Abstract
Aim
The aim of this study was to investigate different intensities of uremic pruritus in the daytime and nighttime, as well as contributing factors, in patients undergoing peritoneal dialysis (PD).
Methods
A total of 46 patients (31 males, 15 females) with a mean age of 59.4±14.7 years and mean PD vintage of 29.2±25.2 months were enrolled in this single-center, prospective, cross-sectional study. The intensity of uremic pruritus in the daytime and nighttime was assessed using a visual analog scale (VAS). The relationships between intensity and various clinical and laboratory parameters were analyzed using multiple linear regression analyses.
Results
The most common site of uremic pruritus was on the back (70%), followed by lower limbs (67%), chest and abdomen (59%), upper limbs (28%), and head and neck (22%). Mean VAS scores were higher in the nighttime compared with the daytime (4.5±3.3 vs. 3.5±2.7, P=0.02). Only male sex was correlated with higher uremic pruritus intensity in the daytime (standard coefficient [β]=0.310, P=0.036). PD vintage (β=0.415, P=0.004) and topical medicines, including moisturizer and topical corticosteroid use (β=0.345, P=0.019), were independently correlated with higher uremic pruritus intensity in the nighttime.
Conclusion
Uremic pruritus intensity was greater in the nighttime than in the daytime in PD patients. Male sex was associated with higher uremic pruritus intensity in the daytime, whereas PD vintage and topical medicine use were associated with higher uremic pruritus intensity in the nighttime.
Keywords:
Introduction
Uremic pruritus is a common complication in patients with end-stage kidney disease.Citation1 The discomfort caused by pruritus can cause sleep disturbance,Citation2,Citation3 depression,Citation3,Citation4 and lack of concentration.Citation2 In hemodialysis patients, moderate and severe uremic pruritus is associated with higher mortality risk.Citation5 The prevalence of uremic pruritus in patients undergoing peritoneal dialysis (PD) has been reported to be higher compared with hemodialysis.Citation6 Furthermore, patients undergoing PD have been reported to have more severe uremic pruritus in degree, distribution, frequency, and sleep disturbance compared with patients undergoing hemodialysis.Citation6 Therefore, the characteristics of patients with uremic pruritus undergoing PD warrant further investigation.
Several recent studies have shown that a lower weekly total Kt/V, longer PD vintage, a higher level of parathyroid hormone, and a higher level of C-reactive protein are independently associated with uremic pruritus severity in patients undergoing PD.Citation6,Citation7 However, further studies on the details of uremic pruritus in these patients, such as circadian variation and the influence of medicines, are required. Therefore, we investigated the differences in intensity of uremic pruritus during the daytime and nighttime, as well as the various factors, including medicines and PD course, associated with uremic pruritus intensity in patients undergoing PD.
Materials and Methods
Ethical Approval
This study was approved by the Ethical Committee of Saitama Medical Center, Jichi Medical University (RIN 15–34), Japan, and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Patients
Inclusion criteria were: i) age >20 years; ii) chronic kidney disease stage G5D; iii) peritoneal dialysis at the time of the study. Exclusion criteria were: i) past renal transplantation; ii) skin diseases other than uremic pruritus such as atopic dermatitis, allergic dermatitis, chronic urticaria, and collagen disease at the time of study; iii) unwillingness or inability to give consent.
Study Design
This was a single-center, prospective, cross-sectional study conducted between April 1, 2018, and March 31, 2019, at Saitama Medical Center, Jichi Medical University, Saitama, Japan. A diagram of the study design is shown in . The site of uremic pruritus was determined by asking patients to locate their itching on a body map. The intensities of uremic pruritus in the daytime and nighttime were assessed using a visual analog scale (VAS). Demographic and clinical laboratory data were collected at the time of uremic pruritus assessment. Independent factors that were correlated with uremic pruritus intensity were analyzed using multiple linear regression analyses for the daytime and nighttime, respectively.
Assessment of Uremic Pruritus
The site of uremic pruritus was determined by asking patients to indicate their itchy areas on a body map (regions: head and neck, chest and abdomen, back, upper limbs, and lower limbs) (). The intensity of uremic pruritus was assessed using a VAS score, ranging from 0 to 10 (0 = no itching, 10 = worst imaginable itching) as described in a previous study.Citation5
Laboratory Methods
Blood and PD fluid parameters were determined by the Department of Clinical Laboratory, Saitama Medical Center. Serum glycated hemoglobin (HbA1c) levels were shown as National Glycohemoglobin Standardization Program values. The bicarbonate concentration was calculated from measured pH and pCO2 using the Henderson–Hasselbalch equation.Citation8 A weekly Kt/V was assessed for each patient by calculating the weekly Kt/V urea from 24-h urinary and dialysate clearances, using measurements of urea in urine and peritoneal effluent.Citation9
Statistics
Statistical analyses were performed using JMP 11 (SAS Institute Inc., Cary, NC, USA). Data were expressed as means ± standard deviation for continuous variables, and as numbers and percentages for categorical variables. Comparisons of uremic pruritus intensities in the daytime and nighttime were performed using Wilcoxon signed-rank test. Parameters that were significantly correlated with higher intensity of uremic pruritus in simple linear regression analyses were included in multiple linear regression analyses to identify factors correlating with a higher intensity of uremic pruritus in patients undergoing peritoneal dialysis. A P-value of <0.05 was considered statistically significant.
Results
Patient Characteristics
All the patients undergoing PD in our hospital during the study period were screened for study entry. Of these, 7 patients were excluded on the basis of exclusion criteria, and the remaining 46 patients were enrolled in the study. The patients’ characteristics and medications are summarized in . There were 31 males and 15 females with a mean age of 59.4±14.7 years and PD mean vintage of 29.2±25.2 months. Fourteen patients (30.4%) were on continuous ambulatory PD (CAPD), 4 patients (8.7%) on automated PD (APD), and 28 patients (60.9%) on a combination of CAPD and APD. The proportions of patients using icodextrin solution, lactate-buffered solution, and bicarbonate-buffered solution were 63.0%, 37.0%, and 67.4%, respectively. The mean weekly total Kt/V was 1.7±0.3. Seven patients (15.2%) were treated with combined PD and hemodialysis. Polysulfone membrane was used in 5 patients and cellulose triacetate membrane was in 2 patients. The percentages of patients with diabetes mellitus and liver disease were 41.3% and 2.2%, respectively. The proportions of patients with medication use were: corticosteroids (2.2%), antihistamines (19.6%), nalfurafine (17.4%), topical medicines (including moisturizing cream and steroid creams) (30.4%), calcium-containing phosphate binders (56.5%), calcium-free phosphate binders (76.1%), vitamin D analogs (63.0%), cinacalcet (19.6%), polaprezinc (10.9%), and erythropoiesis-stimulating agents (95.7%).
Table 1 Patients’ Characteristics
Site and Intensity of Uremic Pruritus
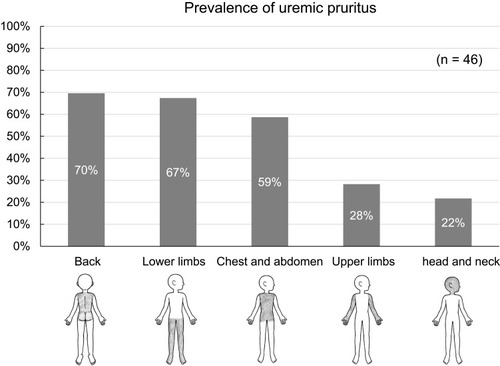
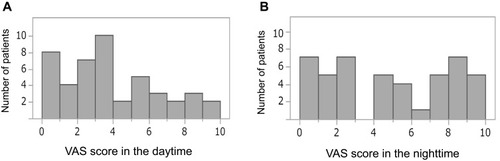
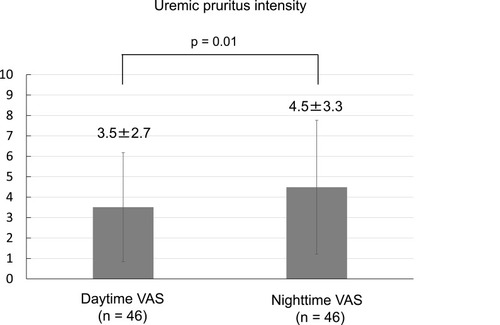
The most common site of uremic pruritus was on the back (70%), followed by lower limbs (67%), chest and abdomen (59%), upper limbs (28%), and head and neck (22%) (). The distribution of patients with each range of VAS scores in the daytime and nighttime is shown in . The intensity of uremic pruritus assessed by VAS score was higher in the nighttime compared with the daytime (4.5±3.3 vs. 3.5±2.7, P=0.02) ().
Independent Factors Correlated with Uremic Pruritus Intensity
Simple linear regression analyses revealed that uremic pruritus intensity in the daytime was significantly correlated with male sex only (standard coefficient [β] = 0.310, P = 0.036) (). Uremic pruritus intensity in the nighttime was significantly correlated with PD vintage and topical medicine. We performed multiple linear regression analyses on variables that showed significant correlations with nighttime uremic pruritus intensity on linear regression analyses (). Multiple linear regression analyses revealed that PD vintage (β = 0.415, P = 0.004) and topical medicine use (β = 0.345, P = 0.019) were independently correlated with uremic pruritus intensity in the nighttime. We additionally conducted multiple linear regression analyses using variables that have a high pre-test probability (P<0.10) and that have been implicated in prior literature (male sex,Citation5 peritoneal dialysis vintage,Citation7 weekly total Kt/V,Citation7 liver disease,Citation10 serum albumin,Citation10 blood urea nitrogen,Citation5 calcium,Citation5,Citation10 phosphate,Citation5,Citation10 ferritin,Citation10 β2 microglobulin,Citation5 and intact-parathyroid hormoneCitation5) (Supplemental Table 1). As a result, PD vintage (β = 0.450, P = 0.016) was independently correlated with uremic pruritus intensity in the nighttime.
Table 2 Simple Linear Regression Analyses of the Variables Correlated with Uremic Pruritus Intensity in the Daytime
Table 3 Simple and Multiple Linear Regression Analyses of the Variables Correlated with Uremic Pruritus Intensity in the Nighttime
Discussion
In this study, we found that uremic pruritus intensity as assessed by VAS score was higher in the nighttime compared with the daytime in patients undergoing PD. We also found that male sex was positively correlated with uremic pruritus intensity in the daytime, whereas PD vintage and topical medicine use were positively correlated with uremic pruritus intensity in the nighttime.
A previous study showed that uremic pruritus intensity was higher for the 12-h nighttime period than for the 12-h daytime period in patients undergoing hemodialysis.Citation2 In the present study, uremic pruritus intensity in the nighttime was greater than that in the daytime, which is consistent with the previous report.Citation2 It has been reported that secretion of inflammatory mediators (such as IL-2 and IL-8),Citation11 skin temperature elevation,Citation12 transepidermal water loss,Citation12 and increased concentration of beta-endorphinCitation13 may contribute to nocturnal uremic pruritus in patients with advanced stages of chronic kidney disease. These mechanisms may be responsible for stronger uremic pruritus intensity in the nighttime in patients undergoing PD in this study; however, further studies are warranted on the mechanism of intra-day variability of uremic pruritus in this population.
A previous study reported the back as the most common site of uremic pruritus in patients with end-stage renal disease.Citation14 Male sex was reported to be associated with the development of severe uremic pruritus in patients undergoing hemodialysis.Citation5 In addition, PD vintage was reported to be associated with uremic pruritus intensity in patients undergoing PD.Citation7,Citation15 Residual renal function declines in accordance with increasing vintage on PD.Citation16 Therefore, a decrease in the elimination of pruritogenic substances such as parathyroid hormone and β2-microglobulin might contribute to increase uremic pruritus intensity with PD vintage.Citation17 The results in these previous reports are consistent with the results of this study. The back and lower limbs are the favored sites of uremic pruritus, and male sex and PD vintage may be influential factors for uremic pruritus intensity. In the present study, 15% of patients were treated with combined PD and hemodialysis because of insufficient removal of water or solute on PD. A combination of PD and hemodialysis showed a tendency to be correlated with uremic pruritus intensity. This might be due to hemodialysis-related conditions including complement activation by hemodialysis membrane, ethylene oxide gas sterilization, and drugs administered intravenously during hemodialysis.Citation18 The degree of biocompatibility of the dialysis membrane might contribute to increase uremic pruritus intensity in the patients of this study;Citation19 however, these effects need to be investigated in further large-scale study. In the present study, diabetic neuropathy and angiopathy were not associated with uremic pruritus intensity, nor was the type of PD modality and PD solution. Topical medicine, including moisturizer and topical corticosteroid, has been the most frequently used for the treatment of uremic pruritus.Citation18 Therefore, the correlation between uremic pruritus intensity and topical medicine might be just because the patients with higher pruritus intensity use more topical medicine. Nalfurafine is a selective κ-opioid receptor agonist that has a potent antipruritic effect on various types of pruritus through central κ-opioid receptor activation. Several studies have shown that nalfurafine decreased uremic pruritus intensity in patients undergoing hemodialysis.Citation20 However, in the present study, there was no association between uremic pruritus intensity and nalfurafine use. These results suggested that nalfurafine could not completely inhibit uremic pruritus intensity in patients undergoing PD.
Recently, several studies have investigated factors associated with the intensity of uremic pruritus in patients undergoing PD.Citation6,Citation7 A prospective cohort study conducted in China reported that lower weekly total Kt/V was associated with a higher intensity of uremic pruritus.Citation7 Another prospective cohort study conducted in Korea showed that the intensity of uremic pruritus was independently associated with total weekly Kt/V and serum creatinine in patients undergoing PD.Citation6 In the present study, however, weekly total Kt/V was not associated with uremic pruritus intensity. Total weekly Kt/V in this study (1.7±0.3) was lower than those in previous studies (2.11 ± 0.27, 3.1 ± 1.3).Citation6,Citation7 A previous study suggested that total weekly Kt/V ≥1.88 may be needed to reduce the intensity of uremic pruritus in PD patients.Citation7 These findings might explain this discrepancy between our results and those of previously published reports. Further studies are required to clarify the mechanisms for uremic pruritus in patients undergoing PD.
This study has several advantages compared with previous studies.Citation7,Citation15 First, we compared the intensity of uremic pruritus during the daytime and nighttime. Second, this study analyzed various factors associated with uremic pruritus intensity in patients undergoing PD in each of the daytime and nighttime. Therefore, the results of this study might be helpful for further studies of uremic pruritus in patients undergoing PD.
This study had several limitations. First, this study was conducted with a small number of patients from a single center, which might have reduced the statistical power to detect significance. Second, all of the participants were Japanese patients from a single center, which might limit the generalizability of our findings. Third, more than half of the patients were taking phosphate binders or vitamin D analogs and approximately 20% were taking cinacalcet, which might affect the study results because a significant association between uremic pruritus and chronic kidney disease mineral and bone disorder has been reported.Citation5 Therefore, further large-scale, multicenter, multiethnic studies are required to confirm our findings.
In conclusion, uremic pruritus intensity in the nighttime was greater than that in the daytime in patients undergoing PD. Male sex was associated with uremic pruritus intensity in the daytime, whereas peritoneal PD vintage and topical medicine use were associated with uremic pruritus intensity in the nighttime.
Acknowledgments
The authors thank all patients and clinicians who participated in the study. We thank Katrina Krogh, MD from Edanz Group for editing a draft of this manuscript.
Disclosure
The authors declare that they have no conflicts of interest.
References
- Hu X, Sang Y, Yang M, Chen X, Tang W. Prevalence of chronic kidney disease-associated pruritus among adult dialysis patients: a meta-analysis of cross-sectional studies. Medicine (Baltimore). 2018;97(21):e10633. doi:10.1097/MD.000000000001063329794739
- Mathur VS, Lindberg J, Germain M, et al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(8):1410–1419. doi:10.2215/CJN.0010011020558560
- Tessari G, Dalle Vedove C, Loschiavo C, et al. The impact of pruritus on the quality of life of patients undergoing dialysis: a single centre cohort study. J Nephrol. 2009;22(2):241–248.19384842
- Lopes GB, Nogueira FC, de Souza MR, et al. Assessment of the psychological burden associated with pruritus in hemodialysis patients using the kidney disease quality of life short form. Qual Life Res. 2012;21(4):603–612. doi:10.1007/s11136-011-9964-x21744033
- Narita I, Alchi B, Omori K, et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69(9):1626–1632. doi:10.1038/sj.ki.500025116672924
- Min JW, Kim SH, Kim YO, et al. Comparison of uremic pruritus between patients undergoing hemodialysis and peritoneal dialysis. Kidney Res Clin Pract. 2016;35(2):107–113. doi:10.1016/j.krcp.2016.02.00227366666
- Wu HY, Huang JW, Tsai WC, et al. Prognostic importance and determinants of uremic pruritus in patients receiving peritoneal dialysis: a prospective cohort study. PLoS One. 2018;13(9):e0203474. doi:10.1371/journal.pone.020347430183756
- Ramsay AG. Clinical application of the Henderson-Hasselbalch equation. Appl Ther. 1965;7(9):730–736.5318252
- Lysaght MJ, Pollock CA, Hallet MD, Ibels LS, Farrell PC. The relevance of urea kinetic modeling to CAPD. ASAIO Trans. 1989;35(4):784–790.2611047
- Pisoni RL, Wikstrom B, Elder SJ, et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2006;21(12):3495–3505. doi:10.1093/ndt/gfl46116968725
- Steinhoff M, Bienenstock J, Schmelz M, Maurer M, Wei E, Biro T. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J Invest Dermatol. 2006;126(8):1705–1718. doi:10.1038/sj.jid.570023116845410
- Yosipovitch G, Xiong GL, Haus E, Sackett-Lundeen L, Ashkenazi I, Maibach HI. Time-dependent variations of the skin barrier function in humans: transepidermal water loss, stratum corneum hydration, skin surface pH, and skin temperature. J Invest Dermatol. 1998;110(1):20–23. doi:10.1046/j.1523-1747.1998.00069.x9424081
- Glinski W, Brodecka H, Glinska-Ferenz M, Kowalski D. Increased concentration of beta-endorphin in the sera of patients with severe atopic dermatitis. Acta Derm Venereol. 1995;75(1):9–11. doi:10.2340/00015555759117747553
- Mistik S, Utas S, Ferahbas A, et al. An epidemiology study of patients with uremic pruritus. J Eur Acad Dermatol Venereol. 2006;20(6):672–678. doi:10.1111/jdv.2006.20.issue-616836494
- Li J, Guo Q, Lin J, Yi C, Yang X, Yu X. Prevalence and associated factors of uraemic pruritus in continuous ambulatory peritoneal dialysis patients. Intern Med. 2015;54(22):2827–2833. doi:10.2169/internalmedicine.54.451626567994
- Menon MK, Naimark DM, Bargman JM, Vas SI, Oreopoulos DG. Long-term blood pressure control in a cohort of peritoneal dialysis patients and its association with residual renal function. Nephrol Dial Transplant. 2001;16(11):2207–2213. doi:10.1093/ndt/16.11.220711682669
- Attia EA, Hassan AA. Uremic pruritus pathogenesis, revisited. Arab J Nephrol Transplant. 2014;7(2):91–96.25366503
- Takahashi N, Yoshizawa T, Kumagai J, et al. Response of patients with hemodialysis-associated pruritus to new treatment algorithm with nalfurafine hydrochloride: a retrospective survey-based study. Renal Replacement Ther. 2016;2(1). doi:10.1186/s41100-016-0039-x
- Aucella F, Vigilante M, Gesuete A, Maruccio G, Specchio A, Gesualdo L. Uraemic itching: do polymethylmethacrylate dialysis membranes play a role? Nephrol Dial Transplant. 2007;22(Suppl 5):v8–v12. doi:10.1093/ndt/gfm29317586845
- Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant. 2010;25(4):1251–1257. doi:10.1093/ndt/gfp58819926718