Abstract
Retained placenta after vaginal delivery is diagnosed when a placenta does not spontaneously deliver within a designated amount of time, variably defined as a period of 18–60 mins. It may also be diagnosed if a patient experiences significant hemorrhage prior to delivery of the placenta. Normal placenta delivery requires adequate uterine contractions, with shearing of the placenta and decidua from the uterine wall and expulsion of the tissue. Thus, retained placenta can occur in the setting of significant uterine atony, abnormally adherent placenta, as with placenta accreta spectrum (PAS), or closure of the cervix prior to placental expulsion. Risk factors for retained placenta parallel those for uterine atony and PAS and include prolonged oxytocin use, high parity, preterm delivery, history of uterine surgery, and IVF conceptions. History of a prior retained placenta and congenital uterine anomalies also appear to be risk factors. Management entails manual removal of the placenta with adequate analgesia, as medical intervention alone has not been proven effective. Complications can include major hemorrhage, endometritis, or retained portions of placental tissue, the latter of which can lead to delayed hemorrhage or infection. Prophylactic antibiotics can be considered with manual placenta removal, though evidence regarding effectiveness is inconsistent. If hemorrhage is encountered, deployment of a massive transfusion protocol, uterine evacuation with suction, and use of intrauterine tamponade, as with an intrauterine balloon, should be initiated immediately. When a separation plane between the placenta and uterus is particularly difficult to create, PAS should be considered, and preparations should be made for hemorrhage and hysterectomy. Patients with risk factors for retained placenta should have a laboratory sample sent for blood type and antibody screening on admission to labor and delivery, and plans should be made for appropriate analgesia and preparations for hemorrhage if a retained placenta is encountered.
Introduction
Retained placenta after vaginal delivery, which occurs in around 1–3% of deliveries, is a relatively common cause of obstetrical morbidity. This is typically diagnosed when the placenta fails to spontaneously separate during the third stage of labor when a patient experiences excessive bleeding in absence of placenta separation or if there is confirmation of placenta tissue remaining after the majority of the placenta delivers spontaneously.Citation1–Citation3 Placentas that fail to spontaneously separate can be a cause of significant surgical and hemorrhagic morbidity.Citation4,Citation5 Untreated, retained placenta is considered the second leading cause of postpartum hemorrhage (PPH).Citation5,Citation6
Although retained placenta is an obstetrical complication encountered relatively infrequently on the labor and delivery floor, recognizing patient risk factors and understanding management are important steps in mitigating this morbidity.
Pathophysiology
Normal placentation begins with blastocyst implantation into the maternal endometrium. In preparation for this implantation, the endometrium develops the decidua under the influence of progesterone and estrogen in early pregnancy. As the blastocyst invades this decidua, the layer of cells forming the surface of the blastocyst develops into the chorionic membrane. Cytotrophoblast cells proliferate from the chorionic membrane and form multinucleated aggregates called syncytiotrophoblast cells. These cells form the placental villi, allowing fetal–maternal interchange between the villi–decidual interaction. With delivery of the infant, both a hormonal cascade and uterine contractions allow for separation of these layers and expulsion of the placenta.Citation7
Retained placenta is generally attributed to one of three pathophysiologies. First, an atonic uterus with poor contraction may prevent normal separation and contractile expulsion of the placenta.Citation2,Citation8,Citation9 Second, an abnormally adherent or invasive placenta, as seen with placenta accreta spectrum (PAS), may be incapable of normal separation. Finally, a separated placenta may be trapped or incarcerated due to closure of the cervix prior to delivery of the placenta.Citation2,Citation8–Citation10 Placental hypoperfusion disorders, such as with preeclampsia, and infection have also been proposed as mechanisms for retained placenta, although little is known about the specific mechanism.Citation9,Citation11
Epidemiology
Estimates of retained placenta put the incidence at between 0.1% and 3%.Citation5,Citation8 Prospective investigations of retained placenta confirm these estimates, with one study of >45,000 patients showing that overall for all gestational ages, retained placenta happened in about 3% of deliveries, with gestational ages of <26 weeks and <37 weeks having a significantly increased risk of retained placenta requiring manual removal.Citation1 Generally, incidence seems to be higher in developed countries where practices tend toward earlier manual removal of the placenta in the third stage of labor.Citation8,Citation12
Risk factors
Many studies have attempted to define risk factors for retained placenta, which are listed in . Established risk factors include prior retained placenta, preterm delivery, prior uterine surgery, previous pregnancy termination, miscarriage or curettage, grand multiparity (greater than five prior deliveries), and congenital uterine anomalies (often unrecognized prior to delivery).Citation3,Citation5,Citation11
Table 1 Risk factors for retained placenta
Some studies have suggested that prolonged oxytocin use could be a potentially modifiable risk factor for retained placenta, with one study reporting that oxytocin use for over 195 mins increased the odds ratio of the retained placenta by 2.0, and oxytocin use over 415 mins increased the odds ratio by 6.5.Citation5 It is less clear whether oxytocin is directly involved in placental retention, or if the association is mediated by uterine atony or infection due to prolonged labor.
Placental under perfusion disorders have been implicated as risk factors for retained placenta.Citation11 In a case–control study of all singleton primiparous vaginal deliveries in Sweden between 1997 and 2009, the authors found an increased association between placental under perfusion disorders (such as preeclampsia, small for gestational age, and stillbirth) and retained placenta; however, they could not designate a common pathophysiology.
Some research suggests that women may be predisposed to retained placenta. Retained placenta in a prior delivery appears to be an important risk factor for recurrence. In one study of over 280 women in Denmark, prevalence of retained placenta was found to be consistent with previously reported numbers (approximately 3%) using strict diagnostic criteria. The authors found that in subsequent vaginal deliveries, the risk of recurrence was substantially increased to about 25%.Citation3 There has even been some suggestion that tendency toward retained placenta may even be inherited. In one study, authors used the Swedish Medical Birth Register to identify women with retained placenta after 1992 whose mothers’ own birth records were also in the Register (after 1973). The authors found that the risk of retained placenta increased if retained placenta had occurred at the mother’s own birth (aOR 1.66 95% CI 1/52–1/82), at the birth of one of her siblings (aOR 1.58, 95% CI 1.43–1.76), or both (aOR 2.75, 95% CI 2.18–3.46).Citation13
Because of its relationship to PAS, assisted reproductive technologies (IVF or ICSI) have been proposed and studied as an additional risk factor for retained placenta.Citation14 Elenis et al, in a 2015 study from Sweden, looked specifically at oocyte donation IVF and the risk of poor obstetrical outcomes in otherwise healthy women.Citation15 The authors found a positive association between retained placenta and oocyte donation, as well as between PPH and oocyte donation.Citation15 In another 2016 study by Aziz et al, seeking to determine whether or not length of third stage was related to IVF, the authors concluded that cryopreserved embryo transfer (donated or autologous) without controlled ovarian hyperstimulation was not related to longer third stage, but did significantly increase the risk for manual removal of the placenta.Citation16
Morbidity
Retained placenta requiring invasive procedures is associated with obstetrical morbidities. Of arguably greatest significance is the risk of postpartum hemorrhage, with retained placenta the second leading cause of significant and even fatal hemorrhage in the obstetric population.Citation5,Citation17 One group found that the odds ratio related to estimated blood loss exceeding 500 mL, 1000 mL, and 2000 mL with retained placenta, respectively, is as high as 33.07 (95% CI 20.57–53.16), 43.44 (95% CI 26.57–71.02), and 111.24 (95% CI 27.26–454.00).Citation5 In another case–control study of 114 women with manual removal for retained placenta, the authors found that the case group required significantly more blood transfusions (13% in the case group versus 0% in the controls).Citation18 Large cohort studies have confirmed this elevated risk.Citation17
Further research additionally suggests that the longer the third stage of labor, the greater the risk of postpartum hemorrhage.Citation19 A study by Dombrowski et al in 1995 tried to determine gestational age–specific data for the length of the third stage, retained placenta, hemorrhage, and manual removal. The authors found that both manual removal of the placenta and PPH decreased with increasing gestational age, and that the two were related. However, causal association could not be determined.Citation1
If the placenta or pieces of the placenta remain in situ following attempt at manual removal, a patient may require surgical management. In a study of >20,000 patients in Norway, 3% of women requiring manual removal of retained placenta needed additional surgical management with dilation and curettage.Citation17 Another case–control study of 114 women found that cases required more dilation and curettage than controls, although with their study number they could not confirm significance.Citation18 Occasionally portions of the placenta or membranes may remain in the uterus after manual extraction, leading to delayed complications from retained products of conception. These can include delayed postpartum hemorrhage or endomyometritis.
Evidence of infection risk, particularly endometritis, following manual or surgical removal of retained placenta has been inconsistently demonstrated.Citation20 A large 1995 retrospective cohort study at University of Iowa compared over 1000 patients requiring manual extraction after vaginal delivery with those who did not.Citation20 After controlling for confounders, the authors found that manual removal of retained placenta was significantly associated with postpartum endometritis.Citation20 Alternatively, in the large cohort study of >20,000 patients from Norway mentioned above, patients requiring intervention for retained placenta did not show a significantly increased risk of infection, despite varying practices regarding antibiotic administration and timing.Citation17 Other studies have similarly found a relationship but could not prove a significant association between manual removal or surgical placental removal and endometritis.Citation18,Citation21 The discrepancies may in part be due to the lack of rigorous distinction between postpartum fever and true uterine infection.
Diagnosis
Retained placenta is clinically diagnosed when the placenta fails to spontaneously separate during the third stage of labor, with or without active management, or in the setting of severe bleeding in the absence of placental delivery.Citation18,Citation22 The first diagnostic criterion requires a time cutoff, though there is no uniform consensus as to timing for diagnosis of retained placenta in the third stage in the absence of postpartum hemorrhage. Selection of a clinical time definition can be based either on a population curve of observed spontaneous placental delivery times or on a time at which morbidity significantly increases. Thirty minutes have been used as a loose guideline, which comes from a 1991 study by Combs et al.Citation2 The researchers found that the third stage had a log-normal distribution, with a mean length of 6.8 minutes, with only 3.3% of deliveries having greater a greater than 30 minutes third stage. This timing has been supported by other studies as well.Citation8 Interestingly, the authors calculated that the incidence of PPH, transfusion, and dilation and curettage remained constant during this period, increasing only after 30 minutes and plateauing at 75 minutes for both manually and spontaneously delivered placentas. Because PPH incidence did not increase until after 30 minutes, Combs et al recommended this timing for initiation of manual removal of the placenta.
However, this guidance is not uniformly supported. In a subsequent study by Deneux-Tharaux, surveys from 14 European countries exhibited wide variations in wait time prior to manual placental removal, largely by country but also by the hospital.Citation23 In countries such as Finland and Denmark, obstetricians tended to wait 60 minutes or more prior to manual removal of the placenta, versus in countries such as Spain and France, where providers removed the placenta after 30 minutes. Practices also varied considerably depending on whether or not the patient in question had prior epidural anesthesia.Citation23 National and worldwide guidelines similarly have no consensus on when to intervene on an undelivered placenta. For instance, the National Institute for Health and Clinical Excellence suggests a wait time of 30 minutes in the United Kingdom prior to manual removal of the placenta,Citation24 while the World Health Organization guidelines propose a wait time of 60 minutes.Citation12,Citation25
The most significant risk of waiting a prolonged amount of time before removing the placenta is postpartum hemorrhage. In 2005, Magann and colleagues undertook a prospective observational study in which all women delivering vaginally were assessed for PPH.Citation15 Using receiver operating characteristic curves, the authors showed that 95% of normal placental delivery occurs within 18 minutes, and that a third stage of labor longer than 18 minutes was associated with a significant risk of PPH.Citation19 The authors followed up this paper in 2012 with a randomized controlled trial assigning vaginal deliveries to manual removal at either 10 or 15 minutes (as opposed to the traditional 30) if the placenta had not yet spontaneously delivered.Citation26 The findings supported the authors’ initial study, showing that removal at 15 minutes had a significantly greater likelihood of hemorrhage compared to 10 minutes, opening up the discussion on earlier intervention.Citation26
At times the bulk of the placenta will deliver spontaneously or manually, but small portions or an accessory lobe may be retained. This may be suspected when the placenta appears fragmented after delivery or when there is ongoing heavy uterine bleeding. In this situation, the uterine cavity may be evaluated with manual exploration or with ultrasound. The utility of ultrasound in this situation has yet to be established, with a focal endometrial mass, particularly with Doppler flow, being the findings of interest. In one study of routine ultrasound immediately after vaginal delivery, the sensitivity for diagnosing retained placental fragments was only 44% with a positive predictive value (PPV) of 58%.Citation27 An alternate study showed a 75–80% sensitivity of postpartum ultrasound, though the mean time for evaluation was 21 days postpartum, when less blood and decidua are expected to be seen.Citation28 While immediate ultrasound’s PPV will be higher when there is clinical suspicion of retained POCs, a negative ultrasound should not deter manual or suction curettage when there is a strong clinical suspicion, especially in the setting of hemorrhage.
Management
After delivery of the infant and prior to diagnosis of retained placenta, active management is recommended to facilitate spontaneous placental separation, including oxytocin, controlled cord traction, and uterine massage.Citation4 These maneuvers have been shown to decrease the risk of postpartum hemorrhage, though it has not been shown that active management will prevent retained placenta.Citation22
Once diagnosed, the placenta is usually manually extracted from the uterus.Citation22,Citation29 lists items that should be readily available if needed during the extraction process. Because this procedure is painful, adequate analgesia should be achieved via epidural, conscious sedation, or general anesthesia prior to an attempt at extraction. Once the patient is comfortable, she should be appropriately positioned in lithotomy. A conical drape, preferably one that is graduated and marked to allow for quantitative blood loss, should be placed under the patient’s buttocks. The operator should make every attempt to wear gown and gloves and maintain sterility, both for personal and for patient protection. The patient’s bladder should be drained. The provider should then use one hand to follow the umbilical cord through the vagina and cervix until the placenta is palpated. If the placenta is separated but not expelled, such as in the case of uterine atony, the tissue can be firmly grasped and brought through the cervix. Uterotonic medications, such as oxytocin, methylergonovine, carboprost, or other prostaglandins, should be given to facilitate contraction once the placenta is removed.Citation4
Table 2 Items that should be available for manual placental extraction
Nitroglycerine (NTG) has been used to facilitate manual extraction by relaxing uterine smooth muscle.Citation30 This may be particularly helpful when the placenta is trapped behind a partially closed cervix, though the use of NTG alone does not appear to facilitate spontaneous placental expulsion.Citation31 It can be given as a 1 mg sublingual dose, or as sequential 50 mcg intravenous boluses, up to a total dose of 200 mcg. The medication can produce hypotension and tachycardia, which can confound assessments of maternal stability. Once the placenta is delivered, uterotonics should be promptly given to restore uterine tone and avoid significant atony.
If the placenta remains attached to the uterine decidua, an attempt should be made to separate it manually. Using one hand to provide counter pressure on the fundus through the maternal abdomen, the provider should then use the internal hand to manually create a cleavage plane between the placenta with the attached decidua and the myometrium. Once separated, the placenta can be removed as described above. If a separation plane cannot be created behind all or part of the placenta, the provider should suspect a morbidly adherent placenta (MAP) and prepare for potential hemorrhage.
If placental removal is refractory or only partially successful (ie the placenta or parts of the placenta remain in the uterus), or if bleeding persists despite placental delivery, often the next step is surgical management with curettage. This may be best achieved in an operating room, with optimal access to surgical equipment, analgesia, and patient resuscitation aids, if needed. Suction curettage is generally used, though a sharp curette may be needed to facilitate a separation plane. Access to uterine tamponade supplies with either a large intrauterine balloon or surgical packs should be immediately accessed in the event of hemorrhage. Crossmatched blood products should be made imminently available if placental separation is difficult or blood loss exceeds 1 L, and the care team should attend to uterotonic administration and attention to coagulopathy as the extraction is performed.Citation4
Due to the risk of endometritis, routine antibiotics are generally administered just before or shortly after manual removal of the placenta.Citation20 Prophylaxis can parallel cesarean prophylaxis with a first-generation cephalosporin. Patients who are febrile at the time of extraction should be fully treated for chorioamnionitis with broad-spectrum antibiotics.Citation32 Despite these guidelines, few studies have been undertaken examining the effectiveness of antibiotics in reducing infectious morbidity. A 2015 systematic review by Chibueze and colleagues attempted to summarize the literature on the efficacy of antibiotics for preventing adverse maternal outcomes related to manual placenta removal following vaginal birth.Citation21 The authors reported on three retrospective cohort studies examining endometritis and puerperal fever after manual extraction for retained placenta. None of the three studies found evidence to suggest beneficial effects for routine antibiotic use in women undergoing intervention for retained placenta. The authors concluded that further research is required to adequately answer this question.Citation21 Due to mixed data regarding prophylaxis, as well as the increasing risk of postpartum hemorrhage with prolonged third stage of labor, administration of antibiotics should not delay manual removal of retained placenta.
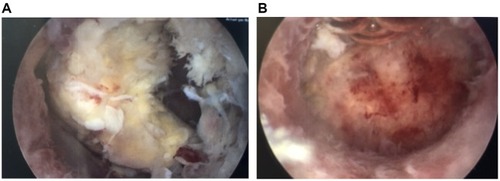
Occasionally, a portion of placental tissue may remain in the uterus, either knowingly or unbeknownst to the providers. This can present as abnormal bleeding days to weeks after delivery and should be suspected in the setting of a delayed postpartum hemorrhage. Recently, studies have examined the usefulness of hysteroscopic morcellation devices in aiding with retained placenta left in situ postpartum ( [before] and [after]). In a series of case reports, Lee and colleagues reported a higher risk of complications with blind curettage compared to hysteroscopic morcellation.Citation33 They additionally reported complete resection in 90% of hysteroscopic cases and reduction of both perforation and intrauterine adhesion risk.Citation33 In another randomized control trial by Hamerlynck et al, the authors randomized patients to undergo hysteroscopic resection of retained placenta with either hysteroscopic morcellation versus loop resection with rigid bipolar resectoscopes.Citation34 These authors in comparison found that when comparing the two modalities, complete resection was comparatively high in both groups, and intrauterine adhesions were comparatively low.Citation34 The one significant difference between the two groups was that the hysteroscopic group had significantly faster operative times.Citation34 The ability to perform hysteroscopic removal depends on the amount of active bleeding, with suction curettage often needed when bleeding is heavy.
Figure 1 The photo on the left (A) shows a retained portion of placenta approximately 8 weeks after delivery. The photo on the right (B) shows the same uterus following hysteroscopic morcellation of the retained placenta.
Other studies have examined alternative, nonsurgical, management for retained placenta, none of which have been successful. In 2012, 99 women in a large teaching hospital in the Netherlands with retained placenta (>60 mins after delivery) were given either 800 mcg misoprostol or placebo orally.Citation35 The author’s primary outcomes were number of manual removals of retained placenta and blood loss. The authors found that oral misoprostol reduced neither the need for manual removal nor the overall amount of blood loss. Both groups were observed for additional 45 mins after administration of misoprostol or placebo. While the authors found that 50% of remaining placentas at 60 mins delivered in the intervening 45 mins, it came at the expense of additional significant blood loss.Citation35
For a time, umbilical vein oxytocin was thought to be a promising alternative or adjunct to manual extraction of the placenta. A 2011 Cochrane Review summarized available data on the subject to assess the use of umbilical vein oxytocin either alone or in conjunction with intravenous oxytocin to reduce the need for manual removal of retained placenta.Citation36 While inexpensive and easy to do, the authors found that all well-designed randomized control trials showed no significant effect of umbilical vein oxytocin on retained placenta.Citation36
Morbidly adherent placenta
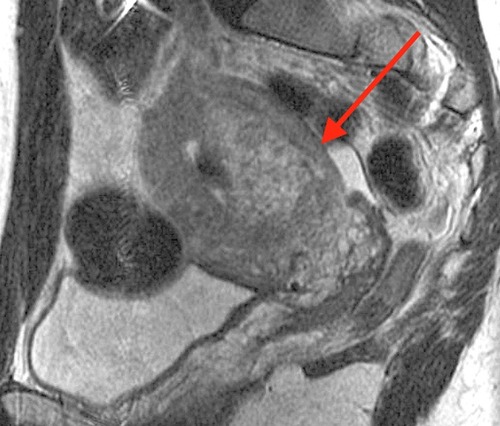
In the unusual event that manual extraction does not result in delivery of the entire or partial placenta, MAP must be considered as an etiology. The PAS, which includes accreta, increta, or percreta, can be causes of significant surgical and hemorrhagic morbidity on the labor and delivery floorCitation4,Citation37 (). While PAS is relatively rare, particularly in the absence of a placenta previa, it can occur at vaginal delivery when there is no previa. Given the excess morbidity, providers should consider this pathology when a placenta is retained in the setting of significant PAS risk factors. These include prior uterine surgeries, including hysteroscopic resections, IVF conception, a history of intrauterine adhesions, or a prior history of MAP or pathologic findings of accreta.Citation38,Citation39
Figure 2 Magnetic resonance image showing a portion of retained placenta 6 weeks postpartum. The arrow indicates an area where the light-gray placenta is deeply invasive into the darker-gray myometrium. Placenta accreta spectrum was confirmed pathologically following hysterectomy.
When a separation plane cannot be created or extraction attempts begin to invert the uterus, MAP should be suspected. In this case, further attempts to extract the placenta should cease, as forcible removal of a MAP can lead to massive hemorrhage.Citation40 At this point, consideration should be made for hysterectomy, which will be necessary if the patient has an undeliverable placenta with significant hemorrhage. Alternative treatment has been described including expectant management or uterine conservation.Citation41 Expectant management has been described in small studies and refers to the placenta left in situ after diagnosis of PAS.Citation38,Citation41,Citation42 Such management requires careful patient selection and counseling, as this risks delayed hemorrhage or infection. Nevertheless, successful conservative management has been described, with placental expulsion, resorption, or removal at a median of 3 months and up to 1 year postpartum.Citation43
Uterine conservation with placental removal is an alternative technique that likewise has been described in only small studies. This refers to resection of the placental bed at the area of suspected PAS and requires conversion to laparotomy after vaginal delivery.Citation41 The resultant defect in theory can be repaired via over-sewing and/or uterine repair or alternatively attempting tamponade with a Bakri balloon. Only one small study has evaluated the latter in a randomized control trial, and only with the lesser invasive types of PAS.Citation41,Citation44
Conclusion
Retained placenta after vaginal delivery can be a source of significant hemorrhagic and surgical morbidity to the mother. In considering ways to lesson morbidity, the clinician should have a knowledge of risk factors for both retained placenta and MAP, allowing them to triage those patients most at risk of hemorrhage and prepare by ensuring blood products are easily available. When managing the patient with retained placenta, 30 minutes of elapsed third stage have been traditionally used as a guideline for timing manual removal; however, recent research has suggested that shorter duration of third stage may in fact be less morbid. Further research should be pursued to determine the best timing and infection prophylaxis for this etiology. Regardless, prompt diagnosis and management with appropriate personnel, access to blood for massive transfusion protocol, and surgical equipment such as uterine suction and tamponade can be required to treat retained placenta and lessen its morbidity.
Disclosure
The authors report no conflicts of interest in this work.
References
- Dombrowski MP, Bottoms SF, Saleh AA, Hurd WW, Romero R. Third stage of labor: analysis of duration and clinical practice. Am J Obstet Gynecol. 1995;172(4 Pt 1):1279–1284. doi:10.1016/0002-9378(95)91493-57726270
- Combs CA, Murphy EL, Laros RK. Factors associated with postpartum hemorrhage with vaginal birth. Obstet Gynecol. 1991;77(1):69–76.1984230
- Nikolajsen S, Løkkegaard ECL, Bergholt T. Reoccurrence of retained placenta at vaginal delivery: an observational study. Acta Obstet Gynecol Scand. 2013;92(4):421–425. doi:10.1111/j.1600-0412.2012.01520.x22882191
- American College of Obstetricians and Gynecologists. ACOG practice bulletin: clinical management guidelines for obstetrician-gynecologists number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039–1047. doi:10.1097/01.AOG.0000214671.19023.6817012482
- Endler M, Grünewald C, Saltvedt S. Epidemiology of retained placenta: oxytocin as an independent risk factor. Obstet Gynecol. 2012;119(4):801–809. doi:10.1097/AOG.0b013e31824acb3b22433344
- Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368–1373.20237047
- Kraus FT, Redline RW, Gersell DJ, Nelson DM, Dicke JM. Placental Pathology. American Registry of Pathology in collaboration with the Armed Forces Institute of Pathology. Atlas Nontumor Pathol. 2004;1:10–16.
- Urner F, Zimmermann R, Krafft A. Manual removal of the placenta after vaginal delivery: an unsolved problem in obstetrics. J Pregnancy. 2014;2014:274651. doi:10.1155/2014/23940624812585
- Greenbaum S, Wainstock T, Dukler D, Leron E, Erez O. Underlying mechanisms of retained placenta: evidence from a population based cohort study. Eur J Obstet Gynecol Reprod Biol. 2017;216:12–17. doi:10.1016/j.ejogrb.2017.06.03528692888
- Kramer MS, Berg C, Abenhaim H, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209(5):449.e1–7. doi:10.1016/j.ajog.2013.07.007
- Endler M, Saltvedt S, Cnattingius S, Stephansson O, Wikström A-K. Retained placenta is associated with pre-eclampsia, stillbirth, giving birth to a small-for-gestational-age infant, and spontaneous preterm birth: a national register-based study. BJOG Int J Obstet Gynaecol. 2014;121(12):1462–1470. doi:10.1111/1471-0528.12752
- Joseph KS, Rouleau J, Kramer MS, et al. Investigation of an increase in postpartum haemorrhage in Canada. BJOG Int J Obstet Gynaecol. 2007;114(6):751–759. doi:10.1111/j.1471-0528.2007.01316.x
- Endler M, Cnattingius S, Granfors M, Wikström A-K. The inherited risk of retained placenta: a population based cohort study. BJOG Int J Obstet Gynaecol. 2018;125(6):737–744. doi:10.1111/1471-0528.14828
- Esh-Broder E, Ariel I, Abas-Bashir N, Bdolah Y, Celnikier DH. Placenta accreta is associated with IVF pregnancies: a retrospective chart review. BJOG Int J Obstet Gynaecol. 2011;118(9):1084–1089. doi:10.1111/j.1471-0528.2011.02976.x
- Elenis E, Svanberg AS, Lampic C, Skalkidou A, Åkerud H, Sydsjö G. Adverse obstetric outcomes in pregnancies resulting from oocyte donation: a retrospective cohort case study in Sweden. BMC Pregnancy Childbirth. 2015;8(15):247. doi:10.1186/s12884-015-0687-9
- Aziz MM, Guirguis G, Maratto S, Benito C, Forman EJ. Is there an association between assisted reproductive technologies and time and complications of the third stage of labor? Arch Gynecol Obstet. 2016;293(6):1193–1196. doi:10.1007/s00404-015-3943-326525699
- Tandberg A, Albrechtsen S, Iversen OE. Manual removal of the placenta. Incidence and clinical significance. Acta Obstet Gynecol Scand. 1999;78(1):33–36. doi:10.1080/j.1600-0412.1999.780108.x9926889
- Titiz H, Wallace A, Voaklander DC. Manual removal of the placenta–a case control study. Aust N Z J Obstet Gynaecol. 2001;41(1):41–44. doi:10.1111/ajo.2001.41.issue-111284645
- Magann EF, Evans S, Chauhan SP, Lanneau G, Fisk AD, Morrison JC. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105(2):290–293. doi:10.1097/01.AOG.0000159040.51773.bf15684154
- Ely JW, Rijhsinghani A, Bowdler NC, Dawson JD. The association between manual removal of the placenta and postpartum endometritis following vaginal delivery. Obstet Gynecol. 1995;86(6):1002–1006. doi:10.1016/0029-7844(95)00327-N7501321
- Chibueze EC, Parsons AJQ, Ota E, Swa T, Oladapo OT, Mori R. Prophylactic antibiotics for manual removal of retained placenta during vaginal birth: a systematic review of observational studies and meta-analysis. BMC Pregnancy Childbirth. 2015;26(15):313. doi:10.1186/s12884-015-0752-4
- Rogers J, Wood J, McCandlish R, Ayers S, Truesdale A, Elbourne D. Active versus expectant management of third stage of labour: the Hinchingbrooke randomised controlled trial. Lancet Lond Engl. 1998;351(9104):693–699. doi:10.1016/S0140-6736(97)09409-9
- Deneux-Tharaux C, Macfarlane A, Winter C, et al. Policies for manual removal of placenta at vaginal delivery: variations in timing within Europe. BJOG Int J Obstet Gynaecol. 2009;116(1):119–124. doi:10.1111/j.1471-0528.2008.01996.x
- National Collaborating Centre for Women’s and Children’s Health (UK). Intrapartum Care: Care of Healthy Women and Their Babies during Childbirth [internet] London: RCOG Press; 2007 [ cited Jun 2, 2019] (National Institute for Health and Clinical Excellence: Guidance) Available from: http://www.ncbi.nlm.nih.gov/books/NBK49388/. Accessed 93, 2019.
- Ronsmans C, Graham WJ; Lancet Maternal Survival Series steering group. Maternal mortality: who, when, where, and why. Lancet Lond Engl. 2006;368(9542):1189–1200. doi:10.1016/S0140-6736(06)69380-X
- Magann EF, Niederhauser A, Doherty DA, Chauhan SP, Sandlin AT, Morrison JC. Reducing hemodynamic compromise with placental removal at 10 versus 15 mins: a randomized clinical trial. Am J Perinatol. 2012;29(8):609–614. doi:10.1055/s-0032-131198522566115
- Carlan SJ, Scott WT, Pollack R, Harris K. Appearance of the uterus by ultrasound immediately after placental delivery with pathologic correlation. J Clin Ultrasound. 1997;25(6):301–308. doi:10.1002/(SICI)1097-0096(199707)25:6<301::AID-JCU3>3.0.CO;2-G9142625
- Durfee SM, Frates MC, Luong A, Benson CB. The sonographic and color Doppler features of retained products of conception. J Ultrasound Med Off J Am Inst Ultrasound Med. 2005;24(9):1181–6; quiz 1188–9.
- Rogers MS, Yuen PM, Wong S. Avoiding manual removal of placenta: evaluation of intra-umbilical injection of uterotonics using the Pipingas technique for management of adherent placenta. Acta Obstet Gynecol Scand. 2007;86(1):48–54. doi:10.1080/0001634060108857017230289
- Chedraui PA, Insuasti DF. Intravenous nitroglycerin in the management of retained placenta. Gynecol Obstet Invest. 2003;56(2):61–64. doi:10.1159/00007273412900527
- Abdel-Aleem H, Abdel-Aleem MA, Shaaban OM. Nitroglycerin for management of retained placenta. Cochrane Database Syst Rev. 2015;12(11):CD007708.
- Committee on Obstetric Practice. Committee opinion no. 712: intrapartum management of intraamniotic infection. Obstet Gynecol. 2017;130(2):e95–101. doi:10.1097/AOG.000000000000223628742677
- Lee MHM. Surgical management of retained placental tissue with the hysteroscopic morcellation device. Gynecol Minim Invasive Ther. 2019;8(1):33–35. doi:10.4103/GMIT.GMIT_66_1830783587
- Hamerlynck TWO, van Vliet HAAM, Beerens A-S, Weyers S, Schoot BC. Hysteroscopic morcellation versus loop resection for removal of placental remnants: a randomized trial. J Minim Invasive Gynecol. 2016;23(7):1172–1180. doi:10.1016/j.jmig.2016.08.82827590568
- van Stralen G, Veenhof M, Holleboom C, van Roosmalen J. No reduction of manual removal after misoprostol for retained placenta: a double-blind, randomized trial. Acta Obstet Gynecol Scand. 2013;92(4):398–403. doi:10.1111/aogs.1206523231499
- Nardin JM, Weeks A, Carroli G. Umbilical vein injection for management of retained placenta. Cochrane Database Syst Rev. 2011;11(5):CD001337.
- Bjurström J, Collins S, Langhoff-Roos J, et al. Failed manual removal of the placenta after vaginal delivery. Arch Gynecol Obstet. 2018;297(2):323–332. doi:10.1007/s00404-017-4579-229101608
- Carusi DA. The placenta accreta spectrum: epidemiology and risk factors. Clin Obstet Gynecol. 2018;61(4):733–742.30204619
- Roeca C, Little SE, Carusi DA. Pathologically diagnosed placenta accreta and hemorrhagic morbidity in a subsequent pregnancy. Obstet Gynecol. 2017;129(2):321–326. doi:10.1097/AOG.000000000000184328079779
- Kayem G, Davy C, Goffinet F, Thomas C, Clément D, Cabrol D. Conservative versus extirpative management in cases of placenta accreta. Obstet Gynecol. 2004;104(3):531–536. doi:10.1097/01.AOG.0000136086.78099.0f15339764
- Placenta accreta spectrum. Obstetric care consensus no. 7. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e259–e275. doi:10.1097/AOG.000000000000298330461695
- Fox KA, Shamshirsaz AA, Carusi D, et al. Conservative management of morbidly adherent placenta: expert review. Am J Obstet Gynecol. 2015;213(6):755–760. doi:10.1016/j.ajog.2015.04.03425935779
- Sentilhes L, Ambroselli C, Kayem G, et al. Maternal outcome after conservative treatment of placenta accreta. Obstet Gynecol. 2010;115(3):526–534. doi:10.1097/AOG.0b013e3181d066d420177283
- Pala Ş, Atilgan R, Başpınar M, et al. Comparison of results of Bakri balloon tamponade and caesarean hysterectomy in management of placenta accreta and increta: a retrospective study. J Obstet Gynaecol J Inst Obstet Gynaecol. 2018;38(2):194–199. doi:10.1080/01443615.2017.1340440
