 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The purpose of this study was to assess the effect of various formulation parameters on anti-CD205 antibody decorated poly(d, l-lactide co-glycolide) (PLGA) nanoparticles (NPs) in terms of their ability to target dendritic cells (DCs). In brief, emulsification solvent evaporation technique was adapted to design NP formulations using two different viscosity grades (low and high) of both ester and carboxylic acid terminated PLGA. Incorporation of ligand was achieved following physical adsorption or chemical conjugation processes. The physicochemical characterizations of formulations were executed to assess the effects of different solvents (chloroform and ethyl acetate), stabilizer percentage, polymer types, polymer viscosities, ligand-NP bonding types, cross-linkers, and cryoprotectants (sucrose and trehalose). Modification of any of these parameters shows significant improvement of physicochemical properties of NPs. Ethyl acetate was the solvent of choice for the formulations to ensure better emulsion formation. Infrared spectroscopy confirmed the presence of anti-CD205 antibody in the NP formulation. Finally, cytotoxicity assay confirmed the safety profile of the NPs for DCs. Thus, ligand modified structurally concealed PLGA NPs is a promising delivery tool for targeting DCs in vivo.
Introduction
Dendritic cells (DCs) are known as the potent antigen presenting cells to induce adaptive immune responses. Manipulating DCs by targeted antigen delivery through various endocytic and secretory pathways is a consequence of delivering site-specific therapeutic delivery system. C-type lectin receptor CD205 (molecular weight of 205 kDa), exclusively expressed on DCs; is a widely studied DC target molecule for induction of immune response. Anti-CD205 monoclonal antibody (mAb) linked delivery system can efficiently deliver its cargo to the processing compartments of DCs in vivo.Citation1 CD205 receptor possesses a fast internalization speed, where over 80% of surface CD205 are internalized within 90 minutes.Citation2,Citation3 The proportion of targeted molecules endocytosed by this receptor in both immature and mature DCs is exceptionally higher compared to other surface receptors. In addition to internalization, antigen presentation on major histocompatibility complex (MHC)-I and MHC-II, CD205 receptors elicited superior presentation compared to CD11c receptor. Thus, targeting this receptor would be promising in both steady-state and inflammatory conditions.Citation2,Citation4 Therefore, CD205 specific antibodies can induce efficient antigen processing and presentation, notably eliciting both T helper1 CD4+ T cell and CD8+ T cell responses. Engagement of anti-CD205 mAb to target CD205 receptors shows high consensus to deliver vaccine utilizing an appropriate delivery system.Citation5
Over the past decade, nanoparticles (NPs) have gained increasing attention in the field of drug delivery. Particularly, polyester based NPs offer the advantage of effective delivery of drug to the target site, ensuring therapeutic benefit with minimum side effects. Industry has recently focused on the US Food and Drug Administration (FDA) approved poly(d, l-lactide co-glycolide) (PLGA) based NPs because of their biodegradability, biocompatibility, low toxicity, controlled release, and surface-modification properties.Citation6,Citation7 Hence, functionalization of PLGA NPs with ligands such as anti-CD205 antibody presents an opportunity for an innovative antibody-targeted vaccine delivery system. This coupling aims to provide increased payload of drug/antigen, thereby increasing response and reducing the number of doses required. The ligand itself might function in a non-activating manner, which is important for immunotherapeutic diseases.Citation8 PLGA polymers are commercially available with different terminal groups, namely, free carboxylic acid (COOH) end groups (uncapped) or esterified terminal groups (capped). The end groups of PLGA can influence drug encapsulation efficiency, degradation, stability, and conjugation of ligands. For example, COOH terminated NPs can result in a slightly acidic environment, that may cause degradation of encapsulated antigen during formulation process or inside endosomal compartment.Citation9
The present study focuses on the formulation optimization with anti-CD205 ligand using both capped and uncapped PLGA; each type offered with low and high viscosity grades ().Citation10 Discussions are based on the comparison and evaluation of how different process parameters affect these two subtypes of ester and COOH ended PLGA NPs for in vitro experiment setups. To serve this purpose, standardization of various parameters was executed to obtain NPs with suitable particle size, surface charge, polydispersity index (PDI), surface display, toxicity profile, and structural modification. Therefore, a structure-activity relationship is concluded after analyzing the results. As a consequence, the ultimate goal is to develop a delivery system with suitable formulation strategy that could simulate the in vitro responses in an animal model. Altogether, our results support the potential use of PLGA NPs as therapeutic delivery system to design a cancer vaccine.
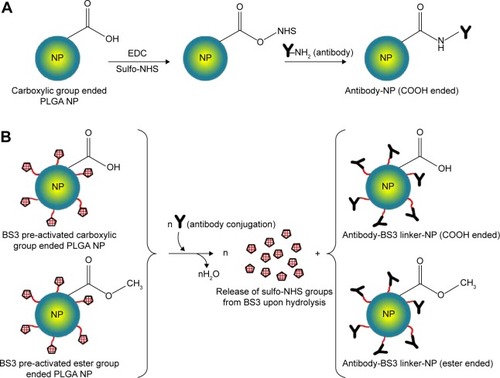
Figure 1 Reaction schemes to prepare targeted PLGA NP.
Notes: (A) Carbodiimide method, where EDC/sulfo-NHS was used as the cross-linker. COOH terminated PLGA reacts with EDC/sulfo-NHS to form NHS-ester that reacts with antibody to obtain a stable amide bond. (B) Using BS3 spacer, where covalent amide bond is formed between ligand and BS3 molecules embedded on pre-activated NPs’ surface. This method is applicable for both ester and COOH terminated PLGA NPs.
Abbreviations: PLGA, poly(d, l-lactide co-glycolide); NP, nanoparticle; EDC, carbodiimide hydrochloride; NHS, N-hydroxysuccinimide; COOH, carboxylic acid; BS3, bis(sulfo-succinimidyl) suberate.
Materials and methods
Materials
Ester terminated PLGA (inherent viscosity 0.15–0.25 dL/g and 0.55–0.75 dL/g) and COOH terminated PLGA (inherent viscosity 0.18 dL/g and 0.55–0.75 dL/g) were purchased from LACTEL Absorbable Polymers, Birmingham, AL, USA. Polyvinyl alcohol (PVA), bis(sulfo-succinimidyl) suberate (BS3), alpha minimum essential medium, fetal bovine serum, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay kit, and bicinchoninic acid (BCA) assay kit were purchased from Sigma-Aldrich Co., St Louis, MO, USA. Other reagents used were N-hydroxysuccinimide esters (sulfo-NHS) and 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) from Thermo Fisher Scientific, Waltham, MA, USA. Biotin anti-mouse CD205 antibody was purchased from Biolegend (San Diego, CA, USA). JAWSII DC line was obtained from American Type Culture Collection (ATCC), Manassas, VA, USA. GM-CSF was purchased from Thermo Fisher Scientific. Solvents like chloroform and ethyl acetate were of analytical grade.
Preparation of NPs by emulsification solvent evaporation method
Method 1
PLGA NPs were prepared by the double emulsification solvent evaporation technique as previously reported.Citation11 Briefly, PLGA was dissolved in chloroform (25% weight/volume [w/v]). Aqueous phase comprised of 5% w/v PVA was added to the oil phase in a drop wise manner and sonicated by probe-sonicator. The resulting primary emulsion was transferred into water to form the secondary emulsion and stirred to evaporate the chloroform. NPs were then collected by centrifugation, washed with distilled water to remove the residual PVA, and resuspended in distilled water. Cryoprotectant was used in the formulations to minimize freeze-drying stress after washing.Citation12
Method 2
The NPs were prepared following single oil in water emulsification solvent evaporation technique.Citation13 In brief, PLGA was dissolved in ethyl acetate (6.5% w/v) and transferred into 2.2% of PVA to form the emulsion. The procedure was then followed as mentioned in Method 1 with some minor modifications. To prepare pre-activated NPs for the covalent attachment of ligand, presence of BS3 in the aqueous phase is mandatory. The prepared NPs were lyophilized with cryoprotectants to avoid aggregation. Parameters mentioned in were considered in the preparation and evaluated for both methods 1 and 2. Discussion will be based on the effect of these parameters on the physicochemical properties of NPs.
Table 1 Variable parameters considered to prepare the NPs
Antibody coupling to the particle surface
Antibody coupling was carried out through two approaches, physical adsorption and covalent conjugation.
Physical adsorption of antibody to NPs’ surface
Biotinylated anti-CD205 mAb was added at a certain concentration to previously lyophilized PLGA NPs in phosphate buffered saline (PBS) and stirred for 4 hours in ice. After that, excess PBS was centrifuged and washed out. The NPs were freeze-dried upon re-suspension followed by storage at −20°C. At each washing step, the obtained supernatants were stored to determine the amount of un-conjugated antibody by BCA assay. This is an indirect method to measure the actual amount of antibody attached to the NPs. The control groups for BCA assay were the supernatants obtained from antibody-free NPs of each formulation through the similar conditions maintained in antibody-NP conjugation process.Citation14
Covalent attachment of antibody with NPs
Two different approaches were followed for covalent attachment where, one was using carbodiimide chemistry with EDC and sulfo-NHS (for method 1); and the other one with the use of BS3 (for method 2). For the first method, EDC/sulfo-NHS solution was added to antibody solution. The resulting suspension was stirred for 4 hours in ice, after which, centrifugation was done twice to remove excess reagents and soluble isourea by-product. Therefore, the amide bonds were formed between the primary amine groups of antibodies with the free carboxylic end groups of PLGA NPs.Citation14 For the second method, BS3 pre-activated freeze-dried NPs were resuspended in PBS for the covalent attachment of antibody.Citation13 Upon addition of antibody, the activated NPs underwent the process of conjugation followed by washing twice with PBS of pH 7.2. The covalent amide linkage was formed by replacing lysine groups of the antibody with free carboxylic group of BS3 upon releasing sulfo-NHS groups. The obtained NPs were further freeze-dried followed by storage at −20°C. The reaction schemes for both approaches are shown in .
Determination of particle size, zeta potential (ZP), and PDI
Dynamic light scattering technique was used to measure the particle size, ZP, and PDI using Malvern ZetaSizer, Nano ZS (Malvern Instruments, Malvern, UK).Citation15 ZP was measured on the basis of electrophoretic mobility in an electric field.Citation16 The measurements were performed for both unmodified and modified NPs before and after freeze-drying. Recovery percentage or yield of the preparation technique was calculated from the amount of NPs obtained divided by the total amount of initial PLGA polymer used to prepare NPs (the amount of PVA is negligible).
Morphology by scanning electron microscopy (SEM)
Morphology of the NP surface was analyzed by SEM. In brief, PLGA NPs were dispersed in distilled water (0.2% w/v). Appropriate portion of nano-suspension was placed on the carbon tape of the metal stub and allowed to air-dry. The samples were then placed in a sputter coater (S150B; BOC Edwards, Sussex, UK) for 1 minute to produce a gold coating witĥ20 nm thickness; and viewed under a scanning electron microscope at voltage of 20–25 kV (Carl Zeiss Evo 60; Carl Zeiss Meditec AG, Jena, Germany).Citation17
Structural characterization by Fourier transform infrared spectroscopy (FTIR)
FTIR analysis of NPs was recorded on a Bruker IFS 66v/S infrared spectrophotometer (Bruker Optics Inc, Billerica, MA, USA) in the mid-infrared range at the Canadian Light Source (CLS), University of Saskatchewan, Saskatoon, SK, Canada. All samples were mixed with spectroscopic grade potassium bromide and mulled to prepare pellets. The spectra were taken for potassium bromide pellets in the range of 4,000–400 cm−1 in absorbance mode.Citation13,Citation18 Data analysis was performed with Bruker Opus software. Baseline correction was performed on all raw absorbance spectra.
Determination of amount of antibody attached to the NPs
A BCA protein assay was performed to measure the amount of antibody attached per mg of NPs. The antibody was analyzed both directly (on the NP surface) and indirectly (in the supernatant). Briefly, a sample of modified NP was taken and resuspended in water to assess the antibody attached to the surface. Unmodified NPs were used as controls. In indirect method, the collected supernatants during washing steps were stored for BCA assay to quantify the amount of unbound antibody. Ninety-six well plate was used and the instructions from the BCA assay kit were followed for the quantification. The standard curve was generated by plotting the absorbance versus various concentrations of the standard solution, bovine serum albumin. The standard curve was found to be linear over the range of 0–32 μg/mL. The absorbance was measured at a wavelength of 562 nm in a microplate reader.Citation19
DC (JAWS II) culture
The DC line obtained from American Type Culture Collection was initiated as recommended. The cell culture was maintained at 37°C and 5% CO2 in complete media for few days to reach the optimum confluency. After that, the cells were stored in cryo-vials containing freezing solution (dimethyl sulfoxide). The complete media is as recommended by ATCC.
In vitro cytotoxicity assay
MTT assay was performed to investigate the cytotoxicity of NPs on DC lines using corresponding untreated cells as control. On day 1, the cells were seeded in 96-well plate at a density of 10,000 cells per mL. After overnight incubation, cells were exposed to various formulation treatments. On day 3, 10 μL of MTT solution was added to each well and further incubated for 4 hours. Finally, 100 μL of MTT solvent was added to dissolve the formazan crystals. The absorption intensity of the purple–blue color formed was measured at a wavelength of 570 nm by microplate reader.Citation15 Cell viability was calculated using the following equation:Citation20
Statistics
All data are presented as mean ± standard deviation. The significance of the differences between groups was analyzed by unpaired Student’s t-test or one-way analysis of variance followed by the Tukey’s post hoc test for multiple comparisons. P-value of <0.05 was considered as statistically significant. All the statistical analyses were performed with GraphPad Prism 5.03 software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Effect of polymers’ end groups and viscosities
The influence of polymer composition (functional end group and inherent viscosity) on physicochemical properties of NPs was investigated. Particle size is an important parameter that can affect the biopharmaceutical and biodistribution properties of formulations. The smaller particle size will lead to a higher total surface area relating to faster release of its payload.Citation21 PDI is an index for size distribution where an enhanced PDI value indicates that the particles do not have a uniform distribution.Citation22
In method 1, the average particle size and PDI for plain NPs prepared with COOH and ester end groups were found to range from 202 to 237 nm and 231 to 281 nm, respectively as shown in . Polymer viscosity (0.15 and 0.55 inherent viscosity) was indicated as a limiting factor for particle size in both COOH and ester terminated NPs. With an increase in polymer viscosity the particle size also increased as shown in .Citation23 After statistical evaluation, there was no significant effect observed for the variable end groups of PLGA on plain NPs’ particle size, PDI, and ZP () values ( and ). However, the effect of variable end groups and viscosities of the polymer on particle size was found significant for ligand modified NPs () although these two variables had no effect on their PDI values.
Table 2 Particle size, PDI, and ZP, and antibody loading for anti-CD205 modified NPs (n=4)
Figure 2 Particle size, PDI, and ZP of NPs prepared with different PLGA polymer end groups and viscosities following method 1.
Notes: The bar diagram and line plots represent particle size and PDI value, respectively. (A) Represents the NP formulations prepared without cryoprotectant. (B) Represents the same formulations preserved with two different cryoprotectants namely sucrose and trehalose. (C) Represents the comparative ZP of all NP formulations with or without cryoprotectant. The level of significance was set to P<0.05 (one-way ANOVA followed by Tukey’s multiple comparison test method). Each bar and line represents mean ± SD (n=12). C = COOH ended PLGA; E = ester ended PLGA, S = sucrose (10%); T = trehalose (10%).
Abbreviations: PDI, polydispersity index; ZP, zeta potential; NP, nanoparticle; PLGA, poly(d, l-lactide co-glycolide); ANOVA, analysis of variance; SD, standard deviation; COOH, carboxylic acid; FD, freeze-drying.
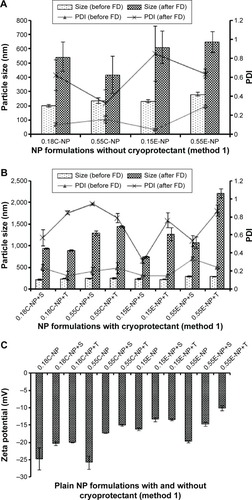
Figure 3 Particle size, PDI, and ZP of NPs prepared with different PLGA polymer end groups and viscosities following method 2.
Notes: The bar diagram and line plots represent particle size and PDI value, respectively. (A) Represents all the NP formulations with or without BS3 prepared without cryoprotectant. (B) Represents the same formulations preserved with sucrose (10%) as cryoprotectant. (C) Represents the comparative ZP of all NP formulations with or without cryoprotectant. The level of significance was set to P<0.05 (one-way ANOVA followed by Tukey’s multiple comparison test method). Each bar and line represents mean ± SD (n=12). C = COOH ended PLGA; E = ester ended PLGA; S = sucrose (10%).
Abbreviations: PDI, polydispersity index; ZP, zeta potential; NP, nanoparticle; PLGA, poly(d, l-lactide co-glycolide); ANOVA, analysis of variance; SD, standard deviation; COOH, carboxylic acid; FD, freeze-drying; BS3, bis(sulfo-succinimidyl) suberate.

In method 2, both particle size and PDI values were in a fairly desirable range for all NPs before freeze-drying (). A proportional relation between polymer viscosity and particle size was observed for plain ( and ) and modified () NPs. In contrary, no significant correlation could be made between the variable polymer end groups and physicochemical properties.
Effect of ligand-NP bonding types
In method 1, low viscosity COOH terminated NP had a significantly higher (P<0.05) amount of antibody adsorbed compared to other groups and the particles were in an appropriate size range below 350 nm. The high viscosity COOH terminated NPs had relatively larger size with wide PDI values. In addition, ZP values showed a significant drop toward positive value upon attachment of ligand with NPs as shown in (P<0.05). NPs obtained after covalent attachment of antibody, had the same particle size trend as observed in the adsorbed groups. While larger aggregates were formed with antibody-adsorbed NPs for ester terminated NPs, which was further minimized using cryoprotectant. The original particle size was above the nanometer range, indicating the presence of aggregates (PDI value >0.99).
In method 2, there was a significant decrease in particle size for the covalently modified NPs compared to antibody-adsorbed formulations. But no concrete correlation could be drawn to compare the antibody loading through adsorption and covalent attachment of ligand with NPs. In addition, the inclusion of antibody to NPs shifted the ZP toward positive values, which indirectly confirms the presence of antibody on the NP surface, as summarized in and . This could be ascribed to the amphiphilic properties of antibody or shielding the negative charges on the surface by positively charged antibody.Citation24,Citation25 represents the overall data for particle size, PDI, ZP, and antibody quantification for modified NPs. Furthermore, the SEM photographs confirmed that NPs form spherical shaped particles within the desired size range. SEM images of NPs were taken at a voltage of 20–25 kV at various magnifications as shown in .
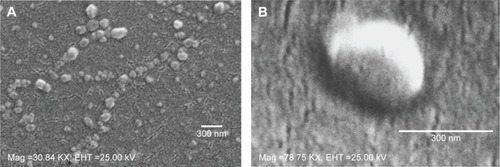
Figure 4 SEM images.
Notes: SEM images of NPs (A) 0.15 iv ester terminated PLGA NPs at magnification of 30.84 KX at EHT (extra high tension) 25 kV; (B) antibody modified 0.15 iv ester terminated PLGA NPs at magnification of 78.75 KX at EHT 25 kV.
Abbreviations: SEM, scanning electron microscopy; NPs, nanoparticles; PLGA, poly(d, l-lactide co-glycolide); iv, inherent viscosity; Mag, magnification.
Effect of cryoprotectants
The NPs were in a well-dispersed suspension form before freeze-drying; however they formed aggregates upon reconstitution after freeze-drying, which could not be re-dispersed even after sonication. The reason behind this irreversible aggregation could be attributed to freeze-drying stress on the particles rendering a wide range of PDI value before ligand attachment.Citation26 Use of cryoprotectants could overcome this stress to obtain aggregation-free fine suspension after ligand attachment. However, all the formulations had significant difference in particle size before and after freeze-drying (P<0.05) irrespective of the presence of cryoprotectant.
Notably, the use of trehalose (10%) could not fully minimize the aggregation produced between the prepared NPs following method 1 before ligand attachment compared to sucrose (10%). As a consequence, the rest of the formulations were continued with sucrose (10%). However, 10% sucrose was preferably chosen after comparative optimization based on the different percentages (1%, 5%, and 10%) of sucrose used as cryoprotectant (data not shown). Sucrose was found to be a better cryoprotectant to retain particle size when compared with trehalose used for plain NP formulations prepared following method 1. Thus, the effect of cryoprotectants was partially or not pronounced in formulations under method 1 as represented in . The ZP value for plain NPs without cryoprotectants was found to be more negative compared to formulations with sucrose. Even though the presence of sucrose should cause a higher negative charge on particles, the opposite was found.Citation27
For method 2, there was significant change in ZP after addition of cryoprotectant (P<0.05). Also, the presence of cryoprotectant showed significant difference in particle size for antibody-adsorbed groups (P<0.05). However, the change in particle size was not significant when cryoprotectant was used in covalently modified formulations. demonstrates the effect of sucrose after antibody attachment, where PDI was found to be below or equal to 0.43. Whereas formulations that were freeze-dried without cryoprotectant showed higher PDIs (highest PDI =0.95). There was significant reduction in particle size for all modified NPs after use of cryoprotectant, except the covalently modified formulations of method 2.
Effect of cross-linkers
EDC/sulfo-NHS cross-linker was considered to conjugate antibody ligand with prepared NPs following method 1.Citation28,Citation29 The presence of cross-linkers could be attributed to larger particles after antibody attachment. In method 2, the BS3 (spacer) pre-activated NP formulations showed fairly considerable physicochemical properties after antibody modification. In both methods, successful attachment of antibody was obtained which was confirmed by BCA assay ().
Confirmation of structural modification by FTIR
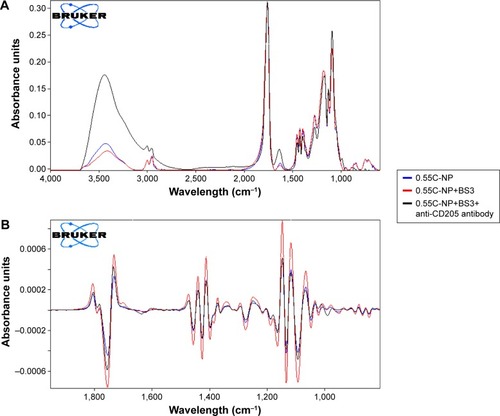
The peak around 1,750 cm−1 is a marked peak that elicits the presence of a carbonyl bond (C=O stretching vibration), which is characteristic of PLGA.Citation30 An amide stretching is present between 3,310 and 3,250 cm−1 in anti-CD205 antibody modified PLGA NP spectra, which corresponds to C=O stretching bond. Theoretically, amide-I vibrations result from C=O stretching vibration near 1,610 cm−1 as observed in the spectra for antibody modified NP.Citation31 Amide-I bond is the most sensitive to prove the structural change in any compound containing proteins. Some contribution in the spectrum from the C=O groups in both BS3 free and BS3 containing NP formulations could also be observed.Citation32,Citation33 To confirm the presence of antibody on the NPs, the spectra for only high viscosity COOH ended PLGA NPs and their modification are represented here (). Spectra for other polymer types and their subtypes are not shown here.
Figure 5 Infrared spectrum of NPs.
Notes: Primary (A) and secondary derivative (B) of IR spectrum for 0.55C-NP (0.55 iv COOH terminated plain PLGA NPs) (blue), 0.55C-NP+BS3 (BS3 containing 0.55 iv COOH terminated PLGA NPs) (red), and 0.55C-NP+BS3+anti-CD205 antibody (BS3 containing 0.55 iv COOH terminated Ab modified PLGA NPs) (black). Data are represented in absorbance unit versus wavelength (cm−1).
Abbreviations: IR, infrared; NP, nanoparticle; COOH, carboxylic acid; PLGA, poly(d, l-lactide co-glycolide); BS3, bis(sulfo-succinimidyl) suberate; iv, inherent viscosity; Ab, anti-CD205 antibody.
Comparison of safety profiles for method 1 and 2
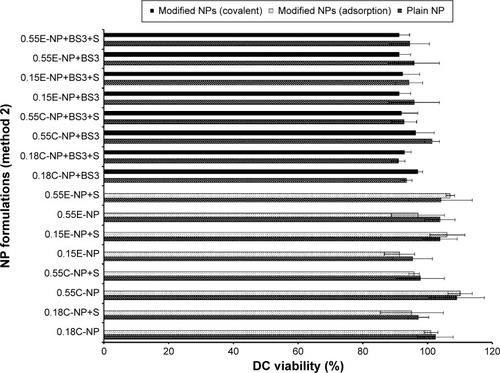
All NP formulations prepared by method 1 (both ester and COOH terminated NPs) retained DC viability (≥80%). The findings indicate that both plain and modified NPs were not toxic to the DCs confirming the safety profile of the NPs as shown in and . Similarly, method 2 based NP formulations demonstrate a viability of >90%.Citation11 There was no significant difference in viability among different groups (plain and modified) for both methods. However, 0.15E-NP+S showed significant difference (P<0.05) compared to other formulations like 0.18C-NP+BS3, 0.55C-NP+BS3, 0.15E-NP+BS3, 0.15E-NP+BS3+S, and 0.55E-NP+BS3+S prepared following method 2.
Figure 6 DC viability (MTT assay) after 24 hours of exposure to plain and antibody modified NPs (method 1).
Notes: The treated NP concentration was 1 mg/mL for cell density of 10,000. Result was calculated based on the absorbance of treated cells in comparison with untreated cells, where blank values were subtracted from each group (n=3). C = COOH ended PLGA, E = ester ended PLGA, S = sucrose (10%), and T = trehalose (10%).
Abbreviations: DC, dendritic cell; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; NPs, nanoparticles; COOH, carboxylic acid; PLGA, poly(d, l-lactide co-glycolide).
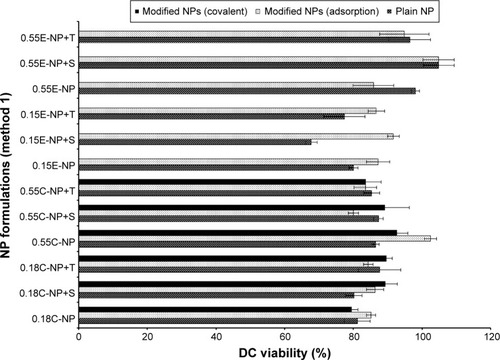
Figure 7 DC viability (MTT assay) after 24 hours of exposure to plain and modified NPs (method 2).
Notes: The treated NP concentration was 1 mg/mL for cell density of 10,000. Result was calculated based on the absorbance of treated cells in comparison with untreated cells, where blank values were subtracted from each group (n=3). C = COOH ended PLGA, E = ester ended PLGA, and S = sucrose (10%).
Abbreviations: DC, dendritic cell; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; NPs, nanoparticles; COOH, carboxylic acid; PLGA, poly(d, l-lactide co-glycolide).
Discussion
The choice of a suitable NP formulation technique is dependent on the desired physicochemical properties. NP size is an important determinant of the formulation efficacy in vivo. Very large particles (around 1 μm) can cause irritant effects after injection.Citation34 On the other hand, particles smaller than 5 nm are likely to be cleared by non-phagocytic cells.Citation35 Alternatively, particles larger than 100 nm have the chance to be taken up by phagocytic cells (for example, DCs).Citation34,Citation36 The formulations prepared by the methods mentioned here, aim to target DC receptors in vivo. Thus the particle size range (309±36 to 544±45 nm) obtained by method 2 could be suitable for DC uptake via subcutaneous delivery.Citation37,Citation38 However, toxicity issue is a concern for emulsification solvent evaporation technique. Chlorinated solvents like chloroform and dichloromethane have issues regarding environmental challenges and human use. Considering these issues, ethyl acetate offers a better solubility and safety profile compared to chloroform. As a polar solvent ethyl acetate with a solubility of 8.1 volume/volume in water is comparatively safer.Citation39 It has lower interfacial tension (1.7 dyne/cm) compared to chloroform (32.8 dyne/cm), which forms more stable emulsions and smaller NPs in PVA solutions.Citation40 A considerable decrease in particle size was observed when ethyl acetate was used as solvent in method 2. In addition, all the formulations had improved PDI indicating mono-disperse NP suspension. Furthermore, method 2 showed a batch-to-batch reproducibility for all the formulations of interest that will be required for in vivo experiments. The represented SEM images confirmed the formation of spherical NPs within the desired size range (). The images confirm the homogenous nature of particles with a uniform and aggregation-free distribution.Citation41
There are different emulsion stabilizers available such as PVA, carbopol and polaxamer. Among these, PVA is found to provide homogenous particles with uniform size distribution.Citation42 It acts on the boundary of aqueous and organic phase to modify the particle size as well as surface charge.Citation43 PVA is able to maintain the interfacial tension at oil-water interface to obtain small sized particles.Citation44 In addition, it prevents the aggregation and preserves the hydrophilicity of particles when used above a concentration of 2%.Citation45 A high concentration of PVA is expected to produce particles with narrow granulometric distribution. Failure to maintain that would lead to the aggregated particles.Citation46 Between method 1 (5% PVA) and method 2 (2.2% PVA), the difference in particle size could be attributed to not only the difference of PVA concentration but also the effect of solvents. Decrease in PVA concentration from method 1 to method 2 could lead to larger particles. But, PVA concentration of 5% did not play a better role to reduce particle size due to PVA’s optimum packing.Citation47 In addition, PVA also renders slightly negative charges on the particles.Citation48
ZP value is an important parameter that reflects stability of a colloidal suspension.Citation49 ZP values for COOH terminated NPs were found to be more negative (−25 to −24 mV) compared to ester terminated (−19 to −16 mV) NPs. This could be attributed to the presence of carboxyl group on the COOH terminated NPs. When the viscosity of the polymers increased, ZP value showed slightly more negative charge for uncapped NPs than the capped formulations.Citation50 When the polymer of higher viscosity is used it increases the concentration of the media resulting in semi-folded particles.Citation51 Increase in concentration of the organic phase also resulted in viscosity resistance against the net shear stress. Ultimately coalescence of particles occurs to provide increase in NP size.Citation52,Citation53 Particles with more positive (above +30 mV) or more negative (below −30 mV) ZP are considered colloidally stable but not pharmaceutically stable.Citation16,Citation54 This indicates that ZP beyond this range needs to be kept in solid and dry form rather than colloidal state. Our results show that ZP of antibody modified NPs was about neutral or slightly positive or negative. The prepared NPs were therefore lyophilized for pharmaceutical stability and should be reconstituted immediately before the administration.Citation16,Citation55
Freeze-drying is an essential part in retaining the stability of formulations after preparation. This process could be stressful leading to colloidal preparations having large aggregates, although there are several cryoprotectants available to increase the physical stability of NPs.Citation56 Cryoprotectants could be water-soluble sugars such as glucose, trehalose, which are added to prevent NPs from aggregating during drying process. Hence, it is advantageous to prepare NPs with addition of cryoprotectants. There are two types of cryoprotectants: intracellular that prevents crystal formation and cell membrane rupture; and extracellular that minimizes hyperosmotic effect during freeze-drying process. Sucrose and trehalose are extracellular cryoprotectants that minimize freeze-drying stress of the formulations.Citation57 For method 1, antibody modified NPs had higher particle size compared to the plain NPs which was predictable. The PDI value remained fairly high even after using cryoprotectants. No significant differences in the effect of sucrose and trehalose could be identified for these formulations. Addition of cryoprotectant might have formed hydrogen bond between their hydroxyl group and NP surface resulting in masking of the negative charges of the particles.
It has been reported that presence of stabilizers could also play a major role in NP aggregation in the drying process.Citation58 The percentage of stabilizer is critical to maintain its influence on both particle size and ZP.Citation59 In the NP formulations (following method 2), the PVA percentage was 2.2% below the optimum range (2.5%–5%) which necessities the use of cryoprotectant. A minimum of 5% cryoprotectant is necessary to ensure stability of formulations.Citation60 In another study, aggregation-free PLGA NP suspension was obtained with 1%–2% PVA where 10% of different cryoprotectants (sucrose, trehalose, glucose) were used.Citation61 Therefore, studies were continued with method 2 formulations using 10% sucrose as cryoprotectant due to its better cryopreservation.
The highest antibody incorporation was obtained with low viscosity ester terminated PLGA NPs (formulations with cryoprotectants for either conjugation method), whereas low viscosity COOH terminated PLGA NPs provided the lowest antibody incorporation (except antibody-adsorbed formulations of method 1). The low viscosity ester terminated PLGA had higher antibody attachment due to higher surface area (smaller size) of NPs. Besides, with the same amount of initial antibody, the COOH terminated PLGA had lower antibody incorporation which could be attributed to the rapid erosion of the polymer.Citation62 For method 2 based formulations, covalently attached NPs were found to be smaller than physically adsorbed NPs although the antibody attachment between these groups was not significantly different. In addition, ZP did not show any rational correlation between antibody loading and conjugation method for both COOH and ester terminated NPs. It was observed that covalently attached low viscosity COOH and ester terminated polymers resulted in smaller particle size compared to high viscosity polymers, which is expected. Moreover, all the formulations had particle size within desired range for DC uptake.Citation11,Citation37 It was found that, the 4 hours stirring time during antibody-NP attachment should be enough to break down the aggregates and to form a fine suspension leaving the surface available for antibody attachment. This is observed from the smaller particle size with acceptable PDI obtained for the low viscosity ester terminated NPs after 4 hours incubation with continuous stirring. However, the particle size was above the target range with poor PDI values for the high viscosity ester terminated NPs prepared following method 1. On the contrary, ligand modified NP formulations prepared by method 2 showed equally acceptable range for PDI values with the highest of 0.43±0.05 and lowest of 0.22±0.07. The antibody attachment on NP surface was calculated to be in a desired range (2 μg/mg of NP). However, no significant correlation could be made between antibody-NP conjugation methods (adsorption and covalent attachment) and polymers’ viscosities. Considering the viability, it was slightly higher with high viscosity grade PLGA for most of the formulations; and conjugation method could not affect DC viability.
The structural modification of BS3 activated formulations was well confirmed by infrared study. In the case of the anti-CD205 conjugated NPs spectrum, presence of N-H stretching of high intensity confirmed the conjugation. Second derivative of all spectra () clearly shows the presence of an amide bond between BS3 and antibody. This characteristic peak stands out, reflecting the establishment of the antibody conjugation on the NPs.
The results from method 2 were more reproducible and consistent, whereas the formulations prepared by method 1 showed higher deviation from the average in the DC viability tests. Based on the confirmed safety profile of the NP formulations, we can further use them for DC uptake and targeting efficiency study.
Conclusion
In this research, the effects of various processing parameters were investigated. The formulation variables evaluated here could be manipulated to enhance the efficiency of the PLGA NPs. Based on the optimum potential parameters it is concluded that formulations prepared using ethyl acetate as solvent (method 2) are shown to be promising for NP preparation and will be further utilized in our in vitro experiments. Further in vitro investigations such as targeting efficiency, maturation of DCs, cytokine secretion profile, and activation of immune response leading to in vivo studies are being conducted in ongoing studies. Therefore, the structural characterizations reflected in those formulations will direct us to obtain optimum in vivo effects. This optimization would help other researchers to select the optimal parameters in their study. In conclusion, this systematic investigation could promote the development of PLGA NPs for further application to design a cancer vaccine.
Acknowledgments
This project was supported by funding from Natural Sciences and Engineering Research (NSERC) Discovery Grant and Saskatchewan Health Research Foundation (SHRF) New Investigator Establishment Grant. The authors thank Canadian Light Source (CLS), Saskatoon, Saskatchewan, for providing assistance with infrared spectroscopy and SEM.
Disclosure
The authors report no conflicts of interest in this work.
References
- BandyopadhyayAFineRLDementoSBockenstedtLKFahmyTMThe impact of nanoparticle ligand density on dendritic-cell targeted vaccinesBiomaterials201132113094310521262534
- ReuterAPanozzaSEMacriCCriteria for dendritic cell receptor selection for efficient antibody-targeted vaccinationJ Immunol201519462696270525653426
- ButlerMMorelASJordanWJAltered expression and endocytic function of CD205 in human dendritic cells, and detection of a CD205-DCL-1 fusion protein upon dendritic cell maturationImmunology2007120336237117163964
- PlatzerBStoutMFiebigerEAntigen cross-presentation of immune complexesFront Immunol2014514024744762
- BarbutoSIdoyagaJVila-PerelloMInduction of innate and adaptive immunity by delivery of poly dA:dT to dendritic cellsNat Chem Biol20139425025623416331
- CooperDLHarirforooshSDesign and optimization of PLGA-based diclofenac loaded nanoparticlesPloS One201491e8732624489896
- DanhierFAnsorenaESilvaJMCocoRLe BretonAPreatVPLGA-based nanoparticles: an overview of biomedical applicationsJ Control Release2012161250552222353619
- LewisJSZaveriTDCrooksCP2ndKeselowskyBGMicroparticle surface modifications targeting dendritic cells for non-activating applicationsBiomaterials201233297221723222796161
- HaddadiAHamdySGhotbiZSamuelJLavasanifarAImmuno-adjuvant activity of the nanoparticles’ surface modified with mannanNanotechnology2014253535510125119543
- SahHThomaLADesuHRSahEWoodGCConcepts and practices used to develop functional PLGA-based nanoparticulate systemsInt J Nanomedicine2013874776523459088
- GhotbiZHaddadiAHamdySHungRWSamuelJLavasanifarAActive targeting of dendritic cells with mannan-decorated PLGA nanoparticlesJ Drug Target201119428129220590403
- KeumCGNohYWBaekJSPractical preparation procedures for docetaxel-loaded nanoparticles using polylactic acid-co-glycolic acidInt J Nanomedicine201162225223422114486
- ThamakeSIRautSLRanjanAPGryczynskiZVishwanathaJKSurface functionalization of PLGA nanoparticles by non-covalent insertion of a homo-bifunctional spacer for active targeting in cancer therapyNanotechnology201122303510121149963
- KocbekPObermajerNCegnarMKosJKristlJTargeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibodyJ Control Release20071201–2182617509712
- ZouWLiuCChenZZhangNStudies on bioadhesive PLGA nanoparticles: A promising gene delivery system for efficient gene therapy to lung cancerInt J Pharm20093701–218719519073241
- MukherjeeBSantraKPattnaikGGhoshSPreparation, characterization and in-vitro evaluation of sustained release protein-loaded nanoparticles based on biodegradable polymersInt J Nanomedicine20083448749619337417
- Al-NemrawiNKDaveRHFormulation and characterization of acetaminophen nanoparticles in orally disintegrating filmsDrug Deliv201511025013958
- KaurRChitandaJMMichelDLysine-functionalized nanodiamonds: synthesis, physiochemical characterization, and nucleic acid binding studiesInt J Nanomedicine201273851386622904623
- ValenciaPMHanewich-HollatzMHGaoWEffects of ligands with different water solubilities on self-assembly and properties of targeted nanoparticlesBiomaterials201132266226623321658757
- LiFSunJZhuHWenXLinCShiDPreparation and characterization novel polymer-coated magnetic nanoparticles as carriers for doxorubicinColloids Surf B Biointerfaces2011881586221764271
- DillenKVandervoortJVan den MooterGVerheydenLLudwigAFactorial design, physicochemical characterisation and activity of ciprofloxacin-PLGA nanoparticlesInt J Pharm20042751–217118715081148
- TsaiYMChienCFLinLCTsaiTHCurcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetrationInt J Pharm2011416133133821729743
- MundargiRCBabuVRRangaswamyVPatelPAminabhaviTMNano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivativesJ Control Release2008125319320918083265
- CoppJAFangRHLukBTClearance of pathological antibodies using biomimetic nanoparticlesProc Natl Acad Sci U S A201411137134811348625197051
- SperlingRAParakWJSurface modification, functionalization and bioconjugation of colloidal inorganic nanoparticlesPhilos Trans A Math Phys Eng Sci201036819151333138320156828
- HermansKVan den PlasDEveraertAWeyenbergWLudwigAFull factorial design, physicochemical characterisation and biological assessment of cyclosporine A loaded cationic nanoparticlesEur J Pharm Biopharm2012821273522634236
- FontePSoaresSCostaAEffect of cryoprotectants on the porosity and stability of insulin-loaded PLGA nanoparticles after freeze-dryingBiomatter20122432933923507897
- IkedaJSunYLAnKNAmadioPCZhaoCApplication of carbodiimide derivatized synovial fluid to enhance extrasynovial tendon gliding abilityJ Hand Surg Am201136345646321371626
- ByrneJDBetancourtTBrannon-PeppasLActive targeting schemes for nanoparticle systems in cancer therapeuticsAdv Drug Deliv Rev200860151615162618840489
- SampathMLakraRKorrapatiPSengottuvelanBCurcumin loaded poly(lactic-co-glycolic) acid nanofiber for the treatment of carcinomaColloids Surf B Biointerfaces201411712813424646452
- KountzSLThe effect of bioscience and technological momentum on the surgical treatment of chronic illnessSurgery19757767357401145439
- BarthAInfrared spectroscopy of proteinsBiochim Biophys Acta2007176791073110117692815
- GlassfordSEByrneBKazarianSGRecent applications of ATR FTIR spectroscopy and imaging to proteinsBiochim Biophys Acta20131834122849285823928299
- Cohen-SelaEChornyMKoroukhovNDanenbergHDGolombGA new double emulsion solvent diffusion technique for encapsulating hydrophilic molecules in PLGA nanoparticlesJ Control Release20091332909518848962
- ChoiHSLiuWMisraPRenal clearance of quantum dotsNat Biotechnol200725101165117017891134
- KettigerHSchipanskiAWickPHuwylerJEngineered nanomaterial uptake and tissue distribution: from cell to organismInt J Nanomedicine201383255326924023514
- HamdySHaddadiAShayeganpourASamuelJLavasanifarAActivation of antigen-specific T cell-responses by mannan-decorated PLGA nanoparticlesPharm Res20112892288230121560020
- GutierroIHernandezRMIgartuaMGasconARPedrazJLSize dependent immune response after subcutaneous, oral and intranasal administration of BSA loaded nanospheresVaccine2002211–2677712443664
- SoppimathKSAminabhaviTMEthyl acetate as a dispersing solvent in the production of poly(DL-lactide-co-glycolide) microspheres: effect of process parameters and polymer typeJ Microencapsul200219328129212022494
- SahanaDKMittalGBhardwajVKumarMNPLGA nanoparticles for oral delivery of hydrophobic drugs: influence of organic solvent on nanoparticle formation and release behavior in vitro and in vivo using estradiol as a model drugJ Pharm Sci20089741530154217722098
- JoseSSowmyaSCinuTAAleykuttyNAThomasSSoutoEBSurface modified PLGA nanoparticles for brain targeting of Bacoside-AEur J Pharm Sci201463293525010261
- YonchevaKVandervoortJLudwigAInfluence of process parameters of high-pressure emulsification method on the properties of pilocarpine-loaded nanoparticlesJ Microencapsul200320444945812851045
- VandervoortJLudwigABiocompatible stabilizers in the preparation of PLGA nanoparticles: a factorial design studyInt J Pharm20022381–2779211996812
- WangHJiaYHuWJiangHZhangJZhangLEffect of preparation conditions on the size and encapsulation properties of mPEG-PLGA nanoparticles simultaneously loaded with vincristine sulfate and curcuminPharm Dev Technol201318369470022676257
- MobarakDHSalahSElkheshenSAFormulation of ciprofloxacin hydrochloride loaded biodegradable nanoparticles: optimization of technique and process variablesPharm Dev Technol201419789190024032531
- MakadiaHKSiegelSJPoly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery CarrierPolymers (Basel)2011331377139722577513
- SanadRAAbdel MalakNSEl-BayoomyTSBadawiAAPreparation and characterization of oxybenzone-loaded solid lipid nanoparticles (SLNs) with enhanced safety and sunscreening efficacy: SPF and UVA-PFDrug Discov Ther20104647248322491313
- VandervoortJYonchevaKLudwigAInfluence of the homogenisation procedure on the physicochemical properties of PLGA nanoparticlesChem Pharm Bull (Tokyo)200452111273127915516745
- Nabi-MeibodiMVatanaraANajafabadiARThe effective encapsulation of a hydrophobic lipid-insoluble drug in solid lipid nanoparticles using a modified double emulsion solvent evaporation methodColloids Surf B Biointerfaces201311240841424036624
- Gomez-GaeteCBustosGLGodoyRRSuccessful factorial design for the optimization of methylprednisolone encapsulation in biodegradable nanoparticlesDrug Dev Ind Pharm201339231032023323873
- PremalethaKLicyCDJoseSSaraladeviAShirwaikarAShirwaikarAFormulation, characterization and optimization of hepatitis B surface antigen (HBsAg)-loaded chitosan microspheres for oral deliveryPharm Dev Technol201217225125821108582
- Awotwe-OtooDZidanASRahmanZHabibMJEvaluation of anticancer drug-loaded nanoparticle characteristics by nondestructive methodologiesAAPS PharmSciTech201213261162222535519
- LiXXuYChenGWeiPPingQPLGA nanoparticles for the oral delivery of 5-Fluorouracil using high pressure homogenization-emulsification as the preparation method and in vitro/in vivo studiesDrug Dev Ind Pharm200834110711518214762
- WestesenKNovel lipid-based colloidal dispersions as potential drug administration systems expectations and realityColloid Polym Sci20002787608618
- Pinto ReisCNeufeldRJRibeiroAJVeigaFNanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticlesNanomedicine20062182117292111
- RizkallaNRangeCLacasseFXHildgenPEffect of various formulation parameters on the properties of polymeric nanoparticles prepared by multiple emulsion methodJ Microencapsul2006231395716830976
- Janz FdeLDebes AdeACavaglieri RdeCEvaluation of distinct freezing methods and cryoprotectants for human amniotic fluid stem cells cryopreservationJ Biomed Biotechnol2012201264935322665987
- ZhangXGuanJNiRLiLCMaoSPreparation and solidification of redispersible nanosuspensionsJ Pharm Sci201410372166217624840928
- MuraSHillaireauHNicolasJInfluence of surface charge on the potential toxicity of PLGA nanoparticles towards Calu-3 cellsInt J Nanomedicine201162591260522114491
- AbdelwahedWDegobertGStainmesseSFessiHFreeze-drying of nanoparticles: formulation, process and storage considerationsAdv Drug Deliv Rev200658151688171317118485
- JeongYIShimYHKimCLimGTChoiKCYoonCEffect of cryoprotectants on the reconstitution of surfactant-free nanoparticles of poly(DL-lactide-co-glycolide)J Microencapsul200522659360116401576
- LamXMDuenasETDaughertyALLevinNClelandJLSustained release of recombinant human insulin-like growth factor-I for treatment of diabetesJ Control Release2000672–328129210825561
