Introduction
Diabetes is a leading cause of end-stage renal disease worldwide, and chronic kidney disease (CKD) is estimated to occur in nearly half of patients with type 2 diabetes (T2D) and one-third of patients with type 1 diabetes.Citation1 Glucagon-like peptide-1 receptor agonists (GLP-1 RA) are a vital therapy for glycemic control in T2D, and is being studied for improving renal outcomes in T2D and CKD. In existing clinical trials, dulaglutide was effective in improving glycemic control, but there were limited patients with moderate to advanced CKD included in these studies.Citation2 This need is further highlighted in recent Kidney Disease Improving Global Outcomes guidelines, which calls for further studies on the efficacy and safety of GLP-1 RA in the setting of moderate-advanced CKD.Citation3 Existing real-world studies on dulaglutide have limitations including a homogenous cohort and limited patients with stage 4–5 CKD.Citation4,Citation5 To meet this gap, we conducted a retrospective study of the tolerance and efficacy of dulaglutide in patients with diabetes and moderate-advanced CKD.
Materials and Methods
This retrospective observational study was approved by the Washington University Institutional Review Board (IRB). A full waiver for patient consent prior to review of records was obtained from the IRB as the study posed no more than minimal risk to individual privacy. Patient data confidentiality was maintained, and extracted data were de-identified before analysis. This study complies with the Declaration of Helsinki. We retrospectively identified outpatient dulaglutide prescriptions in patients with T2D, CKD stages 3–5 based on estimated glomerular filtration rate (eGFR), and dulaglutide use for ≥3 months from 2016 to 2023 across the Barnes Jewish Hospital system. T2D was diagnosed based on clinical diagnosis and/or hemoglobin A1c (HbA1c). Primary outcomes included patient reported side effects (gastrointestinal (GI) or allergic reactions) and hypoglycemia as documented in provider notes. Additional outcomes included change in HbA1c, weight, and body mass index (BMI) after first year of use. Continuous variables were presented as mean ± standard deviation. Fisher’s exact test was used to compare categorical CKD stage if the patient stopped due to a side effect. Chi-squared test for equal proportions was used to test for an overall difference among the three stages. Continuous variables were assessed by paired t-test. Results were significant if P≤0.05. Analyses were completed with SAS v9.4, (Cary, NC, USA).
Results
One hundred forty-nine patients with CKD received dulaglutide from 2016–2023. Ninety-seven percent of patients had T2D while 3% had posttransplant diabetes. Mean age was 68.2 ± 9.6 years, 90 (60%) were female. Seventy-eight (52%) patients identified as white, 67 (45%) as black, and four (3%) as other. One hundred nine (73%) patients had concomitant use of angiotensin-converting enzyme inhibitor or angiotensin II receptor blockers, and 28 (19%) used sodium glucose co-transporter 2 inhibitors. Over a treatment duration of 28.5 ± 21.5 months, the mean eGFR changed from 27.8 ± 12.8 to 18.6 ± 7.5 mL/min/1.73 m2 (P<0.05). At the start of the treatment, four (3%) were stage 1–2, 42 (28%) were stage 3, 91 (61%) of patients were stage 4, and 12 (8%) stage 5 while at the end of period, 97 (65%) of patients were stage 4 and 48 (32%) stage 5 (P<0.05).
To evaluate for side effects, we found 28 (19%) of all patients with moderate-severe CKD (stage 3–5) dexperienced one or more side effects (). There was no difference in the frequency of side effects based on stage of CKD (P=0.747). The most common side effects were nausea/vomiting in 16 (57%), diarrhea/constipation in nine (32%), while a smaller cohort had serious side effects including cholecystectomy in three (11%) or pancreatitis in one (4%). Of the 28 patients who experienced side effects, 11 discontinued dulaglutide due to the side effect after an average of 21.1 ± 18.7 months, most commonly due to nausea/vomiting.
Table 1 Side Effects and Hypoglycemia with Dulaglutide
We further evaluated the rate of hypoglycemia in the study population (). Forty-one (28%) of the total population experienced hypoglycemia leading to two patients discontinuing dulaglutide. Of these, 22 (54%) were CKD stage 4, six (15%) stage 5, 10 (24%) stage 3, and three (7%) had unspecified CKD stage (P=0.004) at the time of the hypoglycemic event. From this cohort, 11 (27%) had level 1 hypoglycemia, 13 (32%) with level 2, and 16 (39%) had hypoglycemia of an unspecified level. Importantly, one patient had level 3 hypoglycemia which required external assistance for recovery. Nearly all patients experiencing hypoglycemia had concomitant use of insulin or sulfonylureas (98%).
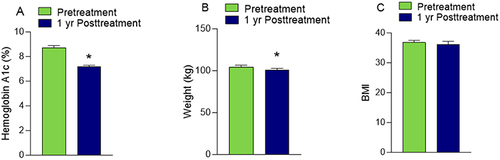
Finally, we evaluated clinical outcomes after the first year of dulaglutide use for the subset of the population with available data. After 1 year, for 128 patients, there was a 1.5% reduction in mean HbA1c from 8.7 ± 2.1 to 7.2 ± 1.3 (P<0.05) (). Furthermore, for 131 patients, there was a 3.7 kg reduction in weight from an average of 104.4 ± 25.1 kg to 100.7 ± 23.8 kg (P<0.05), but there was no significant change in BMI.
Discussion
We present real-world data from a large tertiary-care institution of the tolerability of dulaglutide in patients with diabetes and moderate-advanced CKD from 2016–2023. We found 19% of all patients experienced a side effect during treatment with the most common gastrointestinal-related. We found a small subset of patients experienced serious side effects including cholecystectomy or pancreatitis. There was a high rate of hypoglycemia in nearly one-third of patients with the highest occurrence in patients with stage 4 or 5 CKD. Nearly all patients who developed hypoglycemia had concomitant use of insulin or sulfonylureas. Furthermore, dulaglutide was effective in improving glycemic control with 1.5% reduction in A1c and 3.7 kg weight reduction.
Our study contributes to the existing observational studies on long-acting GLP-1 RA in moderate-advanced CKD, but has several differences. In an observational study by Kim et al, they found dulaglutide had a higher (31.9%) rate of side effects with 5.1% leading to discontinuation, which was higher than the 19% in our study.Citation4 Furthermore, they found a 0.9% A1c reduction compared to 1.5% in our study. Several factors may contribute to these differences including longer study duration, higher rates of patients with moderate-advanced CKD, and inclusion of a more heterogeneous study population in our study compared to the study by Kim et al.Citation4 Another large observational study evaluating GLP-1 RA use in moderate-advanced CKD by Chen et al also was conducted in an Asian population and did not include side effects as a primary outcome.Citation5 Our study highlights the need for future randomized clinical trials with dulaglutide which includes patients with diabetes and moderate-advanced CKD to assess side effects during GLP-1 RA use.
Our study has several limitations including the small sample size from a single institution, which limits its generalizability. Also, the retrospective study limits our ability to conclude all endpoints are directly due to dulaglutide and not confounders. Finally, we did not account for effects of concomitant medications including sodium-glucose cotransporter-2 inhibitors, which can contribute to changes in renal function on initiation.
Conclusion
Dulaglutide is an effective therapy for patients with diabetes and moderate-advanced CKD in the real-world setting. Hypoglycemia occurrence is prevalent in patients with baseline insulin or sulfonylurea use after dulaglutide initiation. Providers should consider appropriate reductions of insulin or discontinuation of sulfonylureas on dulaglutide initiation to prevent this risk. Future randomized trials should include more patients with stage 4–5 CKD to further assess side effects in this population.
Disclosure
RMZ is a sub-investigator for the Bayer CONFIDENCE study and reports grants from American Heart Association, outside the submitted work. The authors report no other conflicts of interest in this work.
Acknowledgments
Statistical analysis was kindly performed by Julia Huecker, MS, via the Biostatistics Consulting Core at Washington University.
Additional information
Funding
References
- Hoogeveen EK. The epidemiology of diabetic kidney disease. Kidney Dial. 2022;2(3):433–442. doi:10.3390/kidneydial2030038
- Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–617. doi:10.1016/S2213-8587(18)30104-9
- Rossing P, Caramori ML, Chan JC, et al. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5S):S1–S127.
- Kim S, An JN, Song YR, et al. Effect of once-weekly dulaglutide on renal function in patients with chronic kidney disease. PLoS One. 2022;17(8):e0273004. doi:10.1371/journal.pone.0273004
- Chen J, Wu C, Jenq C, et al. Association of glucagon-like peptide-1 receptor agonist vs dipeptidyl peptidase-4 inhibitor use with mortality among patients with type 2 diabetes and advanced chronic kidney disease. JAMA Network Open. 2022;5(3):e221169. doi:10.1001/jamanetworkopen.2022.1169