Abstract
Background
Pre-eclampsia is a pregnancy-induced hypertension that occurs after 20 weeks of gestation. It is the leading cause of maternal and perinatal morbidity and mortality globally, but it is higher in developing countries. In Ethiopia, conducting research on the incidence and predictors of pre-eclampsia is crucial due to the paucity of information.
Methods
A prospective cohort study was undertaken using 242 pregnant women between November 1, 2018 and March 30, 2019 at Debre Markos Referral Hospital. All eligible women who fulfilled the inclusion criteria were included in this study. Data were entered into the epic-data Version 4.2 and analyzed using the STATA Version 14.0 software. The Cox-proportional hazard regression model was fitted and Cox-Snell residual test was used to assess the goodness of fit. Pre-eclampsia free survival time was estimated using the Kaplan–Meier survival curve. Both bivariable and multivariable Cox-proportional hazard regression models were fitted to identify predictors of pre-eclampsia.
Results
The overall incidence rate of pre-eclampsia was 3.35 per 100 person-years. Having a pre-existing history of diabetes mellitus [AHR=2.7 (95% CI=1.43–8.81)], having a history of multiple pregnancy [AHR=3.4 (95% CI=2.8–6.9)] and being ≥35 years old age [AHR=2.5 (95% CI=1.42–3.54)] were the significant predictors of pre-eclampsia.
Conclusion
The incidence of pre-eclampsia was high in this study. Having (pre-existing diabetes and multiple pregnancy) and being ≥35 years old age were the significant predictors of pre-eclampsia. Inspiring pregnant women’s health-seeking behavior should provide a chance to diagnose pre-eclampsia early to prevent the medical complication of pre-eclampsia.
Background
Pre-eclampsia is a pregnancy-induced hypertension, characterized by significant proteinuria, with or without edema, and resolves by the 12th post-partial weeks.Citation1 It is a life-threatening multisystem disorder of pregnant mothers and the leading cause of morbidity and mortality of perinatal.Citation2–Citation4 About ten million mothers each year develop pre-eclampsia worldwide.Citation5 The World Health Organization (WHO) estimated that the incidence rate of pre-eclampsia in developing countries is 7-times higher (2.8%) than in developed countries (0.45%).Citation6
Globally, maternal mortality due to pre-eclampsia and related hypertensive disorders were 76,000 per yearCitation5 and 56% of mortality occurring in sub-Saharan countries.Citation7 In developing countries, pre-eclampsia accounted for 40–60% of maternal death.Citation8–Citation11 In Ethiopia, maternal mortality due to pre-eclampsia was 10% per year.Citation12 The incidence rate of pre-eclampsia was 3.3 per 100 person-years in AustraliaCitation13 and 5.2 per 1,000 person-years in Yorkshire.Citation14 The incidence rate of pre-eclampsia in developing countries varies from 1.8–16.7%.Citation6,–Citation15–Citation17 Coming to Addis Ababa, pre-eclampsia increased from 2.2% in 2009 to 5.58% in 2013.Citation18 As the report of the previous studies, factors associated with pre-eclampsia were classified as socio-demographic,Citation12,–Citation19–Citation24 obstetric,Citation19–Citation21,Citation23–Citation26 and clinical factors.Citation21,Citation22,Citation26,Citation27
The result of this study is important for improving the quality-of-life and survival status of mothers and newborn babies and for social-capital and sustainable economic growth of the country at large. There is a paucity of information on the incidence and predictors of pre-eclampsia in Ethiopia; hence, this study aimed to determine the incidence and predictors of pre-eclampsia in Debre Markos referral hospital northwest Ethiopia.
Methods
Study Design, Area, and Periods
A hospital-based prospective cohort study was conducted at Debre-Markos Referral Hospital from November 1, 2018 to March 30, 2019. Debre Markos Referral Hospital is found in Debre Markos Town located at 299 km far from Addis Ababa, the capital city of Ethiopia, and 265 km far from Bahir-Dar, the capital city of Amhara Regional State.
Population
All pregnant women who received antenatal care at Debre Markos Referral Hospital were the target population. Pregnant women who were ≥20 weeks of gestational age during the recruitment period were the study population. All pregnant women having ≥20 weeks of gestational age and not developing pre-eclampsia were the eligible population. Pregnant women free of pre-eclampsia were selected using their antenatal registration number. About 242 pre-eclampsia free pregnant women were drawn using computer generated simple random sampling method.
Sample Size Determination and Sampling Procedure
The minimum required sample size was calculated using a survival sample size determination formula. To calculate the sample size we used the following information and assumptions: level of significance (α)=5%, Za/2=value at 95% confidence interval=1.96, HR: hazard ratio=1.49,Citation19 and power of 80%. We applied sample size calculation for each predictor variable, and the one with the maximum sample size selected for this study. Then, we randomly selected 242 pregnant women using a computer-generated simple random sampling method among those mothers having antenatal care follow-up at Debre Markos referral Hospital since November 1, 2018 to March 30, 2019.
Variables of the Study
The dependent variable of this study was the time to pre-eclampsia. There were three sets of explanatory variables. First, socio-demographic characteristics included age, occupation, physical activity, residence, and educational status. The second set was obstetric factors including history of abortion, null parity, and absence of antenatal care, family history of pre-eclampsia, multigravida, cesarean section, inducted delivery, multiple pregnancy, and gestational age. The third set of explanatory variables was clinical factors such as chronic hypertension, diabetes mellitus, gestational diabetes mellitus, renal and cardiac disease, severe anemia, urinary tract infection, smoking, pyelonephritis, body mass index, pre-existing hypertension, and use of alcohol.
Operational Definition
Pregnant women who experienced pre-eclampsia during their ANC visit were considered as an event and those who did not experience pre-eclampsia (including those who died, lost to follow-up, transferred out, continued on a visit, and gave birth without experience of pre-eclampsia at the end of the study) were censored.
Gestational age was calculated from the last normal menstrual period (LNMP) and, for those women who did not recall their last menstrual period, fundal height and/or ultrasound result was used.
Gravidity was the total number of pregnancies, including abortion, ectopic pregnancy, and any other pregnancies recorded on the follow-up chart.
Parity was recoreded as the number of deliveries after 28 weeks of gestation, including stillbirth and IUFD documented in the chart.
Pre-eclampsia represents pregnant women with blood pressure of ≥140 mmHg systolic or ≥90 mmHg diastolic on two separate readings taken at least 4–6 hours apart after 20 weeks gestation in an individual with previously normal blood pressure and proteinuria.Citation28,Citation29
Proteinuria is detected using the urine dipstick technique. Those women having protein in the urine+1 level and above are classified as proteinuria.
Data Collection Tool and Procedure
The data collection tool was prepared from studies conducted previously. The questionnaire contains socio-demographic factors, obstetric, and clinical characteristics.
To ensure data quality, before data collection, the data collection tool was prepared with care. Likewise, Two days of training were given for both data collectors and supervisor to standardize and agree on the way of interviewing. In addition, we verified, consistent understanding of the prepared questionnaire by study participants through randomly selecting and completing 24 samples, which resulted in slight amendments on the data collection tool. Furthermore, the supervisor and principal investigators supervised the data collectors throughout the entire data collection period.
Two BSc midwives who have been working in the gynecology and obstetrics ward of Debre Markos Referral Hospital were the data collectors. The data collectors had the list of 242 pregnant women with their cell phone numbers and appointment dates. Pregnant women were contacted by the data collectors when they came to Debre Markos referral hospital for their focused antenatal care visit or seeking medical care during the data collection period. Mothers who were missing their respective schedule visit were receiving a call from the data collectors.
Statistical Analysis
Data were entered into the EpidataTM version 4.2, and analyzed using STATA™ version 14.0 statistical software. At the end of the data collection period, the outcome of each study subject was dichotomized into censoring and event. Moreover, to identify the predictor variables, a Cox-proportional hazard regression model was fitted. To check the assumption of the Cox-proportional hazard regression model, we used Schoenfeld residual test for continuous variables and Log–Log plot for categorical variables.
Furthermore, we checked the model fitness using a Cox-Snell residual test (see Supplementary Figure 1). We used the Kaplan–Meier curve to estimate the occurrence of time to pre-eclampsia. A Log rank test was estimated to compare the survival curves for different categorical explanatory variables.
In univariate analysis, we used mean with standard deviations to describe normally distributed continuous data and median with interquartile range for skewed continuous data. In another way, the categorical data collected from pregnant women were described using frequency distribution or percentages. Regarding bivariable analysis the outcome variable (pre-eclampsia) and explanatory variables were entered into the bivariable Cox-proportional hazard regression model to select variables for multivariable Cox-proportional hazard regression model. As a result, variables having a P-value≤0.25 in the bi-variable analysis were fitted into the multivariable Cox-proportion regression model. Finally, adjusted risk ratio with its corresponding 95% confidence interval (CI) was used to declare the presence of a significant association between the explanatory and outcome variables.
Results
Socio-Demographic Characteristics
The mean (SD) age of the study participants was 28 (±5) years. Two hundred and eleven (87.19%) were married and 102 (42.15%) worked as a civil servant in their occupational status. Regarding educational status, 143 (50.09%) had college and above. Slightly less than three-quarters (70.66%) of the pregnant women were physically inactive ( and ).
Table 1 The Characteristics of Pregnant Women
Reproductive and Obstetric Characteristics
The median age at first menstruation was 15 (IQR=2.3) and the mean age at first pregnancy was 23 (±5) years old. During enrollment, the mean gestational age of pregnant women was 30 (±5) weeks and the median gestational age at the time of delivery was 38 weeks, with a 2-week interquartile range. Among study participants, 19 (7.85%) and 24 (9.92%) participants experienced preterm delivery and pre-eclampsia, respectively. About 18 (7.44%), 35 (14.46%), and 26 (10.74%) of the pregnant women had a family history of pre-eclampsia, history of abortion, and history of cesarean section, respectively. About one-third (34.71%) of pregnant women had antenatal care follow-up and one-tenth (9.50) had a history of multiple pregnancy in the previous pregnancy. Approximately four of every ten women (40.50%) where null parity ( and ).
Clinical Characteristics
Fifteen (6.20%) and 16 (6.61%) participants had a family history of chronic hypertension and chronic hypertension themselves, respectively. Less than one in five of the study participants experienced pre-existing diabetes mellitus (14.05%) and renal disease (17.36%), respectively. Slightly more than half of the study women were anemic (55.79%) or experienced urinary tract infections (51.24%) ().
Measured Value of Variables
The mean with the range of systolic blood pressure at baseline was 150±16 mmHg with 129±225 mmHg, and diastolic blood pressure was 105±14.5 mmHg with 85±133 mmHg, respectively. The mean with the range of proteinuria of pregnant women at baseline were 2.8±0.9 and 1–3, correspondingly.
Regarding the follow-up value of blood pressure, the mean with a range of systolic blood pressure was 163.3±18 mmHg and 1234±220 mmHg retrospectively. The mean with the range of proteinuria of pregnant women at baseline were 2.8±0.6 and 1–2.8, correspondingly.
Incidence of Pre-Eclampsia
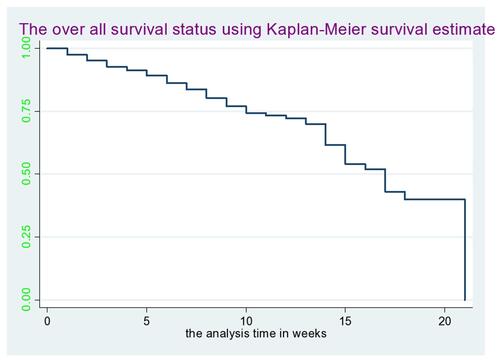
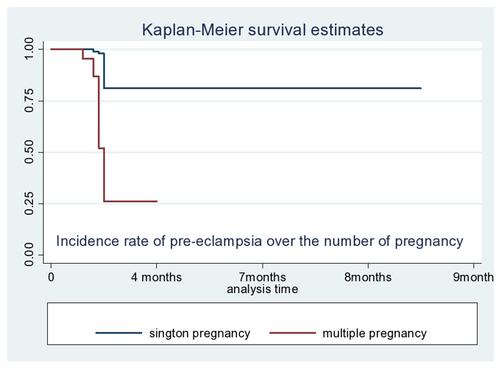
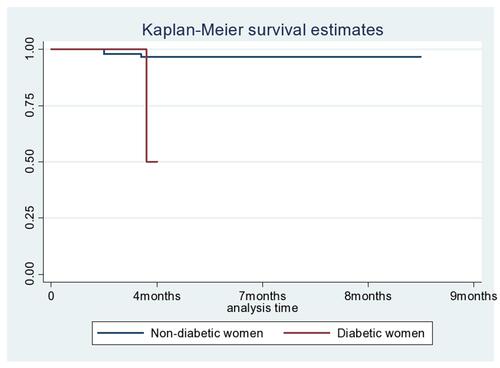
The study participants had 20–40 weeks gestational age during their recruitment. The overall pre-eclampsia free survival time of pregnant women was 1970 person-years. Out of 66 women diagnosed as developing pre-eclampsia, 33 (50%) had mild pre-eclampsia, 20 (30.3%) had severe pre-eclampsia, and 13 (19.69%) showed eclampsia. The overall incidence rate of pre-eclampsia was 3.35 (95% CI=0.026–0.043) per 100 person-years (), the incidence rate over the number of pregnancies () and the incidence rate over the diabetes status () were compared graphically.
Bivariable and Multivariable Cox-Regression Analysis
Having physical activity, a history of infertility, a family history of pre-eclampsia, age at current pregnancy, multiple pregnancy, having a family history of diabetes mellitus, history of hypertension, family history of hypertension, presence of renal disease, pre-existing diabetes mellitus, antenatal follow-up, and history of abortion were the variables having a P-value<0.25 in the bi-variable Cox-regression analysis and were eligible for multivariable analysis. In the multivariable Cox-regression analysis, having a history of pre-existing diabetes mellitus, having a history of multiple pregnancies, and age≥35 years old were statistically significant predictors of pre-eclampsia.
The risk of developing pre-eclampsia among pregnant mothers who had a history of pre-existing diabetes mellitus were 2.7- [AHR=2.7 (95% CI=0.43–0.85)] times higher than those who had no history of pre-existing diabetes mellitus. The chances of developing pre-eclampsia among pregnant women who were ≥35 years old were 2.5- [AHR=2.5 (95% CI=1.42–3.54)] times greater than those who were in the rang of 5–24 years old. Pregnant women who had a history of multiple pregnancy were 3.4- [AHR=3.4 (95% CI=2.8–6.9)] times more likely to experience pre-eclampsia than those who had no history of multiple pregnancy ().
Table 2 Multivariable Cox Regression Analysis
Discussions
Pre-eclampsia is a pregnancy-induced hypertension and defined as high blood pressure with a significant amount of protein in the urine. Pre-eclampsia is noted as the global leading cause of maternal mortality. This study aimed to determine the incidence and predictors of pre-eclampsia using 242 pregnant women who had an antenatal follow-up visit at Debre Markos Referral Hospital. The incidence of pre-eclampsia among pregnant women who had an antenatal follow-up visit at Debre Markos Referral Hospital was 3.35 (95% CI=0.026–0.043) per 100 person-years. The incidence of pre-eclampsia of this study is higher than in Yorkshire, England (5.2 per 1,000 person-years) and Zimbabwe (1.7 per 100 person-years).Citation14,Citation16 This might be due to 29 (11.98%) of the study participants not being formally educated in this study. This might limit their health-seeking behavior and level of understanding, finally creating stress on their current pregnancy, which in turn leads to increasing the occurrence of pre-eclampsia. This finding might have again resulted due to the socio-economic and demographic differences in the study population.
According to the findings of this study, the risk of developing pre-eclampsia among older women (≥35 years old) was 2.5- [AHR=2.5 (95% CI=1.42–3.54)] times higher than their counterparts (15–25 years old women). It is supported by a study conducted in Washington state.Citation19,Citation20 This might be a cardiovascular disorder increased in old age due to increment in cholesterol deposition and high level of lipid profiles, which leads to increasing the risk of pre-eclampsia occurrence due to a narrowing effect on the blood vessels.Citation24,Citation30,Citation31 Likewise, tit could be that more old pregnant women experience pre-eclampsia than young pregnant women due to a hemodynamic adoption problem.Citation32 Furthermore, this could be the gradual loss of cardiovascular vessel compliance due to aging of uterine blood vessels and increased arterial stiffness causing endothelial dysfunction.Citation22,Citation33
This study identified that mothers who had a history of multiple pregnancy were at 3.4- [AHR=3.4 (95% CI=2.8–6.9)] times higher risk of experiencing pre-eclampsia than those pregnant women who had no history of multiple pregnancy. This is supported by studies conducted previously.Citation25,Citation34,Citation35 This might be because experiencing multiple pregnancies can encourage the occurrence of psychological stress, which might lead to placing the woman at risk of developing pre-eclampsia.Citation36 This could be again because women with multiple pregnancy have a large placenta, which has decreased placental perfusion. The excess of placenta tissues, which could not perfuse adequately leads to develop pre-eclampsia.
The risk of developing pre-eclampsia among pregnant women who had a history of pre-existing diabetes mellitus was 2.7- [AHR=2.7 (95% CI=1.43–8.81)] times higher than those pregnant women who had no history of pre-existing diabetes mellitus. The finding of this study is in line with the studies conducted previously.Citation21,Citation27 It could be that diabetic pregnant women develop pre-eclampsia because high levels of blood sugar (diabetes mellitus) cause narrowing of blood vessels and interfere with the normal physiological response during pregnancy according to the World Health Organization evidence.Citation37
Limitations of This Study
Even though this study provided a clue for the policymakers and clinical purpose towards the decrement of the maternal morbidity and mortality, it has its own constraints. First, this study did not considersome essential predictors, like complete blood cell count, electrolyte, and hormonal tests, because it was not considered a laboratory investigation as a data collection method. Second, the incidence rate of pre-eclampsia might be underestimated if mothers who had a tendency to develop pre-eclampsia were lost to their antenatal care visit during the study period.
Recommendations
To overcome stress as well as normalize cardiac output and blood pressure, health professionals should provide health education for pregnant women to have monstrous physical exercise. Inspiring pregnant women’s health-seeking behavior should provide a chance to diagnose pre-eclampsia early to prevent its medical complication.
Conclusion
The incidence rate of pre-eclampsia was high in this study. Pre-existing diabetes mellitus, a history of multiple pregnancy, and being ≥35 years old were statistically significant predictors of pre-eclampsia. Inspiring pregnant women’s health-seeking behavior should provide a chance to diagnose pre-eclampsia early to prevent the medical complication of pre-eclampsia.
Abbreviations
PE, pre-eclampsia; WHO, World Health Organization; LMIC; low- and middle-income countries; ANC, Antenatal care; DM, Diabetes Mellitus; IUFD, Intrauterine fetal death; RR, Relative Risk.
Data Sharing Statement
No additional data are required; all information is stated in the main manuscript.
Ethics Approval and Consent to Participate
Ethical clearance was given from Debre Markos University, health sciences college review committee. During data collection, informed written consent was obtained from all pregnant women who were greater than 18 years old and above, and there were no pregnant women below the age of 18 years old participating in this study. To keep the confidentiality, the information of the study participants was not disclosed to anyone other than the principal investigators. Generally, this study was conducted in accordance with the World Medical Association Declaration of Helsinki.
Author Contributions
MYB: conception of the research idea, study design, data collection, analysis and interpretation, and manuscript write-up. HT, GD, MAA, AAA, MD, MT, DBK, CTL: data collection, analysis and interpretation, and manuscript write-up. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. All authors have read and approved the final manuscript.
Acknowledgment
The authors extend their special thanks for both data collectors and supervisor.
Disclosure
The authors have declared that they have no competing interests.
Additional information
Funding
References
- Watanabe K, Naruse K, Tanaka K, Metoki H, Suzuki Y. Outline of definition and classification of “Pregnancy induced Hypertension (PIH)”. Hypertens Res Pregnancy. 2013;1(1):3–4. doi:10.14390/jsshp.1.3
- WHO U: UNFPA, The World Bank, UN Population Division. Trends in Maternal Mortality: 1990 to 2013. WHO, UNICEF, UNFPA, The World Bank, UN Population Division; 2013.
- Abalos E, Cuesta C, Carroli G, et al.; Maternal WMSo, Network NHR. Pre‐eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on M aternal and N ewborn H ealth. BJOG. 2014;121:14–24. doi:10.1111/1471-0528.12629
- Chen X, Wen S, Smith G, Yang Q, Walker M. General obstetrics: pregnancy‐induced hypertension is associated with lower infant mortality in preterm singletons. BJOG. 2006;113(5):544–551. doi:10.1111/j.1471-0528.2006.00898.x
- Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–1306. doi:10.1097/AOG.0b013e3181a45b25
- Osungbade KO, Ige OK. Public health perspectives of preeclampsia in developing countries: implication for health system strengthening. J Pregnancy. 2011;2011:1–6. doi:10.1155/2011/481095
- Who U. UNFPA, World bank. Trends Maternal Mortality. 1990;2008(2010):1.
- Lakew Y, Reda AA, Tamene H, Benedict S, Deribe K. Geographical variation and factors influencing modern contraceptive use among married women in Ethiopia: evidence from a national population based survey. Reprod Health. 2013;10(1):52. doi:10.1186/1742-4755-10-52
- Moodley J. Maternal deaths associated with hypertensive disorders of pregnancy: a population-based study. Hypertens Pregnancy. 2004;23(3):247–256. doi:10.1081/PRG-200030301
- Health. tMo: Costed Implementation Plan for Family Planning in Ethiopia, 2015/16–2020; 2016. Available from: http://www.healthpolicyplus.com/ns/pubs/2021-2030_EthiopiaCIPNov.pdf. Accessed August 20, 2017.
- Health. TFDRoEMo: Health Sector Transformation Plan; 2017. Available from: https://www.globalfinancingfacility.org/sites/gff_new/files/Ethiopia-health-system-transformation-plan.pdf. Accessed August 20, 2017.
- Gaym A, Bailey P, Pearson L, Admasu K, Gebrehiwot Y; Team ENEA. Disease burden due to pre‐eclampsia/eclampsia and the Ethiopian health system’s response. Int J Gynecol Obstet. 2011;115(1):112–116. doi:10.1016/j.ijgo.2011.07.012
- Thornton C, Dahlen H, Korda A, Hennessy A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000–2008. Am J Obstet Gynecol. 2013;208(6):476.e471–476. e475. doi:10.1016/j.ajog.2013.02.042
- Tuffnell D, Jankowicz D, Lindow S, et al.; Group YOCC. Outcomes of severe pre‐eclampsia/eclampsia in Yorkshire 1999/2003. BJOG. 2005;112(7):875–880. doi:10.1111/j.1471-0528.2005.00565.x
- Fekadu GA, Kassa GM, Berhe AK, Muche AA, Katiso NA. The effect of antenatal care on use of institutional delivery service and postnatal care in Ethiopia: a systematic review and meta-analysis. BMC Health Serv Res. 2018;18(1):577.
- Ngwenya S. Severe preeclampsia and eclampsia: incidence, complications, and perinatal outcomes at a low-resource setting, Mpilo Central Hospital, Bulawayo, Zimbabwe. Int J Womens Health. 2017;9:353. doi:10.2147/IJWH.S131934
- Igbokwe C, Ukwuma M. Incidence of hypertension among pregnant women in Enugu East Local Government Area of Enugu State (2009–2012). Int J Res Arts Soc Sci. 2013;6:303–314.
- Wagnew M, Dessalegn M, Worku A, Nyagero J. Trends of preeclampsia/eclampsia and maternal and neonatal outcomes among women delivering in addis ababa selected government hospitals, Ethiopia: a retrospective cross-sectional study. Pan Afr Med J. 2016;25(Suppl2).
- Lisonkova S, Joseph K. Incidence of preeclampsia: risk factors and outcomes associated with early-versus late-onset disease. Am J Obstet Gynecol. 2013;209(6):544.e541–544. e512. doi:10.1016/j.ajog.2013.08.019
- Sibai B, Ewell M, Levine R, et al. Risk factors associated with preeclampsia in healthy nulliparous women. Am J Obstet Gynecol. 1997;177(5):1003–1010. doi:10.1016/S0002-9378(97)70004-8
- Bej P, Chhabra P, Sharma AK, Guleria K. Determination of risk factors for pre-eclampsia and eclampsia in a tertiary hospital of India: a case control study. J Family Med Prim Care. 2013;2(4):371. doi:10.4103/2249-4863.123924
- Tessema GA, Tekeste A, Ayele TA. Preeclampsia and associated factors among pregnant women attending antenatal care in Dessie referral hospital, Northeast Ethiopia: a hospital-based study. BMC Pregnancy Childbirth. 2015;15(1):73. doi:10.1186/s12884-015-0502-7
- Yakasai IA, Morhason-Bello IO. Risk factors for pre-eclampsia among women at antenatal booking in Kano, Northern Nigeria. Healthcare Low-Resour Settings. 2013;1(1):e12–e12. doi:10.4081/hls.2013.e12
- Ota E, Ganchimeg T, Mori R, Souza JP. Risk factors of pre-eclampsia/eclampsia and its adverse outcomes in low-and middle-income countries: a WHO secondary analysis. PLoS One. 2014;9(3):e91198. doi:10.1371/journal.pone.0091198
- Shambel W, Surender R. Hypertensive disorders of pregnancy and associated factors among admitted pregnant cases in dessie town referral hospital, north east ethiopia. Med Res Chronicles. 2016;3(3):297–306.
- Parretti E, Lapolla A, Dalfra M, et al. Preeclampsia in lean normotensive normotolerant pregnant women can be predicted by simple insulin sensitivity indexes. Hypertension. 2006;47(3):449–453. doi:10.1161/01.HYP.0000205122.47333.7f
- Esakoff TF, Rad S, Burwick RM, Caughey AB. Predictors of eclampsia in California. J Matern Fetal Neonatal Med. 2016;29(10):1531–1535.
- Kintiraki E, Kotronis G, Goulis DG, Kotsis V. Pregnancy-induced hypertension. Hormones. 2015;14(2):211–223. doi:10.14310/horm.2002.1582
- Davey D. The classification and definition of the hypertensive disorders of pregnancy: proposals submitted to the international society for the study of hypertension in pregnancy. Clin Exp Hypertens B. 1986;5(1):97–133.
- Program NHBPE. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):s1–s22. doi:10.1067/mob.2000.107928
- Kolovou GD, Bilianou HG. Influence of aging and menopause on lipids and lipoproteins in women. Angiology. 2008;59(2_suppl):54S–57S. doi:10.1177/0003319708319645
- Van Katwijk C, Peeters LL. Clinical aspects of pregnancy after the age of 35 years: a review of the literature. Hum Reprod Update. 1998;4(2):185–194. doi:10.1093/humupd/4.2.185
- Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179(5):1359–1375. doi:10.1016/S0002-9378(98)70160-7
- Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330(7491):565. doi:10.1136/bmj.38380.674340.E0
- Kumar G, Unnikrishnan B, Nagaraj K, Jayaram S. Determinants of pre-eclampsia: a case–control study in a district hospital in South India. Indian J Community Med. 2010;35(4):502. doi:10.4103/0970-0218.74360
- Bdolah Y, Lam C, Rajakumar A, et al. Twin pregnancy and the risk of preeclampsia: bigger placenta or relative ischemia? Am J Obstet Gynecol. 2008;198(4):428.e421–428. e426. doi:10.1016/j.ajog.2007.10.783
- Villar J, Purwar M, Merialdi M, et al. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre‐eclampsia in populations of low nutritional status from developing countries. BJOG. 2009;116(6):780–788. doi:10.1111/j.1471-0528.2009.02158.x