and soft tissues, these implants must therefore have optimum surface biocompatibility with the host tissue. Accordingly, changing the surface chemistry could also be another option to enhance osteogenesis. Biofunctionalization, ie, immobilization of specific, active biomolecules known for their central role in osteogenesis on the surface of implants, was found to be highly effective in promoting firm and rapid osseointegration.Citation49 Examples of these biomolecules include adhesion molecule (RGD)Citation50 peptide, alkaline phosphatase,Citation49 extracellular matrix (ECM) proteins,Citation51 and calcium phosphate (CaP) coating.Citation52 Bone morphogenetic protein (BMP) has been also immobilized on the surface of dental implants to enhance bioactivity and hence bone formation.Citation53 BMP allows controlled administration and avoids overdosing. In this respect, coating the implant surface with nanocrystalline diamonds increased the surface area and facilitated immobilization of BMP.Citation54 In such a case, the overall texture of the implant can be maintained, while the cellular proliferation and differentiation were improved.Citation55 Immobilization of therapeutic agents that induce osteogenesis, eg, bisphosphonates and simvastatin,Citation56 or prevent bacterial infection, eg, silverCitation57 and zinc oxide nanoparticles,Citation58 into the biofunctionalized implant surface has also been attempted.
Accordingly, for successful dental implants, two conflicting properties (ie, enhancement of protein adsorption for cell adhesion but at the same time inhibition of bacterial colonization and hence biofilm formation) are required.Citation59 To develop this intelligent implant, which will interact with the continuously changing environment, deliver the appropriate molecule or drug or protein when required, and actively direct cell behavior, very challenging robust techniques and reliable in vivo experiments are required.
Preventive nanodentistry
Modern dentistry’s goal is to prevent rather than treat biofilm-dependent oral diseases, eg, dental caries and endodontic and periodontal diseases. Nanotechnology offers new approaches for preventive therapies in oral diseases, particularly dental caries and periodontal diseases.
Dental caries
Dental caries is one of the most prevalent destructive diseases affecting tooth structures. It is caused by bacteria, eg, Streptococcus mutans, Streptococcus sobrinus, and Lactobacillus spp. These bacteria are usually present in aggregates attached to each other and to tooth surfaces in the form of oral biofilms or dental plaques. They produce acids that cause demineralization of tooth structures, ie, loss of calcium (Ca) and phosphate (PO4). Controlling dental caries can be therefore gained through inhibiting the bacterial action, reversing the demineralization process, and promoting remineralization. Development of anti-caries vaccines has also been attempted in this respect.
Antibacterial nanotherapy
Several nanoparticles (eg, zinc oxide,Citation60,Citation61 silver,Citation62–Citation64 and polyethylenimineCitation65) have been incorporated into dental compositesCitation66 or dental adhesivesCitation67 to inhibit the bacterial growth through several mechanisms. These mechanisms include disruption of the bacterial cell membrane,Citation68 inhibition of the active transport as well as the metabolism of sugars, generation of reactive oxygen species,Citation69 displacement of magnesium ions required for the enzymatic activity of oral biofilms,Citation70 disturbance of the electron transportation across the bacterial membrane,Citation63 and prevention of DNA replication.Citation71 These nanoparticles were effective in reducing the S. mutans and Lactobacillus acidophilus biofilms in an in vitro model.Citation72,Citation73 Coating tooth surfaces with antibacterial nanocoating was found to be effective in killing bacteria as well as inhibiting bacterial adhesion and maintaining its integrity in the presence of biological fluids (saliva).Citation74 The antibacterial action of these nanoparticles was shown to be size dependent.Citation75
Biomimetic remineralization – reversing an incipient caries
Owing to their colloidal particle size and potential for delivery of calcium ions, calcium carbonate (CC) nanoparticles can have good retention on oral surfaces. They act as a delivery vehicle for slow continuous release of high concentrations of calcium ions into the surrounding oral fluids (saliva and dental plaque). CC nanoparticles also have the potential to increase the surrounding fluid pH. Accordingly, CC nanoparticles were effective in remineralizing incipient enamel lesions when incorporated into an experimental tooth dentifrice.Citation76 Nanosized calcium fluoride (CaF2), as a labile reservoir for fluoride (F), has been shown to be highly soluble and reactive with dicalcium phosphate dihydrate compared to its macro counterpart. Owing to its high solubility, a great amount of CaF2 could be consumed by its reaction with dicalcium phosphate dihydrate, and accordingly, a high level of F could be incorporated into the stable reaction product (apatite). Accordingly, a mouth rinse containing nanosized CaF2 showed higher F deposition (2.2±0.3 μg/cm2) than the conventional sodium fluoride (NaF) rinse (0.31±0.06 μg/cm2). The CaF2 rinse could potentially be used as an anticaries agent by increasing the F− concentration in oral fluids and thus enhancing tooth remineralization.
Biomimetic remineralization – recurrent decay
Since Ca2+ and PO43− are prerequisites for remineralization, several Ca2+- and PO43−-releasing dental nanocomposites were developed for their remineralizing action; they therefore help in preventing recurrent (secondary) decay around or under restorations. Different forms of nanocalcium phosphates were used as Ca2+- and PO43−-releasing fillers, eg, dicalcium phosphate anhydrous,Citation77,Citation78 tetracalcium phosphate,Citation79 monocalcium phosphate monohydrate,Citation80 and carbonate hydroxyapatite.Citation81 The release of Ca and PO4 is dependent on degradability and volume fraction of CaP form.Citation80 Regardless of Ca and PO4 release, these nanocomposites could be still used for high-stress bearing applications since these nanoparticles are used in combination with other fillers, eg, whiskers fused with nanosized silica. The Ca2+ and PO43− can also be released on demand, ie, when the pH has been reduced from neutral to cariogenic level, and the level of their release increased dramatically with acidic pH.Citation79 Thanks to the chemical binding ability of nanocalcium phosphate fillers, eg, carbonate hydroxyapatite, these nanoparticles bind to eroded enamel and dentin, forming a protective coating, and then reverse the action of acid or bacterial attack.Citation81
Caries vaccine
Several attempts are currently underway in developing an effective anticaries vaccine as a new strategy of preventing the occurrence of dental caries. DNA vaccine was found to be an effective, safe, stable, and inexpensive immunogenic strategy in inducing both humoral and cellular immune responses.Citation82 Many candidate anticaries DNA vaccines have been or are undergoing usage tests in animals or human beings. Examples of these vaccines include pcDNA3-Pac,Citation83 pCIA-P,Citation84 pGJGLU/VAX,Citation85 and pGLUA-P.Citation86 Most of the anticaries vaccines work by preventing bacterial accumulation either by blocking the surface protein antigen PAc or inactivation of glucosyltransferases enzyme.Citation87 Both surface protein antigen PAc and glucosyltransferases are the virulent factors responsible for the adhesion of S. mutans to tooth surfaces.Citation88,Citation89 DNA vaccines, however, have poor immunogenicity in large animals and human beings. Nanotechnology has been employed to tailor delivery vehicles (eg, anionic liposomes in chitosan/DNA nanoparticle complex was used as a delivery vehicleCitation90) to enhance the immunogenicity of anticaries DNA vaccine. Furthermore, the surface charge of the delivery vehicle could be pH dependent to enable the release of the vaccine in a pH-dependent manner.Citation90
Despite the advances in nanotechnology, its clinical application for caries therapy is not yet feasible. Although, the Ca2+- and PO43−-releasing nanocomposites have load-bearing capability, their mechanical behavior as Ca2+ and PO43− ions being released is difficult to estimate and control under identical clinical situations. The profile of ions released under constrained conditions has not been investigated. A balance between the ion release and the rate of caries progression, which is unpredictable, must exist. The optimum particle size of nanofillers required for remineralization, the pattern in which the released ions are precipitated, and the orientation of precipitated nanocrystals in relation to collagen fibrils are important factors that require careful consideration in designing nanomaterials. Until now, all in vitro investigations indicated that nanotechnology could be effective in reversing lesion progression in the outer but not in the deeper part of early caries lesions.Citation91 Controlling deep and large lesions, however, requires a thorough understanding of the caries progression process. Furthermore, incorporation of nanofillers within dental composites might be effective in preventing recurrent caries around restorations; the effect of these nanofillers, however, on bacteria left in affected dentin, which are sometimes left in deep cavities during conservation of tooth structures, has to be studied. Furthermore, regardless of the progress with anticaries vaccinations, their use in human beings has not been tried yet. Accordingly, there is no vaccine available on the market yet. The high diversity of oral flora, high salivary flow, difficult antigen delivery, enzymatic degradation of vaccine, and poor internalization could be limiting factors.
Periodontal diseases
Nanotechnology has been used to prevent bone loss in an experimental periodontal disease model by local application of nanostructured doxycycline gel.Citation92 Nanorobots (dentifrobots) mouthwash or toothpaste left on the occlusal surfaces of teeth with their continuous and fast movement (1–10 μm/second) across the supra and subgingival surfaces continuously removes the organic residues and prevents the calculus accumulation. These nanorobots can be safely deactivated when they are swallowed.Citation93
Diagnostic nanodentistry
Considering the impact of nanotechnology in the fields of drug delivery and diagnostic and novel material design for implantology and tissue regeneration, one may expect the same level of engagement of this emerging field in dentistry. However to date, there have been very few contributions from the scientific and clinical communities reporting an impact of nanotechnology on the diagnostics of dental conditions. Diagnostics of dental conditions is still nowadays routinely undertaken in clinic using a set of guidelines drawn by the best practice in the field. Some of these guidelines have not changed in decades for certain ailments such as caries or tooth crack syndrome for example. It has therefore proven difficult to bring innovative approaches when establishing a treatment plan for patients while ensuring best outcomes. Thus, unlike in medicine, dentistry has not yet seen considerable transition from research laboratory practices to clinical chairs. Nonetheless, over the past few decades, contributions arising from nanotechnology in dentistry have been made mostly in three fields: atomic force microscopy (AFM), imaging contrast enhancers, and biochips.
AFM and oral biofilms
Thorough understanding of bacterial adhesion, the main factor for bacterial colonization and pathogenesis, as well as bacterial nanomechanics is essential for prevention or treatment of biofilm-dependent oral diseases.Citation67 Bacteria have the ability to adhere to others from the same or different species as well as to different substrates, eg, teeth and implants.Citation94,Citation95 With the recent technologies, AFM, with its capability to directly interact with and image live cells without any disruption of their morphology and properties,Citation96 offers a breakthrough in characterization of bacteria as well as measurement of their adhesion to different substrates ().Citation97,Citation98
Figure 3 (A) AFM image (topography) of Bacillus bacterial infection on a gelatine substrate. (B) 3D reconstruction of a single Bacillus bacterium AFM image.
Abbreviation: AFM, atomic force microscopy.
With nanomechanical biosensors, AFM cantilever, a real-time scanning of a live bacterial cell with high sensitivity was made possible.Citation99 Furthermore, information on a cell’s elasticityCitation100 and the membrane-molecules’ propertiesCitation101 become available. The way by which bacteria adhere to tooth surfaces or dental implants has been revealed using AFM. This process usually starts with docking stage where nonspecific reversible attachments between bacteria and substrate through van der Waals and electrostatic forces exist. When the bacteria become close to the substrates, specific ligand–receptor interactions are formed, which is the locking stage. In this stage, molecules on bacterial surfaces and bacterial appendages such as fimbriae, pili, and capsules interact irreversibly with the substrate.Citation102 This adhesion depends primarily on type and properties of both bacteria and the substrate as well as the surrounding environment.Citation103 The formation of a biofilm usually starts with adhesion of initial colonizing species to the host surface; the coaggregation of late colonizing species, which change their phenotype and properties to adapt to the established biofilm, then follows.Citation104,Citation105 A biofilm is therefore a highly organized population of different adherent bacterial species embedded in a matrix of exopolysaccharides and proteins.Citation106,Citation107 The presence of different bacterial species in a biofilm increases its resistance to antibiotic and mechanical removal.Citation108,Citation109 Understanding how the bacterial cells adhere to different cell typesCitation110 and substrates at both nanoscale and picoscale levels,Citation111 however, helps in understanding the biophysics of early phase or oral biofilm formation and pathogenesis of diseases. The adhesion of bacteria to tooth surfaces varies from species to species, eg, microbiomes have higher adhesion forces than planktonic bacteria. This could indicate the importance of early adhering bacteria for biofilm formation.Citation112 AFM also provides precise information on biomechanical interactions of antibacterial drugs with a bacterial cell,Citation113 for example, vancomycin binds to D-Ala–D-Ala terminal of peptidoglycan precursors of bacterial cell walls;Citation99 this could help in the development of antibacterial therapies against drug-resistant bacteria.
Imaging contrast enhancers and oral cancers
One of the most prominent contributions from nanotechnology in the field of dentistry relates to the diagnosis of oral cancer lesions. The ability to image these lesions plays a critical role in overall cancer management and treatment plan design. Standard clinical imaging techniques, such as computer tomography (CT), molecular resonance imaging, and ultrasound, can be categorized as structural imaging techniques. As such, they are able to assist with the identification of anatomical patterns as well as the provision of basic information regarding tumor location, size, and spread based on endogenous contrast. However, in the case of tumors and metastases smaller than 5 mm, these imaging techniques become less reliable to distinguish between benign and cancerous tumors.Citation114,Citation115 In 1895, Wilhelm Roentgen demonstrated serendipitously the use of gold as a contrast agent for X-ray imaging as he imaged one of his wife’s (Anna Berha’s) hands with a gold ring on her finger. Gold as an element exhibits a strong absorption of X-ray and can be used as a contrast agent in the form of gold nanoparticles (GNPs) for example.
GNPs can be synthesized using approaches such as the citrate reduction of aqueous HAuCl4 by the Turkevich methodCitation116 and the Brust–Schiffrin two-phase synthesis method, which uses NaBH4 as a reducing agent and a mercapto-containing binding agent.Citation117 The size distribution of the GNPs produced by both these approaches can be tuned by altering the chemical ratio of gold to reducing substance. Recent progress toward nanotechnology-based CT imaging has been made by Hainfeld et alCitation118 following the wider accessibility of GNPs. Their original work focused on the use of GNPs to induce in vivo vascular contrast enhancement in CT imaging. In their preliminary work,Citation119 the gold particles were not functionalized and consequently did not bind to any specific cell receptors. To increase the effectiveness and the specificity of the targeting of GNPs, it is essential to functionalize these particles. The functionalization of GNPs is usually accomplished either by direct thiolmodification of the targeting ligand or through the attachment of a targeting ligand to GNPs that have been modified with a coating material (eg, polymer, lipid, and DNA). The functionalization of GNPs with a ligand such as polyethylene glycol, for example, promotes both stability and persistence in circulation, allowing greater accumulation in the tumor tissue. It also provides a hydrophobic barrier to reticuloendothelial system phagocytosis and uptake.Citation120 It is however still possible to increase the specificity of GNPs by functionalizing their surface with antibodies to markers overexpressed in tumors, such as EGF, HER2, and folate.Citation121–Citation123 In their approach, Marega et al have used a plasma-polymerized allylamine coating to enable the bioconjugation of tumor-targeting epidermal growth-factor receptor monoclonal antibodies to GNPs.Citation121 Hainfeld et al produced HER2-targeted GNPs by coating 15 nm GNPs with polyethylene glycol and covalently coupling them to anti-HER2 antibodies.Citation118 Popovtzer et al presented an in vitro proof of principle demonstration for head and neck cancer (oral squamous-cell carcinoma [OSCC]),Citation114 showing that the attenuation coefficient for the molecularly targeted cells is over five times higher than for identical but untargeted cells. In their approach, the bioconjugation of the GNPs to the UM-A9 antibody was achieved according to the method described by Kim et al.Citation124
Another contribution from nanotechnology arises in the detection of tumors using near-infrared (NIR) luminescent quantum dots (QDs). QDs are nanometer-scale semiconductor crystals composed of elements of groups II–VI or III–V and act as an inorganic fluorophore when exposed to an excitation wavelength. Metal and semiconductor nanoparticles in the size range of 2–6 nm are of considerable interest, due to their dimensional similarities with biological macromolecules (eg, nucleic acids and proteins). Similar to GNPs, QDs can be coupled to biomolecules, such as transferrin,Citation125,Citation126 immunoglobulin G (IgG),Citation125 biotin,Citation127 streptavidin,Citation128,Citation129 avidin,Citation130,Citation131 nucleic acids,Citation132–Citation134 peptides,Citation135 serotonin,Citation136 adenine,Citation137 adenine monophosphate,Citation137 and wheat germ agglutinin.Citation126,Citation137 These functionalized QDs effectively become luminescent probes that can bind with specificity and sensitivity to a variety of targets (immunoglobulin G, antigens, glycoproteins, nucleic acid sequences, and receptors). The luminescence of QDs is photostable with narrow, symmetric emission spectra and broad continuous excitation, allowing excitation of multiple QDs with a single wavelength.Citation125–Citation127,Citation129,Citation130 These properties make QDs very effective for labeling biological species in vivo. In 1998, Nie’s and Alivisatos’s groups independently reported the use of QDs for imaging of cells and tissues, and these studies have sparked the research growth of QD applications in life science.Citation125,Citation127
Head and neck squamous-cell carcinoma, also known as OSCC, is one of the most common types of cancer in oral oncology, representing ~6% of all cases and accounting for an estimated 650,000 new cancers and ~350,000 cancer deaths globally per year.Citation138,Citation139 Selectively targeting of squamous tumors is a long-standing problem due to the lack of specificity of current drugs. In 2011, Yang et alCitation140 successfully used cell-penetrating peptides to conjugate NIR QDs for cancer diagnostics. In their pioneering approach, they managed to label OSCC with QD conjugates by endocytosis for visual in vivo imaging on a mouse model. Working also on OSCC, Bhirde et alCitation141 used a combination of QDs and CNT-based drug delivery to target and kill HN13 cells. Single wall carbon nanotubes (SWNTs) present remarkable opportunities to meet future advanced drug delivery system challenges. In a similar approach as described before, novel strategies are developed to bind biological molecules such as proteins and DNA and smaller molecules onto SWNTs.Citation142–Citation145 Functionalized, solubilized SWNTs can transport peptides, proteins, genes, and DNACitation146,Citation147 across cell membranes with little cytotoxicity,Citation148 making them an attractive carrier for both treatment and diagnosis strategies.
The use of QDs is not restricted to cancer diagnostics. This technology has also been used in the case of more common ailments such as dental caries and periodontal diseases where biofilms and bacterial infections play an important role. In 2003, Kloepfer et al were the first to use QDs for bacterial labeling.Citation126 Since this pioneering work, the use of QDs has been more widespread and is now used for labeling, detection, and quantification of Mycobacterium bovis, Bacillus Calmette–Guérin,Citation149 Escherichia coli O157:H7,Citation150 and Salmonella enterica and serovar TyphimuriumCitation151 as well as for the simultaneous detection of E. coli O157:H7 and S. enterica and S. typhimurium.Citation152 In 2007, Chalmers et alCitation153 used QDs as luminescent probes to achieve single-cell resolution of oral bacteria biofilms. Using conjugated polyclonal and monoclonal antibodies bound to the QD surface, they were able to label planktonically grown Streptococcus gordonii DL1 cells as well as individual species within mixed-species community biofilms. Although this pioneering work is very promising, there is still a huge amount of work to be done to effectively target the correct species of bacteria for diagnostics purposes. The development of conjugation solution is currently the limiting factor for this approach to be more widespread, and current approaches are only limited to the laboratory rather than frontline patient diagnostics.
Biochips and salivary biomarkers
Possibly one of the most exciting uses of nanotechnology in oral health diagnostics comes from the emergence and development of biochips.Citation154 Biochips are very small devices (less than a few millimeters) on which a collection of miniaturized test sites (microarrays) are arranged. The main advantage of the microchips over more traditional approaches is that many tests (diagnostics) can be performed simultaneously in order to achieve higher throughput and speed. Early work in this field related to dental diagnostics started in 2002 as the National Institute of Dental and Craniofacial Research in the USA initiated a concerted research effort in the area of saliva diagnostics. National Institute of Dental and Craniofacial Research funded several awards to develop microfluidics and microelectromechanical systems (MEMS) for saliva diagnostics with the aim to identify technologically viable systems and support their advancement toward commercialization. This unique venture focused on the development of microfluidic and MEMS technologies for measuring DNA, gene transcripts (mRNA), proteins, electrolytes, and small molecules in saliva, as well as overall profile correlates of a particular disease state, such as cardiovascular disease.Citation155,Citation156 One particular success came from Segal and WongCitation157 who found out that discriminatory and diagnostic human mRNAs are present in saliva of normal and diseased individuals. The salivary transcriptome presents an additional research and clinical resource, the second saliva-based diagnostic alphabet for disease detection. This work led to the development of salivary diagnostic technology using MEMS and nanoelectromechanical system biosensors. These devices exhibit exquisite sensitivity and specificity for analyte detection, down to single-molecule level.Citation158,Citation159 Subsequently, the research consortium led by Wong developed the oral fluid nanosensor test.Citation157,Citation160–Citation162 This handheld, automated, easy-to-use, integrated system enables simultaneous and rapid detection of multiple salivary protein and nucleic acid targets. In 2007, Gau and Wong suggested that the intended use of the oral fluid nanosensor test is for point-of-care multiplex detection of salivary biomarkers for oral cancer.Citation160 In their work, they demonstrated that the combination of two salivary proteomic biomarkers (thioredoxin and interleukin-8 [IL-8]) and four salivary mRNA biomarkers (SAT, ODZ, IL-8, and interleukin-1 beta [IL-1β]) can detect oral cancer with high specificity and sensitivity.
Another important emerging tool in oral health diagnostics involves profiling methods that are available for detecting miRNA expression levels and were recently reviewed by Yoshizawa and Wong.Citation161 miRNAs are short noncoding RNA molecules that play important roles in regulating a variety of cellular processes. Dysregulation of miRNAs is known to be associated with many diseases, such as OSCC. The presence of miRNAs in saliva and their potential as non-invasive biomarkers in oral cancer detection was shown to be an effective marker in the case of OSCC using miR-31over-expression.Citation163 The profiling methods involve biochemical techniques, such as quantitative PCR,Citation164,Citation165 microarray hybridization,Citation166,Citation167 and next-generation sequencing. The miRNAs present in saliva are very low, typically in the nanogram range upon extraction. The challenge of these profiling methods is thus to be able to quantify very low quantities of the miRNAs present in saliva, which is typically in the nanogram range upon extraction. An example of such profiling techniques is Applied Biosystems Stem-loop RT based TaqMan® MicroRNA Assay. This method measures quantitation for mature miRNA expression and is believed to be the gold-standard method with a large dynamic range, high specificity, and high sensitivity.Citation164,Citation168,Citation169
Another venture in the field of biochips comes from the work initiated by Weigum et al on the development of a diagnostic cytology-on-a-chip technique that rapidly detects premalignant and malignant cells with high sensitivity and specificity.Citation170,Citation171 In their approach, they designed a sensor for the diagnostics of OSCC, which integrates multiple laboratory processes onto a microfluidic platform in three primary steps. First, an oral-cytology suspension is delivered to the sensor using pressure-driven flow, where any cells larger than the membrane-pore size are retained on the membrane surface.Citation170,Citation171 The captured cells are then stained with fluorescent dyes and immunoreagents to distinguish the cytoplasm, nucleus, and cancer biomarkers. In their diagnostic approach, they selected epidermal growth-factor receptor as the targeted biomarker, since it is overexpressed and well characterized in OSCC and associated with aggressive phenotypes. Finally, the stained cells are subjected to a 3D fluorescence-microscopy scan of the membrane surface. This is followed by automated image analysis using open-source software. The advantage of this approach is the speed at which a diagnosis can be made.
In the field of periodontal diseases,Citation162 Christodoulides et al have developed an electronic microchip-assay to detect C-reactive protein (CRP), which is a biomarker for inflammation associated with periodontal disease at the picogram per milliliter level.Citation172 CRP is known to be a systemic marker produced as a response to inflammatory stimuliCitation173 that can be used to differentiate in serum, between a healthy state and the presence of periodontitisCitation174,Citation175 and saliva.Citation176 It is the ability of the biochip to detect very low concentrations of CRP present in the saliva compared to serum that has enabled the researchers to distinguish between healthy periodontium and chronic gingival inflammation based directly on salivary CRP levels.Citation177 Moreover, CRP is also classified as a systemic marker of inflammation that has been shown to significantly increase in other conditions, including myocardial infarction, atherosclerosis, and arthritis.Citation178–Citation180 Another device called the integrated microfluidic platform for oral diagnostics was developed to perform immunological assays in less than 10 minutes with low sample volume and concentration requirements to test for periodontal disease.Citation181 This device is able to detect proteins in the picomolar range for tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) that have been spiked into saliva. IL-6 is released in response to IL-1 and TNF and has been shown to increase proportionally with bone loss in adult chronic periodontitis patients.Citation182 The proinflammatory and immune regulatory cytokine, TNF-α, has been identified in saliva and is significantly elevated in people with periodontitis compared with healthy individuals, with increased levels being correlated with increased numbers of sites with bleeding upon probing, pocket depth, and higher clinical attachment levels.Citation183,Citation184
Toward clinical trials
It is clear that there is an effort by the scientific communities to develop new tools to diagnose dental ailments more locally, more precisely, and with a lesser amount of analytes. However, it is also evident that the current level of research undertaken has not yet yielded methods or devices that can be used in a widespread manner for clinical trials. There is a clear lack of clinical trials associated with any nanotechnology-based diagnostic approaches in the current literature. Most of the current nanoscale diagnostic approaches applied to dentistry evolve from the vast number of strategies currently used in medicine, as was seen for cancer. There are a number of isolated clinical cases or reports suggesting that the field of dentistry is slowly appreciating the merits of these approaches. Dentistry is traditionally a very conservative discipline, and this may explain the reason for the current reticence to use these novel approaches. Nanotechnology is yet to find a diagnostics solution for common ailments, such as dental decay and erosion, and has only started to find a solution in the field of periodontology. Until nanotechnology can bring more advanced methods to diagnose or prognose such common conditions, nanotechnology’s impact may be limited in terms of a diagnostic tool in the field of dentistry.
Therapeutic nanodentistry
The use of nanotechnology in treating dental diseases has found a great interest. Its use has been extended from treating dentin hypersensitivity, root canal disinfection, and oral cancer to the most recent use in tissue engineering and drug delivery applications.
Dentin hypersensitivity
Dentin is protected from external stimuli by enamel in the crown or by cementum in the root. The removal of this protective layer exposes the underlying dentinal tubules, changing the fluid pressure hydrodynamics of the fluid inside the dentinal tubules, and is believed to be responsible for dentin hypersensitivity. GNPs were found to be easily adsorbed on the inner dentinal tubule walls; the application of silver staining was then used to help to occlude the open tubules and reduce the dentin sensitivity. After brushing the opened tubules with highly concentrated GNPs, laser irradiation promoted the aggregation of nanoparticles to occlude the exposed tubules.Citation185 Furthermore, dental nanorobots offer a quick and permanent cure to dentin hypersensitivity by selectively and precisely occluding the tubules in minutes using biological materials.Citation186
Root canal disinfection
Several nanoparticles, such as zinc oxide and chitosan alone or in combination, have been incorporated into root canal sealers in an attempt to disinfect root canals. They had no effect on the flow characteristic of the sealers, but they enhanced the antibacterial action, observed by a significant reduction in Enterococcus faecalis adherent to treated dentin.Citation187 Prior surface treatment of root canal dentin with phosphorylated chitosan was effective in maintaining the inhibitory effect of a chitosan-modified root canal sealer on biofilm formation at the sealer–dentin interface.Citation75 Metal oxides such as magnesium oxide nanoparticles could be used as a potential root canal irrigant with promising antibacterial activity in both in vitro and ex vivo studies. Compared to the conventional NaOCl solution (5.25%), magnesium oxide nanoparticles (5 mg/L) showed statistically significant long-term effect in the elimination of E. faecalis adherent to root canal dentin.Citation188
Oral cancers
In cancer radiation therapy, GNPs were conceived as a radio-sensitizer and they caused cell death after gamma radiation. The radiosensitivity of GNPs is dependent on their sizes.Citation189 In cancer chemotherapy, nanodelivery vehicles (eg, naringenin-loaded nanoparticles in 7,12-dimethylbenz(a)anthracene)Citation190 were used for improving the stability as well as targeted and controlled delivery of chemotherapeutic drugs. In photothermal therapy, this relies on the use of a plasmonic probe and NIR light, recently introduced as a minimally invasive technique for the treatment of deep tissue malignancy such as OSCC.Citation91 In this therapy, GNPs, with their enhanced NIR absorbance and ability to convert the absorbed light into heat energy, were used as a plasmonic nanoprobe. The nanodimension of this probe ensures its easy absorption by the localized tissue, reduces its toxicity, and allows its removal from the body after the treatment. Further enhancement of their biocompatibility, coating the gold nanostructures with thermo and pH-responsive polymers, for example poly(N-isopropylacrylamide-co-acrylic acid), has been attempted. RGD (arginine-glycine-aspartate) and NLS (nuclear localization sequence) peptides-conjugated nuclear targeting gold nanostructures can be easily captured by cancerous cells (eg, OSCC), subsequently disturb their functions (DNA damage, induces cytokinesis arrest in cancer cells) inducing cell apoptosis and necrosis.Citation191 The composition and shape of these nanostructures (eg, nanorods,Citation91 nanospheres, and nanocagesCitation192) would further enhance their apoptotic activity. In addition to disruption of cellular functions, gold nanocages produced reactive oxygen species that also kill the cancerous cells.Citation191 Selective inhibition of cancer cell growth through mitochondria-mediated autophagy has been obtained by iron-core–gold shell nanoparticles.Citation193
In addition to treating cancer, nanotechnology has been also extended to offer an effective management of the breakthrough pain associated with cancer. A nanodelivery transbuccal system was developed to rapidly and efficiently deliver the opioid analgesia at a consistent and controlled diffusion into the target tissue. Accordingly, it minimizes the risk of overdosing in patients and also protects the patients from needle injection. It also avoids the enzymatic and spontaneous degradation of the drug associated with oral administration.Citation194
Tissue engineering
Tissue engineering in dentistry, like other medical areas, has been approached to combine the technologies of scaffold matrices with the regenerative power of stem cells, which can be sourced mainly from dental tissues such as dental pulp, periodontal ligament (PDL), and alveolar bone.Citation195 The scaffolding matrices need to have specific functionality, such as to provide surfaces that allow cell attachment, proliferation, and differentiation as well as to present porous networks that enable the penetration of cells and nutrients.Citation196 Furthermore, the matrices need to degrade at an appropriate rate to meet the regeneration speed of tissues while preserving a certain level of mechanical integrity.Citation196
Nanotechnology in tissue engineering has allowed significant improvement of these scaffolding materials to present unique 3D matrix conditions for cells and tissues.Citation197,Citation198 Nanoengineered scaffolds possess intrinsic mechanical properties of materials that cannot be readily achieved by conventional technologies. The nanotopological surfaces provide more definitive physicochemical cues to cells that can recognize the nanoscale structures in their adhesion and differentiation. Furthermore, many biological proteins and active molecules can be integrated with the scaffolding materials at the nanoscale level to exert therapeutic efficacy and to spatially control the cellular behavior. Therefore, the nanotechnology-driven tissue engineering can achieve native tissue mimicking architectures and/or evoke the phenomena that occur in nature, ultimately to create constructs that are equivalent to tissues in dentistry, including dentin, pulp, PDL, cementum, and alveolar bone.
Some examples in biology highlight the hierarchical organization of tissues at the nanoscale, which ultimately informs insights into the design rationale for scaffolding matrices by nanotechnology. For instance, the ECM of many connective tissues is composed of collagen fibers that are formed through self-assembling of smaller subunits. The collagen molecules with a triple helical structure assemble once they are secreted into the extracellular space, forming collagen fibrils, which in turn form collagen fibers that range in sizes between 50 nm and 500 nm. In bone, the collagen self-assembled fibrils/fibers can template the mineralization of HA nanocrystals in a highly ordered manner. To engineer this hierarchical nanocomposite bone structure, a lot of effort has been exerted. The self-assembling peptides could be designed to mimic this, through modifying the amino acid sequences that could act as nucleating sites of the mineral phase.Citation199,Citation200 The process can also control the crystallographic orientation of the HA, which aligns along the c-axes with the long axes of the organic fibers, similar to that found in bone.Citation199
Such a bottom-up approach to create native tissue architecture can provide an engineered construct with unique mechanical properties. In fact, enamel in the teeth is believed to form through the mineralization of an amelogenin-based matrix protein, allowing the precipitation of nanometric crystals with perfect organization that ultimately confers hard and strong mechanical functions placed in tooth surface.Citation201 Furthermore, the unique mechanical properties of dentin can also be created by the HA mineralization in the dentin matrix proteins, which feature the assembly of acidic clusters into beta-sheet for hierarchically oriented mineral formation.Citation202 Therefore, exploiting the nanoarchitecture of the native dental tissues has often been a topic of research in dental tissue engineering scaffolds. The electrospun nanofibers,Citation203–Citation205 self-assembling peptides,Citation206,Citation207 and phase-separation matricesCitation208,Citation209 are the representative cases.
The nanofibrous scaffolds have been widely developed as the matrices for regeneration of dental tissues, including dentin–pulp complex, enamel, PDL, cementum, alveolar bone, and temporomandibular joint. A key aspect of the nanofibrous structures is their morphological trait largely mimicking the native tissue architecture because most of tissue proteins such as collagen and elastin are nanofibrous. Owing to their large surface area relative to volume, the surface-related properties are dominant, and these include surface reactivity, protein adsorption, and surface degradation, which ultimately control the cellular behaviors.Citation210,Citation211
Dentin and pulp are one of the most widely explored dental tissues utilizing the nanofibrous scaffolds. Nanofibrous scaffolds can be produced either by phase-separation, electrospinning, or peptide synthesis approach. For example, nanofibrous scaffolds made of poly-l-lactide acid (PLLA) could be synthesized through phase-separation and porogen leaching methods. The scaffolds exhibited the stimulation of odontogenic differentiation in human dental pulp stem cells when compared to solid-walled PLLA scaffolds.Citation208,Citation209 Furthermore, when ectopically implanted for 8 weeks in nude mice, the nanofibrous matrix provided excellent 3D environments for dental pulp stem cells to regenerate dental pulp as well as dentin.Citation208
Electrospinning is a more common method to generate nanofibers. Some recent studies have developed nanocomposite nanofibrous scaffolds made of biopolymers with bioactive glass, where the bioactive glass role was to provide high bioactivity.Citation204,Citation212 The dental pulp stem cells showed improved proliferation, differentiation, and mineralization on the nanofibers containing bioactive glass when compared to the pure polymer nanofibers.Citation204,Citation212 When combined with nanohydroxyapatite, the electrospun biopolymer nanofibers were also demonstrated to have improved proliferation and odontogenic differentiation of dental pulp stem cells.Citation205,Citation213,Citation214 These studies implicated the importance of bioactive inorganic nanoparticles that possibly play significant roles in improving mineralization and remineralization of dentin matrices that are damaged and diseased.Citation215 An interesting study reported nanofibrous scaffolds that incorporate magnesium phosphate, where the magnesium ions were shown to be sustainably released and to play a role in enhancing the differentiation of dental pulp stem cells.Citation216 Furthermore, when these stem cell-cultured scaffolds were ectopically implanted in nude mice, a greater level of ECM deposition, hard tissue formation, and expression of marker proteins could be achieved when compared to the Mg-free scaffolds, showing the importance of ions in odontogenesis.Citation216
Peptide self-assembly is an intriguing method that enables a bottom-up approach to generate nanofibers. As a representative example for dentin–pulp regeneration, the RGD sequence was designed in the peptide nanofiber scaffolds to control dental pulp cellular behaviors. The dental pulp stem cells were shown to express odontoblast-like phenotypes, and the deposition of mineral was possible.Citation217 Another study to mimic the native dentin structure used a methacrylate-based matrix with interpenetrated silica nanophase, which enabled aligned pores in the micrometer range. The matrices were shown to form a neodentinal cellular pattern distribution once implanted subcutaneously in mice.Citation218 These matrices with nanofibrous structures can be potential scaffolding platforms in the stimulation of dental pulp stem cells and ultimately in the engineering of dentin–pulp complex tissues.
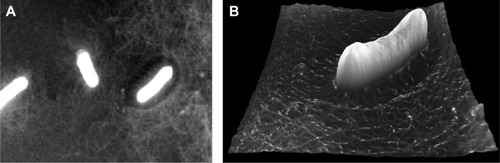
The nanofibrous matrix has also been developed to mimic the enamel. Enamel consists mainly of crystalline HA and is the external layer of tooth; therefore, its erosion or a caries attack may affect the dentin hypersensitivity. A couple of approaches have been proposed using nanomaterials to regenerate enamel. One exemplar study used electrospun polymer nanofibers containing amorphous CaP. In the presence of F ions, the amorphous CaP was able to transform into a contiguous overlayer of crystalline fluoridated apatite of 500 nm in thickness ().Citation219 Another interesting approach was based on self-assembling peptides, which contain high amounts of Arg-Gly-Asp (RGD) sequences. The unique structure allowed the ability to induce proliferation and differentiation of ameloblasts, which were able to synthesize, organize, and biomineralize enamel nodules (). However, compared to other tissues like dentin, the regeneration of enamel structures remains a significant challenge as the erupted enamel is principally acellular, and thus, the involvement of progenitor/stem cells can be considered mainly at the early stage of tooth development.Citation206
Figure 4 Ability of electrospun nanofibers containing ACP to regenerate dentin structure, as revealed by TEM image (A). Dentin cross-section imaged after etching (B) and after treatment with hydrated ACP/PVP (C), showing occlusion of tubules (green arrows). Regeneration of enamel by self-assembling peptides, showing aligned HA crystals sectioned longitudinally (arrows) and in cross-section (arrowheads) (D), representing considerable resemblance to that of authentic enamel (E). Molecular models of designed peptides RADA16 and PRG (F) and the formation of the self-assembled nanofiber present a beta-sheet structure (G). Periodontal ligament fibroblasts cultured in collagen gel in the presence or absence of the functional peptide, showing the ability of cells to penetrate into the gel when the PRG sequences were present in the gel (H). (A–C) Adapted from Fletcher J, Dominic W, Emma Fowler C, Mann S. Electrospun mats of PVP/ACP nanofibres for remineralization of enamel tooth surfaces. Cryst Eng Comm. 2011;13:3692 with permission of The Royal Society of Chemistry.Citation219 (D and E) Adapted from Biomaterials, 31, Huang Z, Newcomb CJ, Bringas P Jr, Stupp SI, Snead ML, Biological synthesis of tooth enamel instructed by an artificial matrix, 9202–9211, Copyright © 2010, with permission from Elsevier.Citation206 (F–H) Adapted from Kumada Y, Zhang S. Significant type I and type III collagen production from human periodontal ligament fibroblasts in 3D peptide scaffolds without extra growth factors. PLoS One. 2010;5:e10305.Citation207
Abbreviations: ACP, amorphous calcium phosphate; TEM, transmission electron microscopy; PVP, poly(vinylpyrrolidone); HA, hydroxyapatite.
The periodontal pockets, including PDL, cementum, and alveolar bone, have also been in great need of nanostructured scaffolds for engineering. The electrospun nanofibers of poly(lactic-co-glycolic acid) (PLGA) or gelatin were used to culture human PDL cells, showing the ability to adhere, proliferate, and osteogenically differentiate.Citation203,Citation220 The incur poration of silica or HA nanoparticles on the surface of these nanofibers has been shown to enhance protein adsorption, which resulted in improved adhesion of PDL fibroblast cells.Citation221 When the scaffolds contained nanosized HA, protein adsorption, cell adhesion, and in vivo bone formation were significantly higher than scaffolds containing conventional HA.Citation222 When PDL stem cells were seeded on the engineered nanosurface, cell viability and differentiation were enhanced compared to the absence of the nanosized HAs. The silk scaffolds were also tailored with nanohydroxyapatite to have mineralized surface.Citation223 The scaffolds constructed with PDL stem cells demonstrated substantial level of periodontal regeneration in an in vivo implantation in dogs, suggesting a potential scaffolding matrix for periodontal tissue engineering.Citation223 In a similar way, the nanosized bioactive glass particles were incorporated into alginate scaffolds, which showed enhanced proliferation and differentiation of human PDL fibroblasts when compared to the alginate-only scaffolds.Citation224 Other than the electrospun nanofibers, the self-assembling peptides containing the RGD-binding sequences (PRG) and laminin cell adhesion motif (PDS) were designed, which demonstrated significant promotion of PDL fibroblast adhesion, proliferation, and protein production ().Citation207
Alveolar bone defects have also been regenerated with nanometric-engineered scaffolds. The nanohydroxyapatite/polyamide nanocomposite scaffolds produced by a phase-separation method were combined with bone marrow stromal cells and then implanted into rabbit jaw bone. The results showed the enhancement of osteogenic markers in the implanted samples with cells, showing the ability of these scaffolds to stimulate osteogenesis, although the amount of new bone formed was not enhanced in the presence of the cells.Citation225 Similar results were obtained using nanosized β-tricalcium phosphate within a collagen matrix, which showed enhanced bone regeneration when implanted with MSCs.Citation226
Drug delivery
The delivery of therapeutically relevant molecules is a promising approach to improve the regeneration ability of damaged tissues and to treat diseases effectively. These molecules are usually loaded in carriers, such as scaffolds or nanoparticles, to allow controlled and sustained release, ultimately influencing a series of biological processes, such as cell homing, attachment, proliferation, and differentiation. The therapeutic molecules should be safely loaded in large quantities and then delivered in a controlled and targeted manner.
Therefore, the design of delivery carriers is of special importance in the success of the delivery systems. When 3D scaffolds are the carriers of the molecules to deliver, the signaling molecules can be combined within the 3D structure or on the surface of the scaffolds. As a result, the therapeutic molecules are released to the surrounding tissues and then act to regulate cellular functions. They can also be used as the tissue engineering matrices in the cultivation and differentiation of stem cells. In these cases, the incorporation of the signaling molecules should be carefully considered on the basis of nanochemistry of scaffolds, for example, molecular bonding between scaffold networks and drug molecules. On the other hand, nanosized particles are the most common form of delivery carriers because the carriers are often intended to enter the cell membrane and sometimes even to the nucleus.Citation227,Citation228 Together with many different nanoparticulates made of either polymers or inorganics, their hybrids have been developed for the delivery of molecules. Those targeted for dental tissues are relatively less explored.
Among the target areas that need a delivery strategy of signaling molecules, periodontal disease is a common problematic issue in dentistry.Citation229 The periodontal disease is primarily due to the bacterial infection that can possibly destroy the host tissues and eventually lead to tooth loosening. The treatment for periodontal diseases is based on two main approaches: the elimination of bacteria to avoid the progression of the disease and the regenerative therapy that may allow the regeneration of the damaged tissue.Citation230 To this end, BMPs have been often used since they are involved in the osteogenic differentiation of stem cells showing the ability to regenerate PDL, cementum, as well as alveolar bone when administered from biomaterials.Citation231–Citation234 In a similar way, when dental pulp is infected, a common clinical treatment involves bacterial control and removal of whole pulpal tissues. Therefore, the successful regeneration of odontoblasts, endothelial cells, fibroblasts, and even neurons is greatly needed to restore pulp tissue. Owing to the complexity of the cells involved in the regeneration process, many different growth factors, such as transforming growth factor (TGF-β1), BMP, and platelet-derived growth factor can be used individually or in combination. The presence of dental caries requires ultimately the regenerative signals of both enamel and dentin, for which antibacterial drugs play a key role at the initial phase. Furthermore, for the regeneration of the temporomandibular joint, delivering molecules to target two different tissues (cartilage and bone) is required; thus, the use of dual factors such as BMP2 and TGF-β may be an appropriate delivery option.
Utilizing nanoparticles to deliver therapeutic molecules has been explored for periodontal regeneration. The most common molecular type is the small drug that can be antibacterial in periodontal diseases. PLGA nanoparticles were developed to encapsulate minocycline for periodontal infections. The nanoparticles of size 85–424 nm have an entrapping efficiency of up to 29.9%.Citation235 The release was shown to be sustained for several weeks, which resulted in a considerable antibacterial effect compared to the minocycline-free nanoparticles.Citation235 Similar nanoparticles composed of PLGA and poly(lactic acid) showed higher entrapment efficiencies of up to 63.8%, and their implantation in dogs showed that the nanoparticles loaded with triclosan were able to penetrate through the junction epithelium.Citation221,Citation236 A more osteoconductive vehicle for the delivery of tetracycline was developed using calcium-deficient HA nanoparticles with varying Ca/P ratios.Citation237 The loaded drug showed sustained release of up to 88% over a period of 5 days and considerable antibacterial effect. Furthermore, when the drug-loaded nanoparticles were placed in contact with PDL cells, the cell proliferation increased, suggesting that the use of HA nanoparticles as local drug delivery agents in periodontal treatment may be a good option.Citation237
Growth factors have also been used for their therapeutic action in dental tissues. Compared to the common drug molecules, growth factors require water-based solutions in the nanoparticle processing. Some of the examples are illustrated in . For this purpose, glycydil methacrylate, derivative dextran, and gelatin nanoparticles were prepared with a size of 53.7 nm to encapsulate BMP, which showed a sustained release of more than 12 days. Furthermore, the nanoparticles were shown to be biocompatible and could be used as a promising vehicle for the delivery of active molecules to the periodontium.Citation238 Nanodiamonds, which are carbon nanoparticles of 4–5 nm, have also been used as a growth-factor carrier for the alveolar ridge augmentation. These have been shown to effectively physisorb and simultaneously deliver BMP2 and fibroblast growth factor and are able to induce the differentiation and proliferation of osteoblasts ().Citation236
Figure 5 (A, B) Effects of BMP2 and FGF delivered by nanodiamonds on osteoblast differentiation and proliferation. ALP activity at 5 days was inhibited by the use of FGF with the nanodiamonds (ND-FGF), but was considerably increased in a dose-dependent manner when the ND-FGF were combined with BMP2 (ND-BF) or when the BMP2 was administered alone (ND-BMP) (A). Osteoblast proliferation shows that BMP did not enhance proliferation (ND-BMP), whereas the presence of FGF alone (ND-FGF) or in combination with BMP significantly enhanced proliferation (ND-BF) (B). (C, D) Mandibular bone loss decreased by siRNA delivery. Histological observation of the mandibular inter-molar alveolar bone height loss significantly decreased in the presence of Sema4d-siRNA-loaded nanoparticles (C) compared to control (D). (A and B) Moore L, Gatica M, Kim H, Osawa E, Ho D, Journal of Dental Research, (92), 976–987. Copyright © 2013 by International & American Associations for Dental Research. Adapted by permission of SAGE Publications.Citation236 (C and D) Zhang Y, Wei L, Miron RJ, Zhang Q, Bian Z, Journal of Dental Research, (93), 1095–1100. Copyright © 2014 by International & American Associations for Dental Research. Adapted by permission of SAGE Publications.Citation241
Abbreviations: BMP, bone morphogenetic protein; FGF, fibroblast growth factor.
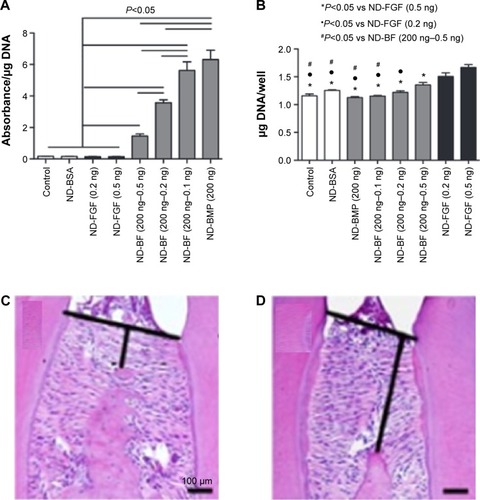
Owing to some concerns related to the lack of continuous supply of growth factors, gene therapy has been proposed as a possible solution.Citation239 For the delivery of genetic molecules, the carriers should be positively charged to enable complexes with highly phosphorylated nucleic acids. Nanosized CaP particles were developed with 30–50 nm in size to load the platelet-derived growth factor gene. Results showed significantly higher PDL fibroblast proliferation when placed in contact with the gene-complexed nanoparticles.Citation239 In a similar way, BMP2 gene was loaded onto CaP nanoparticles. Dental pulp stem cells were able to uptake the complex particles and then express considerable levels of odontogenic differentiation.Citation240 Alveolar bone regeneration has also been achieved through the delivery of polymeric nanoparticles that carry siRNA molecules for the interference of Sema4d, which is considered as a target gene for osteoporosis. The results showed, after injection in mice, that the siRNA-loaded nanoparticles were able to increase significantly the formation of bone in the osteoporotic alveolar bone ().Citation241
These nanoparticles are often incorporated into the 3D matrices to allow scaffolding roles while maintaining the controlled and sustained release of the encapsulated molecules. Some examples are illustrated schematically in . For instance, tetracycline-loaded nanoparticles of size around 130 nm were prepared using an ionic gelation method, which was then incorporated in a calcium sulfate cement matrix.Citation242 The release pattern was sustained and presented an antibacterial effect as well as an enhanced ALP activity of human PDL cells.Citation242 In a similar way, chitosan loaded with tetracycline was prepared as nanoparticles by a solution nebulization method, which was then incorporated into polycaprolactone (PCL) nanofibers. The release pattern of the drug from naked nanoparticles actually showed a burst with ~70% of release initially, which, however, was sustained significantly when incorporated within the PCL nanofiber structure.Citation243 For the regeneration of mandible defects, the nanohydroxyapatite/collagen scaffolds were developed to deliver BMP2-derived peptides. The nanocomposite scaffolds showed sustained and controlled release of the peptide molecules as well as enhanced osteogenic capacity of marrow stromal cells.Citation244 Nanocomposite scaffolds of HA nanoparticles with PCL were also reported to deliver BMP2 and have an excellent ability to regenerate the mandibular bone defect in rabbits ().Citation245 The BMP2-loaded nanohydroxyapatite/collagen/poly(lactic acid) constructs also showed a slow release of BMP2, which resulted in high osteogenic signaling of dental pulp stem cells. Furthermore, when the BMP2-loaded scaffolds in combination with dental pulp stem cells were implanted in rabbits, the bone formation was similar to that of autologous bone, proving that BMP2 was able to promote higher differentiation of the loaded stem cells, which in turn enhanced bone regeneration.Citation246
Figure 6 Three-dimensional micro-CT images of bone defect area where the scaffolds were implanted, showing improved regeneration when BMP2 was delivered from the scaffold (A–C). Long-term S. aureus growth inhibition test for DOXY release from PLA nanospheres, PLA nanospheres within nanofibers scaffolds, and control BSA nanospheres, showing the increased inhibition when DOXY was released from the composite nanofiber scaffolds (D). Quantification of blood vessel formation in the defect region treated with FTY720-loaded nanofibers (E). Micro-CT images of the mandibular ramus on the day of the surgery and 3 weeks and 12 weeks post surgery of control, nanofibers, and FTY720-loaded nanofibers. (A–C) Adapted with permission from Liu X, Zhao K, Gong T, et al. Delivery of growth factors using a smart porous nanocomposite scaffold to repair a mandibular bone defect. Biomacromolecules. 2014;15:1019–1030. Copyright © (2014) American Chemical Society.Citation245 (D) Adapted from Journal of Controlled Release, 146, Feng K, Sun H, Bradley MA, Dupler EJ, Giannobile WV, Ma PX, Novel antibacterial nanofibrous PLLA scaffolds, 363–369, Copyright © 2015, with permission from Elsevier.Citation248 (E and F) Adapted from Biomaterials, 34, Das A, Segar CE, Hughley BB, Bowers DT, Botchwey EA, The promotion of mandibular defect healing by the targeting of S1P receptors and the recruitment of alternatively activated macrophages, 9853–9862, Copyright © 2013, with permission from Elsevier.Citation251
Abbreviations: CT, computer tomography; BMP, bone morphogenetic protein; S. aureus, Staphylococcus aureus; DOXY, doxycycline; PLA, poly(lactic acid); BSA, bovine serum albumin.
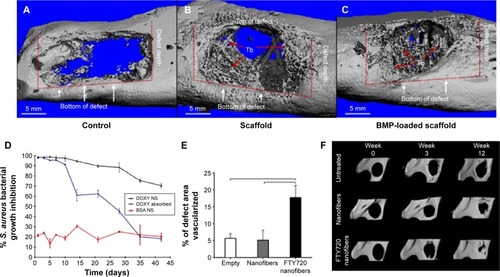
Nanofibers have often been shown to be excellent matrices for the sustained delivery of drugs mainly due to their high surface area. For PDL regeneration, nanofibers composed of PCL effectively encapsulated metronidazole benzoate and presented a low burst and sustained release of the drug up to 19 days.Citation247 Similarly, doxycycline was encapsulated into PLGA nanospheres, which were then incorporated into PLLA nanofibrous scaffolds.Citation248 The drug release was strongly dependent on the physical and chemical composition of the nanospheres, showing a complete release up to 6 weeks and a strong antibacterial effect ().Citation248 Nanofibers have also been used for the treatment of pulp infections, using polydioxanone incorporating metronidazole or ciprofloxacin antibacterial drugs, which proved to be a good candidate for regenerative endodontics.Citation249 In order to regenerate pulp and dentin, self-assembling peptide nanofibers encapsulating fibroblast growth factor, TGF-β1 and vascular endothelial growth factor, and dental pulp stem cells were used. These constructs led to the formation of vascularized connective tissues similar to dental pulp when implanted into immunocompromised mice.Citation250 In an elegant study for the mandibular reconstruction, PLGA nanofibers-incorporated FTY720, which is a targeted agonist of S1P receptors 1 and 3 and is used as a cell recruiter to enhance bone regeneration, was explored ().Citation251 The in vivo results showed that the loaded nanofibers were able to enhance blood vessel growth and the recruitment of macrophages, therefore proving their ability to regenerate critical size defects by recruiting specific macrophages and bone healing cells.Citation251
Collectively, the delivery of therapeutic molecules, including drugs, growth factors, and genes, has been realized in the disease treatment or repair of dental tissues, with the use of different types of nanomaterials, including nanotopological scaffolds, nanoparticulate carriers, or combination of them. For the controllable and effective delivery of these bioactive molecules and the physicochemical properties of the carrier materials, the loading and compartment of the cargo molecules should be controlled at the molecular level, which ultimately is in great need of nanotechnology and nanoscale tailoring approach.
Concluding remarks
It can be seen that nanotechnology has been exploited in dentistry for some time, particularly in the development of restorative materials, with some significant success. Researchers have been able to overcome the technical challenges of processing these materials to exploit the properties at the nanoscale. Other areas offer significant promise, particularly in the prevention and treatment of oral biofilms. One point to note in the treatment of biofilms is that virtually all treatments have focused on the killing of bacteria in the biofilm and yet the bacteria only account for around 20% of the mass of the biofilm. The remainder is a polysaccharide matrix, and it may be judicious to consider targeting this matrix, which holds the bacterial community together rather than the bacteria themselves. Utilization of nanotechnology for therapeutics more generally in dentistry offers significant opportunities as the requirements can be limited in their complexity and thus the therapeutic can be more readily realized. One factor that should be taken into consideration when developing nanotechnologies is the cost of the device or therapy being developed. There are numerous complex therapies being developed to treat a wide range of diseases, not only in dentistry but also in biomedicine more broadly, but they will never see clinical use as they will be too expensive to synthesize and thus too expensive for purchasers to procure. Cost is an important factor that is often forgotten in the development of new therapies and devices, but is ultimately the hurdle that will decide if it progresses or fails.
Acknowledgments
This work was partially supported by Priority Research Centers Program (grant 2009-0093829) through the National Research Foundation and Commercializations Promotion Agency for R&D Outcomes (COMPA) funded by the Ministry of Education, Science and Technology, South Korea. One of the authors (JCK) acknowledges the financial support from the UK ESPRC (EP/L026287/1).
Disclosure
The authors declare that there are no conflicts of interest.
References
- YangJZhangZMenXXuXFabrication of stable, transparent and superhydrophobic nanocomposite films with polystyrene functionalized carbon nanotubesAppl Surf Sci20092552292449247
- Abiodun SolankeIMFAjayiDMArigbedeAONanotechnology and its application in dentistryAnn Med Health Sci Res201443171177
- MantriSSMantriSPThe nano era in dentistryJ Nat Sci Biol Med201341394423633833
- SinghSNalwaHSNanotechnology and health safety – toxicity and risk assessments of nanostructured materials on human healthJ Nanosci Nanotechnol2007793048307018019130
- OzerFBlatzMBSelf-etch and etch-and-rinse adhesive systems in clinical dentistryCompend Contin Educ Dent20133411214 quiz 20, 3023550327
- YoshiharaKYoshidaYNagaokaNNano-controlled molecular interaction at adhesive interfaces for hard tissue reconstructionActa Biomater201063573358220346420
- SolhiLAtaiMNodehiAImaniMGhaemiAKhosraviKPoly(acrylic acid) grafted montmorillonite as novel fillers for dental adhesives: synthesis, characterization and properties of the adhesiveDent Mater201228436937722169675
- PashleyDHTayFRBreschiLState of the art etch-and-rinse adhesivesDent Mater201127111621112620
- TjäderhaneLNascimentoFDBreschiLOptimizing dentin bond durability: control of collagen degradation by matrix metalloproteinases and cysteine cathepsinsDent Mater201329111613522901826
- ScaffaPMVidalCMBarrosNChlorhexidine inhibits the activity of dental cysteine cathepsinsJ Dent Res201291442042522266526
- Van LanduytKLDe MunckJMineACardosoMVPeumansMVan MeerbeekBFiller debonding and subhybrid-layer failures in self-etch adhesivesJ Dent Res201089101045105020631093
- Malacarne-ZanonJde AndradeESilvaSMWangLPermeability of dental adhesives – a SEM assessmentEur J Dent20104442943920922163
- HashimotoMDe MunckJItoSIn vitro effect of nanoleakage expression on resin-dentin bond strengths analyzed by microtensile bond test, SEM/EDX and TEMBiomaterials200425255565557415159072
- ProfetaACMannocciFFoxtonRMThompsonIWatsonTFSauroSBioactive effects of a calcium/sodium phosphosilicate on the resin-dentine interface: a microtensile bond strength, scanning electron microscopy, and confocal microscopy studyEur J Oral Sci2012120435336222813227
- LohbauerUWagnerABelliRZirconia nanoparticles prepared by laser vaporization as fillers for dental adhesivesActa Biomater20106124539454620624492
- WagnerABelliRStotzelCHilpertAMullerFALohbauerUBiomimetically- and hydrothermally-grown HAp nanoparticles as reinforcing fillers for dental adhesivesJ Adhes Dent201315541342223560259
- Di HipolitoVReisAFMitraSBde GoesMFInteraction morphology and bond strength of nanofilled simplified-step adhesives to acid etched dentinEur J Dent20126434936023077413
- MorãesRRGarciaJWWilsonNDImproved dental adhesive formulations based on reactive nanogel additivesJ Dent Res201291217918422019910
- ChiangYSChenYLChuangSFRiboflavin-ultraviolet-A-induced collagen cross-linking treatments in improving dentin bondingDent Mater201329668269223582694
- dos SantosPHKarolSBedran-RussoAKNanomechanical properties of biochemically modified dentin bonded interfacesJ Oral Rehabil201138754154621058972
- Bedran-RussoAKYooKJEmaKCPashleyDHMechanical properties of tannic-acid-treated dentin matrixJ Dent Res200988980781119767576
- DaoodUIqbalKNitisusantaLIFawzyASEffect of chitosan/riboflavin modification on resin/dentin interface: spectroscopic and microscopic investigationsJ Biomed Mater Res A201310171846185623184366
- LiuRRFangMZhaoSJLiFShenLJChenJHThe potential effect of proanthocyanidins on the stability of resin-dentin bonds against thermal cyclingZhonghua Kou Qiang Yi Xue Za Zhi2012475268272 Chinese22883820
- TayFRPashleyDHBiomimetic remineralization of resin-bonded acid-etched dentinJ Dent Res200988871972419734458
- RyouHNiuLNDaiLEffect of biomimetic remineralization on the dynamic nanomechanical properties of dentin hybrid layersJ Dent Res20119091122112821730254
- BreschiLMartinPMazzoniAUse of a specific MMP-inhibitor (galardin) for preservation of hybrid layerDent Mater201026657157820299089
- AlmahdyAKollerGSauroSEffects of MMP inhibitors incorporated within dental adhesivesJ Dent Res201291660561122518030
- SabatiniCEffect of a chlorhexidine-containing adhesive on dentin bond strength stabilityOper Dent201338660961723550910
- OsorioRYamautiMOsorioEEffect of dentin etching and chlorhexidine application on metalloproteinase-mediated collagen degradationEur J Oral Sci20111191798521244516
- ToledanoMYamautiMRuiz-RequenaMEOsorioRA ZnO-doped adhesive reduced collagen degradation favouring dentine remineralizationJ Dent201240975676522659338
- ThompsonJMAgeeKSidowSJInhibition of endogenous dentin matrix metalloproteinases by ethylenediaminetetraacetic acidJ Endod2012381626522152622
- De Moraes PortoICDe AndradeAKAlvesLCBrazREffect of dentin pretreatment with potassium oxalate: analysis of microtensile bond strengths and morphologic aspectsMicrosc Res Tech201275223924421809415
- DundarMOzcanMComlekogluMESenBHNanoleakage inhibition within hybrid layer using new protective chemicals and their effect on adhesionJ Dent Res2011901939820940355
- SauroSMannocciFToledanoMOsorioRPashleyDHWatsonTFEDTA or H3PO4/NaOCl dentine treatments may increase hybrid layers’ resistance to degradation: a microtensile bond strength and confocal-micropermeability studyJ Dent200937427928819155116
- OuyangXHuangXPanQSynthesis and characterization of triethylene glycol dimethacrylate nanocapsules used in a self-healing bonding resinJ Dent2011391282583321925565
- Abou NeelEAChrzanowskiCYoungAMBiointerfaces – Where Materials Meet Biology: Interfaces in Composite MaterialsLondonRoyal Society of Chemistry2014
- DaviesJEUnderstanding peri-implant endosseous healingJ Dent Educ200367893294912959168
- BressanESbricoliLGuazzoRNanostructured surfaces of dental implantsInt J Mol Sci2013141918193123344062
- WeberHPFiorelliniJPThe biology and morphology of the implant-tissue interfaceAlpha Omegan199285461641308344
- AlbrektssonTSennerbyLWennerbergAState of the art of oral implantsPeriodontol 2000200847152618412571
- MendonçaGMendonçaDBAragãoFJCooperLFAdvancing dental implant surface technology – from micron- to nanotopographyBiomaterials200829283822383518617258
- KhanRAParsonsAJJonesIAWalkerGSRuddCDDegradation and interfacial properties of iron phosphate glass fiber-reinforced PCL-based composite for synthetic bone replacement materialsPolym Plast Technol2010491212651274
- BrammerKSOhSCobbCJBjurstenLMvan der HeydeHJinSImproved bone-forming functionality on diameter-controlled TiO2 nanotube surfaceActa Biomater2009583215322319447210
- OhSBrammerKSLiYSStem cell fate dictated solely by altered nanotube dimensionProc Natl Acad Sci U S A200910672130213519179282
- Cavalcanti-AdamEACell spreading and focal adhesion dynamics are regulated by spacing of integrin ligandsBiophys J Vol20079229642974
- OgawaTSaruwatariLTakeuchiKAitaHOhnoNTi nanonodular structuring for bone integration and regenerationJ Dent Res200887875175618650547
- ThakralGRTNSJSPVNanosurface – the future of implantsJ Clin Diag Res201485ZE07ZE10
- BalloAAgheliHLausmaaJThomsenPPetronisSNanostruc-tured model implants for in vivo studies: influence of well-defined nanotopography on de novo bone formation on titanium implantsInt J Nanomedicine201163415342822267926
- AminianAPardunKVolkmannEEnzyme-assisted calcium phosphate biomineralisation on an inert alumina surfaceActa Biomater20151333534325462843
- SchliephakeHScharnweberDDardMSewingAArefARoesslerSFunctionalization of dental implant surfaces using adhesion moleculesJ Biomed Mater Res B Appl Biomater200473B18896
- StadlingerBHintzeVBierbaumSBiological functionalization of dental implants with collagen and glycosaminoglycans – a comparative studyJ Biomed Mater Res B Appl Biomater2012100B233134122102613
- LavenusSLouarnGLayrollePNanotechnology and dental implantsInt J Biomater2010201019
- PuleoDAKisslingRASheuMSA technique to immobilize bioactive proteins, including bone morphogenic protein – 4 (BMP-4), on titanium alloyBiomaterials2002232079208711996050
- KlossFRGassnerRPreinerJThe role of oxygen termination of nanocrystalline diamond on immobilization of BMP-2 and subsequent bone formationBiomaterials2008292433244218316119
- SpechtCGWilliamsOAJackmanRBSchoepferROrdered growth of neurons on diamondBiomater Biomater20042540734078
- YoshinariMMatsuzakaKInoueTSurface modification by cold-plasma technique for dental implants – bio-functionalization with binding pharmaceuticalsJpn Dent Sci Rev20114789101
- UnossonERodriguezDWelchKEngqvistHReactive combinatorial synthesis and characterization of a gradient Ag-Ti oxide thin film with antibacterial propertiesActa Biomater20151150351025281786
- MemarzadehKShariliASHuangJRawlinsonSCAllakerRPNanoparticulate zinc oxide as a coating material for orthopedic and dental implantsJ Biomed Mater Res A20141031924408884
- HanawaTBiofunctionalization of titanium for dental implantJpn Dent Sci Rev20104693101
- GuHFanDGaoJEffect of ZnCl2 on plaque growth and biofilm vitalityArch Oral Biol201257436937522071420
- XieYHeYIrwinPLJinTShiXAntibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuniAppl Environ Microbiol20117772325233121296935
- BlecherKNasirAFriedmanAThe growing role of nanotechnology in combating infectious diseaseVirulence20112539540121921677
- LiQMSLyonDYBrunetLLigaMVLiDAlvarezPJAntimicrobial nanomaterials for water disinfection and microbial control: potential applications and implicationsWater Res200842184591460218804836
- AllakerRPThe use of nanoparticles to control oral biofilm formationJ Dent Res201089111175118620739694
- ShveroDKDavidiMPWeissEISrererNBeythNAntibacterial effect of polyethyleneimine nanoparticles incorporated in provisional cements against Streptococcus mutansJ Biomed Mater Res B Appl Biomater201094236737120583306
- KasraeiSSamiLHendiSAlikhaniMYRezaei-SoufiLKhamverdiZAntibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and LactobacillusRestor Dent Endod201439210911424790923
- ChenCWeirMDChengLAntibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticlesDent Mater201430889190124954647
- KawabataNNishiguchiMAntibacterial activity of soluble pyridiniumtype polymersAppl Environ Microbiol199854253225353202632
- FoldbjergROlesenPHougaardMDangDAHoffmannHJAutrupHPVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytesToxicol Lett200919015616219607894
- SongBLeffLGInfluence of magnesium ions on biofilm formation by Pseudomonas fluorescensMicrobiol Res2006161435536116517137
- RaiMYadavAGadeASilver nanoparticles as a new generation of antimicrobialsBiotechnol Adv200927768318854209
- das NevesPBAgnelliJAKurachiCde SouzaCWAddition of silver nanoparticles to composite resin: effect on physical and bactericidal properties in vitroBraz Dent J201425214114525140719
- LiFWeirMDFouadAFXuHHKEffect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm modelDent Mater201430218219124332270
- BesinisADe PeraltaTHandyRDInhibition of biofilm formation and antibacterial properties of a silver nano-coating on human dentineNanotoxicology20148774575423875717
- LuZRongKLiJYangHChenRSize-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteriaJ Mater Sci Mater Med20132461465147123440430
- NakashimaSYoshieMSanoHBaharAEffect of a test dentifrice containing nano-sized calcium carbonate on remineralization of enamel lesions in vitroJ Oral Sci2009511697719325202
- XuHHSunLWeirMDNano DCPA-whisker composites with high strength and Ca and PO4 releaseJ Dent Res200685872272716861289
- XuHHWeirMDSunLTakagiSChowLCEffects of calcium phosphate nanoparticles on CaPO4 compositeJ Dent Res200786437838317384036
- XuHHWeirMDSunLCalcium and phosphate ion releasing composite: effect of pH on release and mechanical propertiesDent Mater200925453554219101026
- XuHHSunLWeirMDTakagiSChowLCHockeyBEffects of incorporating nanosized calcium phosphate particles on properties of whisker-reinforced dental compositesJ Biomed Mater Res B Appl Biomater200781111612516924611
- RoveriNForestiELelliMSynthetic biomimetic carbonate hydroxyapatite nanocrystals for enamel remineralizationAdv Mater Res200847–50821824
- TalwarGPDiwanMRazviFMalhotraRThe impact of new technologies on vaccinesNatl Med J India199912627428010732430
- WangJLiuJGJiangJWangWBaiGHGuanXYStudy on gene vaccine pcDNA3-PAc against dental caries by intranasal immunization in rabbitsShanghai Kou Qiang Yi Xue201423213313724935831
- SuLKYuFLiZFZengCXuQAFanMWIntranasal co-delivery of IL-6 gene enhances the immunogenicity of anti-caries DNA vaccineActa Pharmacol Sin201435559259824705100
- LiuGFanMGuoJEfficacy of immune responses induced by anti-caries DNA vaccine-loaded bacterial ghost in miceZhonghua Kou Qiang Yi Xue Za Zhi2014491374124697886
- GuoJHJiaRFanMWBianZChenZPengBConstruction and immunogenic characterization of a fusion anti-caries DNA vaccine against PAc and glucosyltransferase I of Streptococcus mutansJ Dent Res200483326027014981131
- KtSKmkMNBJimsonSRSDental caries vaccine – a possible option?J Clin Diagn Res2013761250125323905153
- KogaTOkahashiNTakahashiIKanamotoTAsakawaHIwakiMSurface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans Serotype cInfect Immun19905822892962298480
- YamashitaYBowenWHBurneRAKuramitsuHKRole of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat modelInfect Immun1993619381138178359902
- ChenLZhuJLiYEnhanced nasal mucosal delivery and immunogenicity of anti-caries DNA vaccine through incorporation of anionic liposomes in chitosan/DNA complexesPLoS One201388e71953719517191323977186
- HannigMHannigCNanotechnology and its role in caries therapyAdv Dent Res2012242535722899680
- BotelhoMAMartinsJGRuelaRSQueirozDBRuelaWSNanotechnology in ligature-induced periodontitis: protective effect of a doxycycline gel with nanoparticulesJ Appl Oral Sci201018433534220835566
- SaravanaKRVijayalakshmiRNanotechnology in dentistryInd J Dent Res2006176265
- SubramaniKJungREMolenbergAHammerleCHBiofilm on dental implants: a review of the literatureInt J Oral Maxillofac Implants200924461662619885401
- AbeYSkali-LamiSBlockJCFranciusGCohesiveness and hydrodynamic properties of young drinking water biofilmsWater Res20124641155116622221338
- YangXYangWWangQAtomic force microscopy investigation of the characteristic effects of silver ions on Escherichia coli and Staphylococcus epidermidisTalanta2010814–51508151220441931
- Pinzon-ArangoPANagarajanRCamesanoTAEffects of l-alanine and inosine germinants on the elasticity of Bacillus anthracis sporesLangmuir20102696535654120095533
- ZhangTChaoYShihKLiXYFangHHQuantification of the lateral detachment force for bacterial cells using atomic force microscope and centrifugationUltramicroscopy2011111213113921185457
- McKendryRANanomechanics of superbugs and superdrugs: new frontiers in nanomedicineBiochem Soc Trans201240460360822817702
- LongoGRioLMRoduitCForce volume and stiffness tomography investigation on the dynamics of stiff material under bacterial membranesJ Mol Recognit201225527828422528189
- SullanRMBeaussartATripathiPSingle-cell force spectroscopy of pili-mediated adhesionNanoscale2014621134114324296882
- AnYHFriedmanRJConcise review of mechanisms of bacterial adhesion to biomaterial surfacesJ Biomed Mater Res19984333383489730073
- BushnakIALabeedFHSearRPKeddieJLAdhesion of microorganisms to bovine submaxillary mucin coatings: effect of coating deposition conditionsBiofouling201026438739720182931
- HojoKNagaokaSOhshimaTMaedaNBacterial interactions in dental biofilm developmentJ Dent Res2009881198299019828884
- ZhaoGUsuiMLLippmanSIBiofilms and inflammation in chronic woundsAdv Wound Care (New Rochelle)20132738939924527355
- CostertonJWLewandowskiZCaldwellDEKorberDRLappinscottHMMicrobial biofilmsAnnu Rev Microbiol1995497117458561477
- HassettDJSuttonMDSchurrMJHerrABCaldwellCCMatuJOPseudomonas aeruginosa hypoxic or anaerobic biofilm infections within cystic fibrosis airwaysTrends Microbiol200917313013819231190
- Hall-StoodleyLStoodleyPEvolving concepts in biofilm infectionsCell Microbiol20091171034104319374653
- Van AckerHVan DijckPCoenyeTMolecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilmsTrends Microbiol201422632633324598086
- YounesJAvan der MeiHCvan den HeuvelEBusscherHJReidGAdhesion forces and coaggregation between vaginal staphylococci and lactobacilliPLoS ONE [Electronic Resource]201275e36917
- FlorinE-LMoyVTGaubHEAdhesion forces between individual ligand-receptor pairsScience199426451574154178153628
- WesselSWChenYMaitraAAdhesion forces and composition of planktonic and adhering oral microbiomesJ Dent Res2014931848824186560
- FormosaCGrareMJauvertENanoscale analysis of the effects of antibiotics and CX1 on a Pseudomonas aeruginosa multidrug-resistant strainSci Rep2012257522893853
- PopovtzerRAgrawalAKotovNATargeted gold nanoparticles enable molecular CT imaging of cancerNano Lett20088124593459619367807
- ReuveniTMotieiMRommanZPopovtzerAPopovtzerRTargeted gold nanoparticles enable molecular CT imaging of cancer: an in vivo studyInt J Nanomedicine201162859286422131831
- TurkevichJStevensonPCHillierJA study of the nucleation and growth processes in the synthesis of colloidal goldDiscuss Faraday Soc19511155
- BrustMWalkerMBethellDSchiffrinDJWhymanRSynthesis of thiolderivatised gold nanoparticles in a two-phase liquid-liquid systemJ Chem Soc Chem Comm19947801802
- HainfeldJFO’ConnorMJDilmanianFASlatkinDNAdamsDJSmilowitzHMMicro-CT enables microlocalisation and quantification of HER2-targeted gold nanoparticles within tumour regionsBr J Radiol201184100252653321081567
- HainfeldJFSlatkinDNSmilowitzHMThe use of gold nanoparticles to enhance radiotherapy in micePhys Med Biol20044918N309N31515509078
- ChoWSChoMJeongJSize-dependent tissue kinetics of PEG-coated gold nanoparticlesToxicol Appl Pharmacol2010245111612320193702
- MaregaRKarmaniLFlamantLAntibody-functionalized polymer-coated gold nanoparticles targeting cancer cells: an in vitro and in vivo studyJ Mater Chem201222392130521312
- DixitVVan den BosscheJShermanDMThompsonDHAndresRPSynthesis and grafting of thioctic acid-PEG-folate conjugates onto Au nanoparticles for selective targeting of folate receptor-positive tumor cellsBioconjug Chem200617360360916704197
- SudimackJLeeRJTargeted drug delivery via the folate receptorAdv Drug Deliv Rev200041214716210699311
- KimKHuangS-WAshkenaziSPhotoacoustic imaging of early inflammatory response using gold nanorodsAppl Phys Lett20079022
- ChanWCWNieSMQuantum dot bioconjugates for ultrasensitive nonisotopic detectionScience19982815385201620189748158
- KloepferJAMielkeREWongMSNealsonKHStuckyGNadeauJLQuantum dots as strain- and metabolism-specific microbiological labelsAppl Environ Microbiol20036974205421312839801
- BruchezMMoronneMGinPWeissSAlivisatosAPSemiconductor nanocrystals as fluorescent biological labelsScience19982815385201320169748157
- MånssonASundbergMBalazMIn vitro sliding of actin filaments labelled with single quantum dotsBiochem Biophys Res Commun2004314252953414733939
- ZhuLAngSLiuWTQuantum dots as a novel immunofluorescent detection system for Cryptosporidium parvum and Giardia lambliaAppl Environ Microbiol200470159759814711692
- GoldmanERBalighianEDMattoussiHAvidin: a natural bridge for quantum dot-antibody conjugatesJ Am Chem Soc2002124226378638212033868
- JaiswalJKMattoussiHMauroJMSimonSMLong-term multiple color imaging of live cells using quantum dot bioconjugatesNat Biotechnol2003211475112459736
- DubertretBSkouridesPNorrisDJNoireauxVBrivanlouAHLibchaberAIn vivo imaging of quantum dots encapsulated in phospholipid micellesScience200229855991759176212459582
- XiaoYBarkerPESemiconductor nanocrystal probes for human metaphase chromosomesNucleic Acids Res2004323
- XuHShaMYWongEYMultiplexed SNP genotyping using the Qbead (TM) system: a quantum dot-encoded microsphere-based assayNucleic Acids Res2003318
- AkermanMEChanWCWLaakkonenPBhatiaSNRuoslahtiENanocrystal targeting in vivoProc Natl Acad Sci U S A20029920126171262112235356
- RosenthalSJTomlinsonIAdkinsEMTargeting cell surface receptors with ligand-conjugated nanocrystalsJ Am Chem Soc2002124174586459411971705
- KloepferJAMielkeRENadeauJLUptake of CdSe and CdSe/ZnS quantum dots into bacteria via purine-dependent mechanismsAppl Environ Microbiol20057152548255715870345
- ParkinDMBrayFFerlayJPisaniPGlobal cancer statistics, 2002CA Cancer J Clin20055527410815761078
- SeiwertTYCohenEEState-of-the-art management of locally advanced head and neck cancerBr J Cancer20059281341134815846296
- YangCCHungPSWangPWmiR-181 as a putative biomarker for lymph-node metastasis of oral squamous cell carcinomaJ Oral Pathol Med201140539740421244495
- BhirdeAAPatelVGavardJTargeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug deliveryACS Nano20093230731619236065
- MalhotraRPatelVVaqueJPGutkindJSRuslingJFUltrasensitive electrochemical immunosensor for oral cancer biomarker IL-6 using carbon nanotube forest electrodes and multilabel amplificationAnal Chem20108283118312320192182
- BiancoAKostarelosKPartidosCDPratoMBiomedical applications of functionalised carbon nanotubesChem Commun20055571577
- ChenRJBangsaruntipSDrouvalakisKANoncovalent functionalization of carbon nanotubes for highly specific electronic biosensorsProc Natl Acad Sci U S A200310094984498912697899
- SinghRPantarottoDMcCarthyDBinding and condensation of plasmid DNA onto functionalized carbon nanotubes: toward the construction of nanotube-based gene delivery vectorsJ Am Chem Soc2005127124388439615783221
- KamNWSDaiHJCarbon nanotubes as intracellular protein transporters: generality and biological functionalityJ Am Chem Soc2005127166021602615839702
- PantarottoDBriandJPPratoMBiancoATranslocation of bioactive peptides across cell membranes by carbon nanotubesChem Commun200411617
- SinghRPantarottoDLacerdaLTissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracersProc Natl Acad Sci U S A200610393357336216492781
- OtsukaYHanakiKZhaoJZDetection of Mycobacterium bovis bacillus Calmette-Guerin using quantum dot immuno-conjugatesJpn J Infect Dis200457418318415329454
- SuXLLiYBQuantum dot biolabeling coupled with immunomagnetic separation for detection of Escherichia coli O157: H7Anal Chem200476164806481015307792
- WuXLiuHLiuJImmunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dotsNat Biotechnol2003214452452
- YangLJLiYBSimultaneous detection of Escherichia coli O157: H7 and Salmonella Typhimurium using quantum dots as fluorescence labelsAnalyst2006131339440116496048
- ChalmersNIPalmerRJDu-ThummLSullivanRShiWKolenbranderPEUse of quantum dot luminescent probes to achieve single-cell resolution of human oral bacteria in biofilmsAppl Environ Microbiol200773263063617114321
- LiYDennyPHoCMThe Oral Fluid MEMS/NEMS Chip (OFMNC): diagnostic and translational applicationsAdv Dent Res20051813516000263
- YagerPEdwardsTFuEMicrofluidic diagnostic technologies for global public healthNature2006442710141241816871209
- HerrAEHatchAVThrockmortonDJMicrofluidic immuno-assays as rapid saliva-based clinical diagnosticsProc Natl Acad Sci U S A2007104135268527317374724
- SegalAWongDTSalivary diagnostics: enhancing disease detection and making medicine betterEur J Dent Educ200812222918289265
- HuangTShalciAHoDZhangYHMcCabeERBHoCMRapid bacterial diagnosis: MEMS-based DNA detectionPediatr Res2002514276A277A
- SoongRKBachandGDNevesHPOlkhovetsAGCraigheadHGMontemagnoCDPowering an inorganic nanodevice with a biomolecular motorScience200029054961555155811090349
- GauVWongDOral fluid nanosensor test (OFNASET) with advanced electrochemical-based molecular analysis platformAnn N Y Acad Sci2007109840141017435145
- YoshizawaJMWongDTSalivary microRNAs and oral cancer detectionMethods Mol Biol201393631332423007518
- FuentesLYakobMWongDTWEmerging horizons of salivary diagnostics for periodontal diseaseBr Dent J20142171056757325415010
- LiuCJKaoSYTuHFTsaiMMChangKWLinSCIncrease of microRNA miR-31 level in plasma could be a potential marker of oral cancerOral Dis20101636036420233326
- ChenCRDBroomerAJZhouZReal-time quantification of microRNAs by stem-loop RT-PCRNucleic Acids Res200533e17916314309
- ShiRChiangVLFacile means for quantifying microRNA expression by real-time PCRBiotechniques20053951952516235564
- LiWRuanKMicroRNA detection by microarrayAnal Bioanal Chem20093941117112419132354
- YinJQZhaoRCMorrisKVProfiling microRNA expression with microarraysTrends Biotechnol200826707618191262
- MestdaghPFeysTBernardNHigh-throughput stem-loop RT-qPCR miRNA expression profiling using minute amounts of input RNANucleic Acids Res200836e14318940866
- SchmittgenTDLeeEJJiangJReal-time PCR quantification of precursor and mature microRNAMethods200844313818158130
- WeigumSEFlorianoPNReddingSWNano-bio-chip sensor platform for examination of oral exfoliative cytologyCancer Prev Res201034518528
- WeigumSEFlorianoPNChristodoulidesNMcDevittJTCell-based sensor for analysis of EGFR biomarker expression in oral cancerLab Chip200778995100317653341
- ChristodoulidesNMohantySMillerCSApplication of microchip assay system for the measurement of C-reactive protein in human salivaLab Chip20055326126915726202
- PasceriVWillersonJTYehETDirect proinflammatory effect of C-reactive protein on human endothelial cellsCirculation2000102182165216811056086
- JoshipuraKJWandHCMerchantATRimmEBPeriodontal disease and biomarkers related to cardiovascular diseaseJ Dent Res200483215115514742654
- LoosBGCraandijkJHoekFJWertheim-van DillenPMEvan der VeldenUElevation of systemic markers related to cardiovascular disease in the peripheral blood of periodontitis patientsJ Periodontol200071101528153411063384
- PedersonEDStankeSRWhitenerSJSebastianiPTLambertsBLTurnerDWSalivary levels of alpha(2)-macroglobulin, alpha(1)-antitrypsin, C-reactive protein, cathepsin G and elastase in humans with or without destructive periodontal diseaseArch Oral Biol19954012115111558850655
- ChristodoulidesNFlorianoPNMillerCSLab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitisAnn N Y Acad Sci2007109841142817435146
- MilovanovicMNilssonEJaremoPRelationships between platelets and inflammatory markers in rheumatoid arthritisClin Chim Acta20043431–223724015115702
- RidkerPMGlynnRJHennekensCHCReactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarctionCirculation19989720200720119610529
- RidkerPMStampferMJRifaiNNovel risk factors for systemic atherosclerosis – a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial diseaseJAMA2001285192481248511368701
- HerrAEHatchAVGiannobileWVIntegrated microfluidic platform for oral diagnosticsAnn N Y Acad Sci2007109836237417435142
- IrwinCRMyrillasTTThe role of IL-6 in the pathogenesis of periodontal diseaseOral Dis19984143479655045
- FineDHMarkowitzKFurgangDMacrophage inflammatory protein-1 alpha: a salivary biomarker of bone loss in a longitudinal cohort study of children at risk for aggressive periodontal disease?J Periodontol200980110611319228096
- NgPYDonleyMHausmannEHutsonADRossomandoEFScannapiecoFACandidate salivary biomarkers associated with alveolar bone loss: cross-sectional and in vitro studiesFEMS Immunol Med Microbiol200749225226017328758
- LiuCHCFilling in dentinal tubulesNanotechnology200718475104
- FreitasRAJrNanodentistryJ Am Dent Assoc2000131111559156611103574
- KishenAShiZShresthaANeohKGAn investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfectionJ Endod200834121515152019026885
- MonzaviAEshraghiSHashemianRMomen-HeraviFIn vitro and ex vivo antimicrobial efficacy of nano-MgO in the elimination of endodontic pathogensClin Oral Investig2015192349356
- ZhangXDWuDShenXSize-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapyBiomaterials201233276408641922681980
- SulfikkaraliNKrishnakumarNManoharanSNirmalRMChemopreventive Efficacy of Naringenin-Loaded Nanoparticles in 7,12-dimethylbenz(a)anthracene Induced Experimental Oral CarcinogenesisPathol Oncol Res20131928729623233294
- KangBMackeyMAEl-SayedMANuclear targeting of gold nanoparticles in cancer cells induces DNA damage, causing cytokinesis arrest and apoptosisJ Am Chem Soc201013251517151920085324
- MackeyMASairaFMahmoudMAEl-SayedMAInducing cancer cell death by targeting its nucleus: solid gold nanospheres versus hollow gold nanocagesBioconjug Chem201324689790623777334
- WuYNYangLXShiXYThe selective growth inhibition of oral cancer by iron core-gold shell nanoparticles through mitochondria-mediated autophagyBiomaterials201132204565457321458061
- SprintzMBenedettiCFerrariMFERRARI Applied nanotechnology for the management of breakthrough cancer painMinerva Anestesiol20057141942316012414
- LangerRVacantiJPTissue engineeringScience19932609209268493529
- ChanBPLeongKWScaffolding in tissue engineering: general approaches and tissue-specific considerationsEur Spine J200817suppl 446747919005702
- BalasundaramGWebsterTJNanotechnology and biomaterials for orthopedic medical applicationsNanomedicine (Lond)2006116917617716106
- ShiJVotrubaARFarokhzadOCLangerRNanotechnology in drug delivery and tissue engineering: from discovery to applicationsNano Lett2010103223323020726522
- HartgerinkJDBeniashEStuppSISelf-assembly and mineralization of peptide-amphiphile nanofibersScience20012941684168811721046
- SongJMalathongVBertozziCRMineralization of synthetic polymer scaffolds: a bottom-up approach for the development of artificial boneJ Am Chem Soc20051273366337215755154
- DuCFaliniGFermaniSAbbottCMoradian-OldakJSupra-molecular assembly of amelogenin nanospheres into birefringent microribbonsScience20053071450145415746422
- HeGDahlTVeisAGeorgeANucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1Nat Mater2003255255812872163
- InançBArslanYESekerSElçinAEElçinYMPeriodontal ligament cellular structures engineered with electrospun poly(DL-lactide-co-glycolide) nanofibrous membrane scaffoldsJ Biomed Mater Res A20099018619518491392
- QuTLiuXNano-structured gelatin/bioactive glass hybrid scaffolds for the enhancement of odontogenic differentiation of human dental pulp stem cellsJ Mater Chem B Mater Biol Med201314764477224098854
- YangXYangFWalboomersXFBianZFanMJansenJAThe performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffoldsJ Biomed Mater Res A20109324725719557787
- HuangZNewcombCJBringasPJrStuppSISneadMLBiological synthesis of tooth enamel instructed by an artificial matrixBiomaterials2010319202921120869764
- KumadaYZhangSSignificant type I and type III collagen production from human periodontal ligament fibroblasts in 3D peptide scaffolds without extra growth factorsPLoS One20105e1030520421985
- WangJLiuXJinXThe odontogenic differentiation of human dental pulp stem cells on nanofibrous poly(L-lactic acid) scaffolds in vitro and in vivoActa Biomater201063856386320406702
- WangJMaHJinXThe effect of scaffold architecture on odontogenic differentiation of human dental pulp stem cellsBiomaterials2011327822783021663962
- GaneshNJayakumarRKoyakuttyMMonyUNairSVEmbedded silica nanoparticles in poly(caprolactone) nanofibrous scaffolds enhanced osteogenic potential for bone tissue engineeringTissue Eng Part A2012181867188122725098
- WooKMChenVJMaPXNano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachmentJ Biomed Mater Res A20036753153714566795
- KimGHParkYDLeeSYOdontogenic stimulation of human dental pulp cells with bioactive nanocomposite fiberJ Biomater Appl20152985486625098335
- DengXLXuMMLiDSuiGHuXYYangXPElectrospun PLLA/MWNTs/HA Hybrid Nanofiber Scaffolds and Their Potential in Dental Tissue EngineeringKey Eng Mater2007330–332393396
- XuMMMeiFLiDElectrospun Poly(L-lacticacid)/Nano-Hydroxyapatite Hybrid Nanofibers and Their Potential in Dental Tissue EngineeringKey Eng Mater2007330–332377380
- BesinisAvan NoortRMartinNInfiltration of demineralized dentin with silica and hydroxyapatite nanoparticlesDent Mater2012281012102322721698
- QuTJingJJiangYMagnesium-containing nanostructured hybrid scaffolds for enhanced dentin regenerationTissue Eng Part A2014202422243324593189
- GallerKMCavenderAYuwonoVSelf-assembling peptide amphiphile nanofibers as a scaffold for dental stem cellsTissue Eng Part A2008142051205818636949
- Vallés-LluchANovella-MaestreESancho-TelloMPradasMMFerrerGGBatallaCCMimicking natural dentin using bioactive nano-hybrid scaffolds for dentinal tissue engineeringTissue Eng Part A20101692783279320388038
- FletcherJDominicWEmma FowlerCMannSElectrospun mats of PVP/ACP nanofibres for remineralization of enamel tooth surfacesCryst Eng Comm2011133692
- ZhangSHuangYYangXGelatin nanofibrous membrane fabricated by electrospinning of aqueous gelatin solution for guided tissue regenerationJ Biomed Mater Res A20099067167918563816
- ShalumonKTSowmyaSSathishDChennazhiKPNairSVJayakumarREffect of incorporation of nanoscale bioactive glass and hydroxyapatite in PCL/chitosan nanofibers for bone and periodontal tissue engineeringJ Biomed Nanotechnol2013943044023620999
- HeHYuJCaoJBiocompatibility and osteogenic capacity of periodontal ligament stem cells on nHAC/PLA and HA/TCP scaffoldsJ Biomater Sci Polym Ed Epub2010616
- YangCLLeeJSJungUWSeoYKParkJKChoiSHPeriodontal regeneration with nano-hyroxyapatite-coated silk scaffolds in dogsJ Periodontal Implant Sci20134331532224455445
- SrinivasanSJRChennazhiKPNairSVJayakumarRBiocompatible alginate/nano bioactive glass ceramic composite scaffolds for periodontal tissue regenerationCarbohydr Polym201287274283
- GuoJMengZChenGRestoration of critical-size defects in the rabbit mandible using porous nanohydroxyapatite-polyamide scaffoldsTissue Eng Part A2012181239125222320360
- ZhangXXuMLiuXRestoration of critical-sized defects in the rabbit mandible using autologous bone marrow stromal cells hybridized with nano-β-tricalcium phosphate/collagen scaffoldsJ Nanomater2013201318
- KettlerKVeltmanKvan WezelAJan HendriksACellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell typeEnviron Toxicol Chem20143348149224273100
- TreuelLJiangXNienhausGUNew views on cellular uptake and trafficking of manufactured nanoparticlesJ R Soc Interface2013102012093923427093
- DarveauRPPeriodontitis: a polymicrobial disruption of host homeostasisNat Rev Microbiol2010848149020514045
- PragatiSAshokSKuldeepSRecent advances in periodontal drug delivery systemsInt J Drug Deliv20091114
- GiannobileWVRyanSShihMSSuDLKaplanPLChanTCRecombinant human osteogenic protein-1 (OP-1) stimulates periodontal wound healing in class III furcation defectsJ Periodontol1998691291379526911
- KingGNKingNHughesFJThe effect of root surface demineralization on bone morphogenetic protein-2-induced healing of rat periodontal fenestration defectsJ Periodontol1998695615709623899
- RipamontiUCrooksJPetitJCRuegerDCPeriodontal tissue regeneration by combined applications of recombinant human osteogenic protein-1 and bone morphogenetic protein-2. A pilot study in Chacma baboons (Papio ursinus)Eur J Oral Sci200110924124811531070
- WikesjöUMSorensenSRKinoshitaAJian LiXWozneyJMPeriodontal repair in dogs: effect of recombinant human bone mor-phogenetic protein-12 (rhBMP-12) on regeneration of alveolar bone and periodontal attachmentJ Clin Periodontol20043166267015257745
- KashiTSEskandarionSEsfandyari-ManeshMImproved drug loading and antibacterial activity of minocycline-loaded PLGA nanoparticles prepared by solid/oil/water ion pairing methodInt J Nanomedicine2012722123422275837
- MooreLGaticaMKimHOsawaEHoDMulti-protein delivery by nanodiamonds promotes bone formationJ Dent Res20139297698124045646
- MadhumathiKSampath KumarTSRegenerative potential and antibacterial activity of tetracycline loaded apatitic nanocarriers for the treatment of periodontitisBiomed Mater2014903500224687419
- ChenFMMaZWDongGYWuZFComposite glycidyl methacrylated dextran (Dex-GMA)/gelatin nanoparticles for localized protein deliveryActa Pharmacol Sin20093048549319305420
- ElangovanSJainSTsaiPCMargolisHCAmijiMNano-sized calcium phosphate particles for periodontal gene therapyJ Periodontol20138411712522414259
- YangXWalboomersXFvan den DolderJNon-viral bone morphogenetic protein 2 transfection of rat dental pulp stem cells using calcium phosphate nanoparticles as carriersTissue Eng Part A200814718118333806
- ZhangYWeiLMironRJZhangQBianZPrevention of alveolar bone loss in an osteoporotic animal model via interference of semaphorin 4dJ Dent Res2014931095110025252878
- Sindhura ReddyNSowmyaSBumgardnerJDChennazhiKPBiswasRJayakumarRTetracycline nanoparticles loaded calcium sulfate composite beads for periodontal managementBiochim Biophys Acta201418402080209024561265
- KhodirWWAGuarinoVAlvarez-PerezMCafieroCAmbrosioLTrapping tetracycline-loaded nanoparticles into polycaprolactone fiber networks for periodontal regeneration therapyJ Bioact Compat Polym201328258273
- ZhangXGuoWGCuiHIn vitro and in vivo enhancement of osteogenic capacity in a synthetic BMP-2 derived peptide-coated mineralized collagen compositeJ Tissue Eng Regen Med Epub2013131
- LiuXZhaoKGongTDelivery of growth factors using a smart porous nanocomposite scaffold to repair a mandibular bone defectBiomacromolecules2014151019103024467335
- LiuHCELLWangDSReconstruction of alveolar bone defects using bone morphogenetic protein 2 mediated rabbit dental pulp stem cells seeded on nano-hydroxyapatite/collagen/poly(L-lactide)Tissue Eng Part A2011172417243321563858
- ZamaniMMorshedMVarshosazJJannesariMControlled release of metronidazole benzoate from poly epsilon-caprolactone electro-spun nanofibers for periodontal diseasesEur J Pharm Biopharm20107517918520144711
- FengKSunHBradleyMADuplerEJGiannobileWVMaPXNovel antibacterial nanofibrous PLLA scaffoldsJ Control Release201014636336920570700
- BottinoMCKamockiKYassenGHBioactive nanofibrous scaffolds for regenerative endodonticsJ Dent Res20139296396924056225
- HartgerinkJDCavenderACSchmalzGD’SouzaRNA customized self-assembling peptide hydrogel for dental pulp tissue engineeringTissue Eng Part A20121817618421827280
- DasASegarCEHughleyBBBowersDTBotchweyEAThe promotion of mandibular defect healing by the targeting of S1P receptors and the recruitment of alternatively activated macrophagesBiomaterials2013349853986224064148
