Abstract
Purpose
Peritoneal dialysis (PD) is a successful renal replacement therapy; however, long-term PD leads to structural and functional peritoneal damage. Therefore, the monitoring and estimation of peritoneal function are important in PD patients. Oxidative stress has been implicated as one possible mechanism of peritoneal membrane damage. The aim of this study was to evaluate the association between an oxidative stress marker, 8-hydroxydeoxyguanosine (8-OHdG), and peritoneal damage in PD patients.
Methods
The authors evaluated 8-OHdG in drained dialysate by enzyme immunoassay to investigate the association between 8-OHdG and solute transport rate estimated by peritoneal equilibration test and matrix metalloproteinase-2 (MMP-2) level in 45 samples from 28 PD patients.
Results
The 8-OHdG level was significantly correlated with dialysate:plasma creatine ratio (r = 0.463, P < 0.05) and significantly inversely correlated with D/D0 glucose (where D is the glucose level of peritoneal effluents obtained 4 hours after the injection and D0 is the glucose level obtained immediately after the injection) (r = −0.474, P < 0.05). The 8-OHdG level was also significantly correlated with MMP-2 level (r = 0.551, P < 0.05), but it was not correlated with the age of subjects, the duration of PD, or blood pressure.
Conclusion
The level of 8-OHdG in drained dialysate may be a useful novel marker of peritoneal damage in PD.
Introduction
Peritoneal dialysis (PD) is a successful renal replacement therapy for end-stage renal disease patients.Citation1,Citation2 It is suitable as the first method of renal replacement therapy; however, long-term PD leads to peritoneal damage.Citation3,Citation4 This peritoneal damage has been found to be characterized by progressive increase in the thickness of peritoneal membrane, predominantly in the submesothelial collagenous area, declining ultrafiltration loss, and an increased solute transport rate.Citation4–Citation6 This damage makes it difficult to continue PD therapy and may occasionally cause encapsulating peritoneal sclerosis (EPS), which is a serious, life-threatening complication in PD patients.Citation7,Citation8 Therefore, both monitoring and estimation of peritoneal function are important in PD patients. As an estimator of peritoneal damage, the solute transport rate is usually measured by the peritoneal equilibration test (PET);Citation9,Citation10 however, PET is an invasive and time-consuming method because it requires blood sampling and it takes half a day for patients. Therefore, it is important to estimate peritoneal damage using a conventional and noninvasive method.
The mechanisms involved in structural and functional peritoneal changes remain unclear. Oxidative stress has been implicated as one possible mechanism of peritoneal membrane damage.Citation11 PD fluid containing high concentrations of glucose has been reported to increase cellular reactive oxygen species (ROS).Citation12 In addition, recent studies have reported the beneficial effects of antioxidants on preservation of the structural and functional integrity of the peritoneal membrane.Citation13,Citation14 From these lines of evidence, the authors hypothesized that an oxidative stress marker may be a useful marker of peritoneal damage in PD patients. To test this hypothesis, the authors measured an oxidative stress marker, 8-hydroxydeoxyguanosine (8-OHdG), in drained dialysate and investigated the association between the 8-OHdG level and the solute transport rate estimated by PET and matrix metalloproteinase-2 (MMP-2) level, which was reported to be a marker of peritoneal damage in PD patients.Citation15,Citation16
Material and methods
This study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of Jichi Medical University. Written informed consent was obtained from all patients.
Subjects
A total of 45 drained dialysate samples from 28 PD patients (18 males and 10 females, mean age 56.3 ± 13.1 years, ranging from 25 to 78 years) were investigated. The patients’ initial nephropathies were chronic glomerulonephritis (eleven patients), diabetic nephropathy (eight patients), lupus nephritis (one patient), drug nephropathy (one patient), chronic renal sclerosis (two patients), gout kidney (one patient), and unknown etiology (four patients). Nine patients had a history of peritonitis due to infection with a pathogenic organism and were treated with antibiotics.
PET
Peritoneal solute transport was assessed with the PET.Citation10 Drainage of intra-abdominal fluid was followed by an intraperitoneal injection of 2 L of PD fluid containing 2.27%–2.5% glucose. The creatinine (Cr) level of peritoneal effluents obtained 4 hours after the injection (D) was divided by that of plasma (P) to obtain the D/P Cr ratio. The glucose level of peritoneal effluents obtained 4 hours after the injection (D) was divided by that obtained immediately after the injection (D0) to obtain the D/D0 glucose ratio.
Laboratory methods
The concentrations of 8-OHdGH and MMP-2 in the drained dialysate obtained from the PET were measured by enzyme-linked immunosorbent assay (ELISA).
The 8-OHdG ELISA
The Highly Sensitive 8-OHdG Check ELISA kit (Japan Institute for the Control of Aging [JaICA], Nikken SEIL Co, Shizuoka, Japan) was used according to the manufacturer’s protocol. Briefly, 8-OHdG monoclonal antibody and 50 μL of peritoneal effluents were added to the microtiter plate, which had been precoated with 8-OHdG. Following this, 50 μL of reconstituted primary antibody to 8-OHdG was added per well and incubated at 4°C overnight. After three washes, the reconstituted secondary antibody was added and incubated at room temperature for 1 hour. After three more washes, substrate was added to each well and incubated at room temperature for 15 minutes. The reaction-terminating solution was then added and the absorbance was read at 450 nm.
The MMP-2 ELISA
MMP-2 ELISA was performed using the Biotrak MMP-2 ELISA kit (GE Healthcare, Piscataway, NJ) according to the manufacturer’s protocol. Briefly, standards and samples were incubated in microtiter wells precoated with an anti-MMP-2 antibody. Bound MMP-2 was detected using a peroxidase-labeled Fab’ antibody to MMP-2 and any excess was removed by washing and aspiration. The amount of peroxidase bound to each well was determined by addition of a 3,3′,5,5′-tetram-ethylbenzidine substrate. The reaction was stopped and the resultant color was read at 450 nm.
Statistical analysis
All data are expressed as the mean plus or minus the standard deviation. The means of indicated groups were compared using Student’s t-test. Relationships between continuous variables were analyzed using Pearson correlation tests or linear regression analysis. Differences with a P-value less than 0.05 were considered statistically significant.
Results
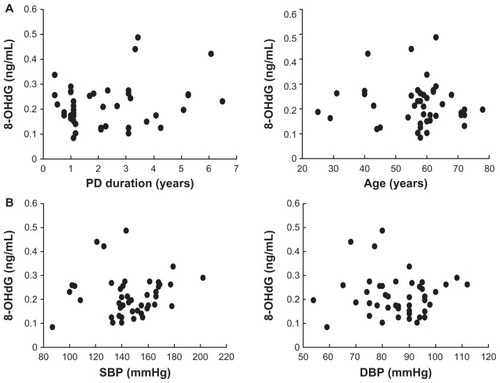
Patient characteristics are presented in . Their duration of PD was 1.9 ± 2.1 years. Their systolic blood pressure (SBP) was 146.5 ± 27.6 mmHg and diastolic blood pressure (DBP) was 83.3 ± 12.8 mmHg. The patients’ hemoglobin and albumin levels were low: hemoglobin was 9.8 ± 1.3 g/dL and albumin was 3.2 ± 0.3 g/dL. The 8-OHdG levels in drained dialysate were not significantly different by sex, initial nephropathy (diabetic or nondiabetic), or past history of peritonitis ().
Figure 1 The level of 8-hydroxydeoxyguanosine (8-OHdG) in drained dialysate in peritoneal dialysis (PD) patients. Comparison of 8-OHdG levels in drained dialysate between (A) males and females, (B) those who had diabetic nephropathy (DMN) and those who did not (non-DMN), and (C) those who had a past history of peritonitis and those who did not.
Abbreviation: NS, no statistical significance.
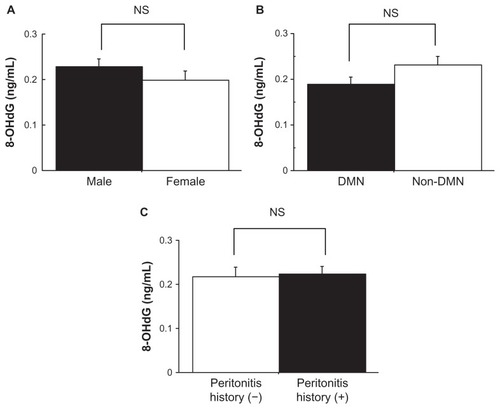
Table 1 Patients’ baseline characteristics (N = 28)
Correlation between the 8-OHdG concentration and the peritoneal solute transport rate and MMP-2 level
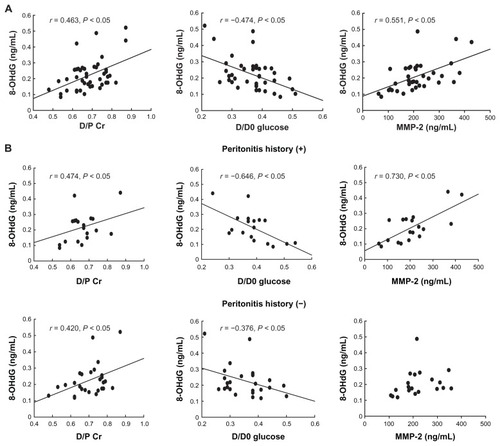
The 8-OHdG level in the drained dialysate was significantly correlated with D/P Cr (r = 0.463, P < 0.05) and significantly inversely correlated with D/D0 glucose (r = −0.474, P < 0.05) in PD patients (). The 8-OHdG level in the drained dialysate was also significantly correlated with MMP-2 level in the drained dialysate (r = 0.551, P < 0.05) (). In the subanalysis based on past history of peritonitis, 8-OHdG level in the drained dialysate correlated with D/P Cr and inversely correlated with D/D0 glucose in both the PD patients who had a past history of peritonitis (D/P Cr: r = 0.474, P < 0.05; D/D0 glucose: r = −0.646, P < 0.05) and the PD patients who did not have a past history of peritonitis (D/P Cr: r = 0.420, P < 0.05; D/D0 glucose: r = −0.376, P < 0.05) (). On the other hand, 8-OHdG level in the drained dialysate was significantly correlated with MMP-2 level in drained dialysate in the PD patients who had a past history of peritonitis (r = 0.730, P < 0.05); however, it was not correlated with MMP-2 level in drained dialysate in those PD patients who did not have past history of peritonitis ().
Figure 2 Correlation between the 8-hydroxydeoxyguanosine (8-OHdG) level in drained dialysate and the peritoneal solute transport rate, determined by peritoneal equilibration test and matrix metalloproteinase-2 (MMP-2) level in drained dialysate: 8-OHdG level and (A) dialysate:plasma creatine ratio (D/P Cr), D/D0 glucose (where D is the glucose level of peritoneal effluents obtained 4 hours after the injection and D0 is the glucose level obtained immediately after the injection), and MMP-2 level in peritoneal dialysis (PD) patients and (B) D/P Cr, D/D0 glucose, and MMP-2 level in those PD patients who had a past history of peritonitis and in those PD patients who did not.
Characteristics of 8-OHdG concentration in drained dialysate in PD patients
The 8-OHdG level in drained dialysate was not correlated with the age of subjects or the duration of PD in PD patients (). The 8-OHdG in drained dialysate was also not correlated with the SBP or DBP in PD patients ().
Discussion
The results of this study show that 8-OHdG level in drained dialysate was significantly correlated with the solute transport rate, which was determined by PET. In addition, 8-OHdG level in drained dialysate was not correlated with age of PD patients, their duration of PD, or their blood pressure (BP). These results suggested that 8-OHdG level in drained dialysate reflects peritoneal damage and could be a novel marker of peritoneal damage. This is the first study to demonstrate a significant correlation between 8-OHdG level in drained dialysate and peritoneal damage in PD patients. The oxidative stress marker 8-OHdG is one of the most abundant oxidative products of DNA. Several studies have reported that 8-OHdG is a useful marker for assessment of oxidative DNA damage by ROS, including that in end-stage renal disease patients.Citation17,Citation18
The mechanisms involved in peritoneal structural and functional changes remain unclear; however, several studies have reported that ROS contributed to multiple factors inducing peritoneal damage.Citation13,Citation14,Citation19 Noh et alCitation13 reported that increase of omental transforming growth factor beta-1 (TGF-β1), vascular endothelial growth factor (VEGF), and type I collagen by conventional PD solution was blocked by an antioxidant, N-acetylcysteine, in an animal model. Kihm et alCitation14 also reported that increase of TGF-β1, alpha smooth muscle actin, VEGF, and vessels per area in peritoneum by PD fluid was attenuated by an antioxidant, benfotiamine, in an animal model. They also reported interleukin (IL)-6- and CD3-positive cells per area in peritoneum were increased by PD fluid and that this increased IL-6 was blocked by benfotiamine.Citation14 Gotloib et alCitation19 reported that oxidative stress induced peritoneal fibrosis and sclerosis characterized by peritoneal adhesions, wrapping of intestinal loops, and the presence of a layer of fibrous tissue in animal PD model. These results suggest that ROS contribute to inflammation, fibrosis, revascularization, and sclerosis in PD.
Cancer antigen 125 (CA125) level can be considered as a marker for mesothelial cell mass and is often used as a marker of peritoneal injury.Citation20–Citation22 Although the downward trend with time of CA125 level in effluent suggested mesothelial cell mass, a single low value is difficult to interpret. MMP-2 level in drained dialysate has been reported to be associated with solute transport rate estimated with PET.Citation16 Furthermore, MMP-2 level in effluent has been reported to be markedly increased in patients with peritoneal injury including EPS.Citation15
In this study of PD patients, 8-OHdG levels in drained dialysate were similar between those who had a past history of peritonitis and those who did not. In addition, these levels were significantly correlated with D/P Cr and inversely correlated with D/D0 glucose, regardless of past history of peritonitis. On the other hand, 8-OHdG level in the drained dialysate was significantly correlated with MMP-2 level in drained dialysate in the PD patients who had a past history of peritonitis; however, it was not correlated with MMP-2 level in drained dialysate in those who did not have a past history of peritonitis. These results suggest that past history of peritonitis may contribute to peritoneal injury by association with oxidative stress, and that 8-OHdG level in drained dialysate may be more strongly correlated with peritoneal injury in PD patients who have a past history of peritonitis than in PD patients who do not, beyond patients’ PD duration, age, and BP. These results also suggest that inhibition of oxidative stress in the peritoneum may be a good therapeutic option to prolong PD duration, especially in PD patients with a past history of peritonitis.
A limitation of this study was that the plasma 8-OHdG level of each sample was not measured to investigate which of local production of 8-OHdG in the peritoneum or increased leakage of plasma 8-OHdG into the dialysate was associated with peritoneal injury in PD patients. Further large and long-term studies will be needed to elucidate the production and regulation of 8-OHdG and the association between 8-OHdG levels in drained dialysate and the development of peritoneal injury including EPS in PD. As it stands, 8-OHdG levels in drained dialysate could be used in combination with PET, MMP-2, and CA125 level in drained dialysate to evaluate peritoneal injury.
Conclusion
In conclusion, the results of the present study suggest that 8-OHdG level in drained dialysate may be a useful novel marker of peritoneal damage in PD.
Disclosure
The authors report no conflicts of interest in this work.
References
- KredietRTAdvances in peritoneal dialysisMinerva Urol Nefrol200759325126017912222
- BurkartJPirainoBKaldasHWhy is the evidence favoring hemodialysis over peritoneal dialysis misleading?Semin Dial200720320020217555481
- Van BiesenWVanholderRLameireNThe role of peritoneal dialysis as the first-line renal replacement modalityPerit Dial Int200020437538311007366
- HungKYHuangJWTsaiTJChenWYNatural changes in peritoneal equilibration test results in continuous ambulatory peritoneal dialysis patients: a retrospective, seven year cohort surveyArtif Organs200024426126410816198
- WilliamsJDCraigKJTopleyNMorphologic changes in the peritoneal membrane of patients with renal diseaseJ Am Soc Nephrol200213247047911805177
- DaviesSJPhillipsLNaishPFRussellGIPeritoneal glucose exposure and changes in membrane solute transport with time on peritoneal dialysisJ Am Soc Nephrol20011251046105111316864
- GandhiVCHumayunHMIngTSSclerotic thickening of the peritoneal membrane in maintenance peritoneal dialysis patientsArch Intern Med19801409120112037406618
- KawanishiHEncapsulating peritoneal sclerosisNephrology (Carlton)200510324925515958037
- KawaguchiYSaitoAKawanishiHRecommendations on the management of encapsulating peritoneal sclerosis in Japan, 2005: diagnosis, predictive markers, treatment, and preventive measuresPerit Dial Int200525Suppl 4S83S9516300277
- TwardowskiZJNolphKDKhannaRLimitations of the peritoneal equilibration testNephrol Dial Transplant19951011216021618643196
- MortierSFaictDSchalkwijkCGLameireNHDe VrieseASLong-term exposure to new peritoneal dialysis solutions: effects on the peritoneal membraneKidney Int20046631257126515327425
- LeeHBYuMRSongJSHaHReactive oxygen species amplify protein kinase C signaling in high glucose-induced fibronectin expression by human peritoneal mesothelial cellsKidney Int20046541170117915086456
- NohHKimJSHanKHOxidative stress during peritoneal dialysis: implications in functional and structural changes in the membraneKidney Int200669112022202816641917
- KihmLPMüller-KrebsSKleinJBenfotiamine protects against peritoneal and kidney damage in peritoneal dialysisJ Am Soc Nephrol201122591492621511829
- HiraharaIInoueMOkudaKAndoYMutoSKusanoEThe potential of matrix metalloproteinase-2 as a marker of peritoneal injury, increased solute transport, or progression to encapsulating peritoneal sclerosis during peritoneal dialysis: a multicentre study in JapanNephrol Dial Transplant200722256056717035369
- HiraharaIInoueMUminoTSaitoOMutoSKusanoEMatrix metalloproteinase levels in the drained dialysate reflect the peritoneal solute transport rate: a multicentre study in JapanNephrol Dial Transplant20112651695170120921293
- TarngDCHuangTPWeiYH8-hydroxy-2′-deoxyguanosine of leukocyte DNA as a marker of oxidative stress in chronic hemodialysis patientsAm J Kidney Dis200036593494411054349
- AustinEWParrishJMKinderDHBullRJLipid peroxidation and formation of 8-hydroxydeoxyguanosine from acute doses of halogenated acetic acidsFundam Appl Toxicol199631177828998956
- GotloibLWajsbrotVCupermanYShostakAAcute oxidative stress induces peritoneal hyperpermeability, mesothelial loss, and fibrosisJ Lab Clin Med20041431314014749683
- RodriguesASAlmeidaMFonsecaIPeritoneal fast transport in incident peritoneal dialysis patients is not consistently associated with systemic inflammationNephrol Dial Transplant200621376376916332703
- RodriguesASMartinsMKorevaarJCEvaluation of peritoneal transport and membrane status in peritoneal dialysis: focus on incident fast transportersAm J Nephrol2007271849117284895
- Van EschSZweersMMJansenMAde WaartDRvan ManenJGKredietRTDeterminants of peritoneal solute transport rates in newly started nondiabetic peritoneal dialysis patientsPerit Dial Int200424655456115559485