?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To compare physician assessment of the next-generation NovaSure® device to the original NovaSure device.
Design
Prospective open-label, unblinded survey utilizing Likert scales.
Setting
Community-based obstetrician and gynecology practices.
Patients
There were 270 evaluations completed by 168 physicians for women undergoing endometrial ablation with the next-generation NovaSure device compared to the previous model.
Interventions
Physician survey across multiple community-based obstetrician and gynecology practices.
Measurements and main results
In general, 53.7% of women had a normal uterus, with an average cavity length of 5.3 cm ± 0.9 cm, and 44.7% of women had a normal cervix. Overall, 98.7% of physicians agreed or strongly agreed that they were satisfied with the performance of the next-generation NovaSure device compared with the previous device. Additionally, 89% of physicians rated the next-generation NovaSure device as superior to the previous device. The best rated features were the SureFit™ Cervical Seal, Smooth Access™ Tips, and the thumb-pusher. No adverse events were reported in this study.
Conclusion
Community-based physicians rated the next-generation NovaSure device as superior to the previous model in a general population of women.
Introduction
For women, normal menstrual bleeding patterns occur at an interval of 21 to 35 days, with bleeding lasting 1 to 7 days.Citation1 This equates to less than one tampon or pad, or <80 mL of blood, for every 3-hour period.Citation1 Menorrhagia is defined as excessive but regular cyclic bleeding of more than 7 days, passage of clots, or iron deficiency anemia.Citation1 Prolonged bleeding over 12 days should be considered abnormal regardless of cyclic pattern.Citation1 Pain and heaviness are the most frequently reported bothersome aspects of excessive or very excessive periods.Citation2 In Western countries, up to 35% of women report excessive or very excessive bleeding.Citation3,Citation4 However, only 5% of women seek medical help for menorrhagia annually.Citation4 Among women with excessive menstrual bleeding, 53% reported that their periods interfered with their life, compared with 23% of community controls.Citation5
Pharmacotherapy treatment options for excessive bleeding include oral contraceptives, danazol, progestens, etc.Citation6 Surgical treatment options for excessive bleeding include endometrial ablation and hysterectomy. Hysteroscopic endometrial ablation offers an effective, less invasive alternative to hysterectomy. Physicians and patients should work together to decide the best treatment option for an individual patient. For patients who wish to avoid major surgery and in whom childbearing is complete, endometrial ablation is a reasonable and effective alternative to hysterectomy. Citation7 The NovaSure® (Hologic, Inc, Marlborough, MA) endometrial ablation procedure is intended to ablate the endometrial lining of the uterus in premenopausal women with excessive bleeding due to benign causes for whom childbearing is complete.Citation8 To date, more than one million women have undergone the NovaSure procedure. Although widely accepted by physicians for use in endometrial ablation procedures, an initiative was undertaken to understand which features could be revised or enhanced to further improve the surgeons experience with the device. The manufacturer made several enhancements to the original device and a next-generation NovaSure device was released for commercial use in 2010. Here we present community-based, physician-reported data evaluating the ease-of-use of the newly designed next-generation NovaSure device compared with the prior model.
Methods
The data presented here represent the findings of community-based, physician-reported assessments on the ease-of-use of the next-generation NovaSure device. The objective was to compare physician assessment of the original NovaSure device to the next-generation NovaSure device. Physicians were asked to participate in the study based upon their use and experience with the NovaSure device and respondents were not compensated for their participation in this study. Participating physicians represented a diverse group that was representative of general, community-based practices. Their procedure volumes ranged from very low (a few per month) to multiple procedures per week. The study was a prospective open-label, unblinded survey utilizing Likert scales to determine whether the updated next-generation NovaSure device design was perceived better than the previous model in ease-of-use or component features. A 5-point Likert scale was used to assess ease-of-use items. Physicians rated the performance of the next-generation NovaSure device (1 = strongly disagree to 5 = strongly agree) and also compared it with the previous model of the device (1 = inferior, 2 = equal, and 3 = superior). Anatomic variability (cervix, uterine position, and cavity length) and procedural information was also collected.
Subjects with benign endometrial pathology, uterine sound <10 cm, intracavitary fibroids <2 cm, and complete childbearing were evaluated. Ease-of-use during endometrial ablation using the new, revised design of the next-generation NovaSure device was compared with the previous NovaSure device.
Internal reliability of the survey was assessed via Cronbach’s alpha. Cronbach’s alpha is def ined as:
where K is the number of components σX2 is the variance of the observed total test scores, and σYi2 is the variance of component i for the current sample of persons. Cronbach’s alpha is a measure of internal consistency, that is, how closely related a set of items are as a group. Higher alpha scores (>0.80) are more desirable and indicate better reliability.
Results
Ease-of-use assessed by a community-based survey
Physicians (n = 168) from community-based obstetrics and gynecology practices rated six specific components of the next-generation NovaSure device. In total, 270 evaluations were completed, with 245 fully completed; 56 physicians completed two evaluations, and 26 physicians completed three or more evaluations. Patients included women undergoing endometrial ablation with the next-generation NovaSure device. In general, 53.7% of women had a normal uterus and 21.8% had an anteverted uterus (). The average cavity length of women was 5.3 cm ± 0.9 cm with 55.1% of women having a cavity length between 4.5 cm and 5.5 cm (). Approximately 45% of women had a normal cervix and 34.2% had a typical multip cervix ().
Table 1 Assessment of uterus type
Table 2 Assessment of cavity length
Table 3 Assessment of cervix type
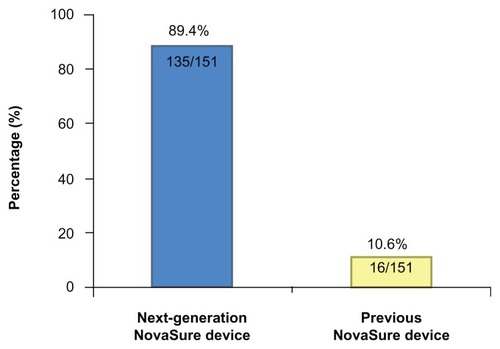
Overall, 98.7% (155 of 157) of physicians agreed or strongly agreed that they were satisfied with the performance of the next-generation NovaSure device compared with the previous device. Additionally, 89% (135 of 151) of physicians rated the next-generation NovaSure device as superior to the previous device (). Physicians also assessed the overall device and specific components of the next-generation NovaSure device compared with the previous model (). Overall satisfaction of the next-generation NovaSure device was evaluated in patients with cavity lengths ranging from 4–9 cm. Additionally, 100% (149 of 149) of physicians agreed that the thumb-pusher on the next-generation NovaSure device was superior in patients with cavity lengths of > 7 cm. The next-generation NovaSure device was perceived as easier to insert in uterine cavities >7 cm (P = 0.16). More than 99% of physicians said that overall, the next-generation NovaSure device was similar or superior to the previous model in patients with a normal cervix. All physicians (100%) agreed that the next-generation NovaSure device was similar or superior to the previous model in patients with stenotic, typical nulip, typical multip, and patulous cervixes.
Figure 2 Community-based physician survey comparing the Next-Generation NovaSure device with the previous model. (A) Overall satisfaction. (B) Ease-of-use.
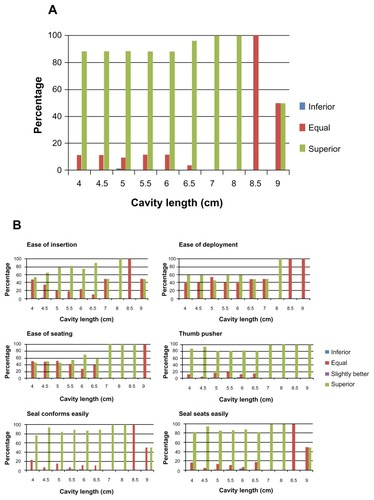
Physicians prefer the next-generation NovaSure device over the earlier design, regardless of patient anatomy. A few features were considered advantageous, including the redesigned cervical collar to provide a more complete seal and improve the ability to pass cavity integrity assessment, Smooth Access™ Tips to enhance array deployment, and a thumb-pusher to overcome a long vaginal canal (). Results of this survey are considered valid, as the Cronbach’s alpha was ≥0.80, indicating good reliability. This survey showed a high validity and reliability based on Cronbach’s alpha calculation for ease-of-use (Cronbach’s alpha = 0.841) and comparison items (Cronbach’s alpha = 0.823).
Table 4 Community-based physician survey: physician-rated best features
Discussion
Excessive menstrual bleeding can have a direct impact on a woman’s quality of life. Performing endometrial ablation is a viable treatment alternative to hysterectomy, and the goal of treatment is to improve the patient’s quality of life.Citation9 The NovaSure endometrial ablation system is intended to ablate the endometrial lining of the uterus in premenopausal women with menorrhagia (excessive bleeding) due to benign causes for whom childbearing is complete. The current data demonstrate that recent enhancements made to the NovaSure device have been well-received by physicians. The next-generation NovaSure device was designed based on surgeon feedback with the goal to enhance features that would further improve the surgeons experience with the device. Several enhancements were made to the original device, and a next-generation NovaSure device was released for commercial use in 2010.
In the community-based survey, 98% of physicians were satisfied with the performance of the next-generation Nova- Sure device, and 89% considered it as superior to the previous model. It is important to note that the data based on anatomic variability support the use of the next-generation NovaSure device in many patient types, which is relevant for general practitioner use. In this survey, more than 50% of women had a normal uterus and almost 22% had an anteverted uterus. The average cavity length in the women was 5.3 cm and 55.1% of women had a cavity length of 4.5 cm, 5.0 cm, or 5.5 cm. Cervix type also indicated that these women were representative of a general population as 44.7% had a normal cervix and 34.2% had a typical multip cervix. Regardless of anatomy, physicians preferred the next-generation device, and did not identify an anatomic configuration for which the prior device would have been preferred. Physicians considered the thumb-pusher, the SureFit™ Cervical Seal, and Smooth Access Tips as the best features of the next-generation device. The thumb-pusher allows more leverage and control over the cervical collar. The next-generation NovaSure device SureFit Cervical Seal is larger, designed from softer material, provides a 70% greater coverage area, and may improve the procedure efficiency and patient comfort. Finally, the Smooth Access Tips allow for easier insertion of the device.
The results presented here are encouraging and support the changes and enhancements made in the next-generation NovaSure device. These data were collected among community- based physicians and appear to be representative of a general population of women based upon their type of uterus and cervix and cavity length. Advantages with the next-generation NovaSure device include a procedure that can be performed in-office, and is quicker than many other hysteroscopic procedures and less invasive than a hysterectomy.
Acknowledgments
Editorial support was sponsored by Hologic, Inc, and provided by Ed Shifflett, PhD at AlphaBioCom.
Disclosure
The authors report no conflicts of interest in this work.
References
- ElyJWKennedyCMClarkECAbnormal uterine bleeding: a management algorithmJ Am Board Fam Med200619659060217090792
- SanterMWykeSWarnerPWhat aspects of periods are most bother-some for women reporting heavy menstrual bleeding? Community survey and qualitative studyBMC Womens Health20077817543131
- SanterMWarnerPWykeSA Scottish postal survey suggested that the prevailing clinical preoccupation with heavy periods does not reflect the epidemiology of reported symptoms and problemsJ Clin Epidemiol200558111206121016223665
- HurskainenRGrenmanSKomiIDiagnosis and treatment of menorrhagiaActa Obstet Gynecol Scand200786674975717520411
- ShapleyMJordanKCroftPRWhy women consult with increased vaginal bleeding: a case-control studyBr J Gen Pract20025247510811311885820
- WhittedRAbnormal Uterine Bleeding and FibroidsAssociation of Minimally Invasive Gynecologic Surgeons Abnormal uterine bleeding and fibroids. Available from: http://www.floridaamigos.com/pdf/AUB_and_Fibroid_Center.pdfAccessed on March 6, 2012
- CoteIJacobsPCummingDCUse of health services associated with increased menstrual loss in the United StatesAm J Obstet Gynecol2003188234334812592237
- HologicIncNovaSure Instructions for Use and Controller Operator’s ManualMarlborough, MAHologic, Inc2009
- ShankarMChiCKadirRAReview of quality of life: menorrhagia in women with or without inherited bleeding disordersHaemophilia2008141152017961167