Abstract
Context
Follicle-stimulating hormone (FSH)-secreting pituitary adenoma is usually a nonfunctioning tumor, but in rare cases it may develop into ovarian hyperstimulation. Several reports have revealed that serum FSH levels are normal to slightly high in patients with combined FSH-secreting pituitary adenoma with ovarian hyperstimulation. This finding is different from iatrogenic ovarian hyperstimulation syndrome (OHSS), which is associated with extremely high levels of FSH.
Objective
To describe the clinical course of two patients who developed OHSS from FSH-secreting pituitary adenoma.
Results
Endocrine studies of the two cases revealed that FSH levels were normal or slightly increased, but luteinizing hormone levels were low to undetectable. Their estradiol (E2) levels were intriguing: levels fluctuated drastically over 6 weeks in Case 1, but stayed flat in Case 2. Ultrasonographic examinations showed bilaterally enlarged multicystic ovaries, and magnetic resonance imaging indicated pituitary tumors. Transsephenoidal resection of the tumors ameliorated the symptoms and pathological diagnosis revealed FSH-secreting pituitary adenomas.
Conclusion
As is not the case in iatrogenic OHSS, even a small to moderate amount of FSH stimulation, which is continuously secreted by a pituitary adenoma, can cause ovarian hyperstimulation. Although FSH-secreting pituitary adenoma can cause ovarian hyperstimulation, an extremely high amount of E2 biosynthesis from granulosa cells seldom occurs.
Introduction
Pituitary adenomas are the third most common intracranial tumors, and about 10%–17% of primary brain neoplasms are due to these tumors.Citation1 Almost all pituitary adenomas are nonfunctioning tumors (40%), prolactinomas (30%), or growth hormone-secreting adenomas (20%). Follicle-stimulating hormone (FSH)-secreting adenomas (FSHomas) are relatively uncommon and rarely cause endocrinological symptoms because the patients are predominantly middle-aged adults. Gonadotropin increase does not cause ovarian hyperstimulation because of ovarian aging. Therefore, intracranial mass effects such as visual-field defects, headache, and cranial nerve palsy preface the detection of FSHoma.
Conversely, the characteristics of FSHoma in reproductive-aged women are enlarged ovaries with multiple cysts, elevated serum estradiol (E2) levels, normal to mildly elevated FSH levels, suppressed luteinizing hormone (LH) levels, and causally induced menstrual disorder, infertility, and ovarian hyperstimulation.Citation2,Citation3 The mechanism by which normal to mildly elevated levels of FSH induce ovarian hyperstimulation is not fully known. Kajitani et al have hypothesized that the bioactivity of the FSH secreted by FSHoma is more active but were unable to prove this.Citation4
Thus, an alternate explanation may exist for ovarian hyperstimulation in patients with FSHoma. We have recently treated two cases of FSHoma in patients of reproductive age. Thorough observation and review of the literature revealed several interesting points about the disease:
normal to moderately high levels of FSH may be enough to elicit abundant follicles and subsequent multicystic ovaries
fluctuation of E2 levels exists in spite of continuous FSH stimulation
there is wide individual variability of E2 levels (normal to extremely high) in spite of ovarian hyperstimulation
decreased LH levels cannot be explained by negative feedback of increased E2 levels or by a compression of normal hypophysis
FSHoma rarely causes massive ascites and thromboembolism, which are typical symptoms of ovarian hyperstimulation syndrome (OHSS).
Case 1
A 32-year-old woman presented at Muroran Municipal Hospital in Muroran, Japan with severe symptoms of OHSS, consisting of multicystic ovarian enlargement, massive ascites, and thromboembolism. The detailed medical history and clinical course of this patient has been reported previously.Citation5 She had become pregnant naturally and developed OHSS in her fifth week of gestation. In her ninth week of gestation, she developed deep vein thrombosis and termination of pregnancy was chosen. After termination, things went well transiently until she developed ovarian enlargement without ascites 1 month later.
As there was suspicion of a malignant ovarian neoplasm, an exploratory laparotomy was performed and pathological specimens revealed that the swollen ovaries were follicular cysts. Due to the diagnostic delay, transition of E2 levels was closely monitored and showed periodical fluctuation (). Several medications that are known to decrease E2 levels – gonadotropin-releasing hormone (GnRH) agonist, aromatase inhibitor, and fluconazole – were tried sequentially but nothing was effective. In addition, GnRH agonist treatment exacerbated the symptoms and increased E2 levels (). A thorough hormonal examination showed the patient’s LH level to be suppressed, but FSH was within the normal range in spite of the extremely high E2 level (). We finally realized that the cause was pituitary neoplasm and this was successfully treated by transsphenoidal resection of the tumor (). Immunohistochemical study confirmed that the tumor was positive for FSH but negative for LH ().
Figure 1 Fluctuation of estradiol (E2) levels after dilatation and curettage.
Abbreviation: D and C, dilatation and curettage.
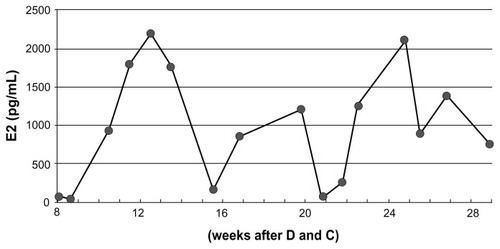
Figure 2 Fluctuation of estradiol (E2) levels after dilatation and curettage. The periodic increase and decrease of the E2 levels are seen in follicle-stimulating hormone (FSH)- secreting adenoma in an approximate a 6-week cycle.
Abbreviations: GnRHa, gonadotropin-releasing hormone agonist; LH, luteinizing hormone.
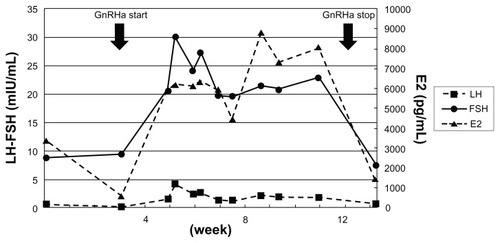
Figure 3 Magnetic resonance imaging (MRI) and immunohistochemical findings of the two cases. (A and D) T1-weighted MRI images of Cases 1 and 2, respectively. Pituitary tumor was seen in each case. (B and E) Immunohistochemistry for follicle-stimulating hormone (FSH) in pituitary tumors derived from Cases 1 and 2, respectively. Positive staining for FSH was done for each specimen. (C and F) Immunohistochemistry for LH in pituitary tumors derived from Cases 1 and 2, respectively.
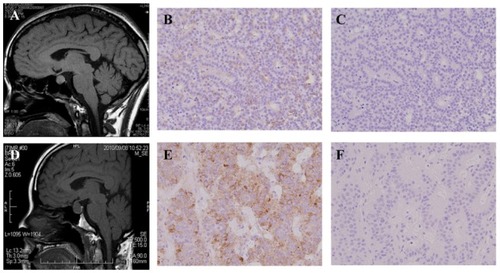
Table 1 Endocrine profile of two cases
Case 2
A 40-year-old woman, gravida 4 para 2, visited the Tonan Hospital in Sapporo, Japan with abnormal genital bleeding. Her medical and obstetrical history was uneventful. She had had regular menses until 5 years before presentation. Pelvic ultrasonography and magnetic resonance imaging revealed bilateral multicystic ovarian enlargement with a scant accumulation of ascites, which could not be excluded as a malignant tumor. Bilateral oophorocystectomy was performed and pathological examination showed follicular cysts.
After the operation, she suffered from abdominal distension again. She consulted with Sapporo Medical University Hospital to investigate the cause. We noticed that her preexisting medical condition was quite similar to that of the Case 1 patient. Her hormonal profile also showed suppressed LH level, but her FSH level was within the normal range (). Her E2 levels were examined several times and were within normal range (less than 300 pg/mL). After an MRI scan revealed a pituitary tumor, transsphenoidal resection of the tumor ameliorated the symptoms (). Immunohistochemical study confirmed that the tumor was positive for FSH but negative for LH ()
Discussion
In this study, we have shown two extremely rare cases of FSHoma in women of reproductive age. Generally, hormone-secreting tumors produce specific hormones at high levels. However, serum FSH levels in cases with FSHoma are normal or only slightly elevated.
In addition, surprisingly, normal or slightly elevated serum levels of continuous FSH stimulation can cause ovarian hyperstimulation. This phenomenon is contrary to iatrogenic OHSS, which is caused by administration of extremely supraphysiological gonadotropin. This is because the diagnosis of FSHoma is somewhat puzzling. The commercially available method for FSH measurement is based on enzyme immunoassay. In this method, FSH level is determined by the amount of FSH, not FSH bioactivity. In other words, at present, mutated FSH, which may have increased bioactivity, and normal FSH are not able to be distinguished from each other by laboratory examination. Therefore, we initially suspected that FSH secreted by a FSHoma was different from the intact FSH secreted by a normal pituitary gland. In these kinds of cases, coding sequences of the FSH in the tumor tissues can be determined, but no somatic mutation found. Judging from our study and the previous report,Citation4 the FSH secreted by a FSHoma was functionally identical to that of a normal pituitary gland.
It is known that approximately 1000 primordial follicles per menstrual cycle become recruited independent of FSH and then pre-antral follicles grow to be small antral follicles, which are sensitive to FSH, 85 days prior to ovulation.Citation6,Citation7 The dominant follicle is FSH-level dependently selected by 7–8 days of the menstrual cycle.Citation8 The dominant follicle avoids atresia due to a decreased dependency on FSH, which is the so-called threshold level required for continued follicular development.Citation9–Citation11 If gonadotropin stimulation is started earlier than usual and is above the threshold level, it is possible to recruit more follicles. The mechanism of ovarian hyperstimulation is that FSH levels produced by the FSHoma are barely above the threshold level, which is designated/described as being within the normal limit. This hypothesis may be further confirmed by a report regarding 3 months of clomiphene citrate stimulation.Citation12 In that report, accidentally prolonged stimulation by clomiphene citrate (50 mg/day for 3 months) resulted in ovarian hyperstimulation. The FSH level of this patient was within the normal range (7.0 mIU/mL).
It is unfortunate that there was a delay in reaching diagnosis in Case 1; however, both cases have given us many insights. Intriguingly, the periodic increase and decrease of the E2 levels were seen in Case 1 over an approximate 6-week cycle, although FSH was continuously steadily secreted. This phenomenon may support cyclic follicle recruitment and atresia. Collins et al have reported that prolonged ovarian hyperstimulation causes follicular atresia in Macaca fascicularis.Citation13 It is known that during exogenous administration of gonadotropins, endogenous LH is suppressed and LH surge hardly occurs. This may result in entrapment of the oocyte and postmaturity (atresia). In humans, follicle recruitment is done every month, where recruited follicles become atretic and subsequent neo-recruitment occurs. This may be the reason for the fluctuating E2 levels.
In FSHoma patients, LH is suppressed to undetectable levels unrelated to E2 levels. Schoemaker et al reported that administration of pure FSH caused a decrease in LH in patients suffering from anovulation and initially elevated LH levels.Citation14
Interestingly, pituitary ability to secrete LH is maintained in FSHoma patients and GnRH analog stimulation causes intrinsic LH secretion and exacerbates the symptoms.Citation15 These findings suggest that suppression of LH secretion is mediated by FSH and not by negative-feedback effects of E2 or pituitary compression.
The E2 levels in our patients were quite different in spite of the same situation; that is, exposure to continuous FSH stimulation. This phenomenon may be explained by the extent of the LH suppression. Administration of recombinant FSH to patients with gonadotropin deficiency causes follicle growth without increasing E2 levels.Citation16 This means that LH is essential for E2 production. In a hypophysectomized rat model, a positive correlation between LH and E2 levels was shown.Citation17 The administration of recombinant FSH did not show elevated E2 levels, but the administration of recombinant FSH plus a small amount of LH showed increased E2 levels, and E2 was dose-dependently increased by LH. The LH level in Case 2 may have been suppressed more aggressively to nearly zero; consequently, E2 production was hampered. Alternatively, the LH level in Case 1 may have been enough to produce E2, although the LH level was low.
Ascite accumulation was seen in Case 1, however, this phenomenon is quite rare in patients with FSHoma. Increased vascular permeability in OHSS is thought to be mediated by vascular endothelial growth factor, which is produced with LH or human chorionic gonadotropin.Citation18,Citation19 Typically, patients with FSHoma display low LH levels and have no ovulation due to impaired LH surge. Therefore, extravasation hardly occurs in FSHoma. It was a highly unusual event that the first patient naturally conceived and developed OHSS with massive ascites. In addition, ascites were not seen after termination of pregnancy, although multicystic ovarian enlargement emerged again. This means that FSH stimulation only is not sufficient for extravasations; rather, LH or human chorionic gonadotropin stimulation in addition to FSH is required.
Conclusion
Thorough observations of two patients with FSHoma were highly informative. We now know some characteristics of functioning FSHoma, the clinical course of continuous FSH stimulation, and the underlying mechanism of ascite accumulation in OHSS. Unfortunately, endocrinologically functioning FSHoma in reproductive-aged women is not well-known among gynecologic clinicians. This report may therefore be helpful for the diagnosis of FSHoma, which has symptoms that are quite rare.
Acknowledgments
We are grateful to our patients who cooperated in frequent medical tests to clarify their clinical course. We thank Drs Shinichiro Kon, Hiroshi Ohyama, Michifumi Kyuma (Muroran Municipal Hospital), Yutaka Sawamura (Sawamura Neurology and Neurosurgery Clinic), and Haruhiko Hotta (Sapporo Syuyukai Hospital) for helpful discussion and appropriate diagnosis and treatment of the patients.
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Disclosure
The authors declare no conflicts of interest in this work.
References
- BeshayVEBeshayJEHalvorsonLMPituitary tumors: diagnosis, management, and implications for reproductionSemin Reprod Med200725538840117710735
- DjerassiACoutifarisGWestVAGonadotroph adenoma in a premenopausal woman secreting follicle-stimulating hormone and causing ovarian hyperstimulationJ Clin Endocrinol Metab19958025915947852525
- Christin-MaitreSRongières-BertrandGKottlerMLA spontaneous and severe hyperstimulation of the ovaries revealing a gonadotroph adenomaJ Clin Endocrinol Metab19988310345034539768644
- KajitaniTLiuSMaruyamaTAnalysis of serum FSH bioactivity in a patient with an FSH-secreting pituitary microadenoma and multicystic ovaries: A case reportHum Reprod200823243543918056718
- BabaTEndoTKitajimaYKamiyaHMoriwakaOSaitoTSpontaneous ovarian hyperstimulation syndrome and pituitary adenoma: incidental pregnancy triggers a catastrophic eventFertil Steril2009921390. e1e319356755
- MacklonNSFauserBCAspects of ovarian follicle development throughout lifeHorm Res199952416117010725781
- GougeonARegulation of ovarian follicular development in primates: facts and hypothesesEndocr Rev19961721211558706629
- GarciaJEJonesGSAcostaAAWrightGJrHuman menopausal gonadotropin/human chorionic gonadotropin follicular maturation for oocyte aspiration: phase I, 1981Fertil Steril19833921671736217993
- BrownJBPituitary control of ovarian function – concepts derived from gonadotrophin therapyAust N Z J Obstet Gynaecol19781814654278588
- BairdDTA model for follicular selection and ovulation: lessons from superovulationJ Steroid Biochem1987271–315233121918
- FauserBCVan HeusdenAMManipulation of human ovarian function: physiological concepts and clinical consequencesEndocr Rev1987181711069034787
- SillsESPoynorEAMoomjyMOvarian hyperstimulation and oophorectomy following accidental daily clomiphene citrate use over three consecutive monthsReprod Toxicol200014654154311099879
- CollinsRLWilliamsRFHodgenGDEndocrine consequences of prolonged ovarian hyperstimulation: hyperprolactinemia, follicular atresia, and premature luteinizationFertil Steril19844234364456432589
- SchoemakerJWentzACJonesGSDubinNHSappKCStimulation of follicular growth with “pure” FSH in patients with anovulation and elevated LH levelsObstet Gynecol1978513270277628528
- CastelbaumAJBigdeliHPostKDFreedmanMFSnyderPJExacerbation of ovarian hyperstimulation by leuprolide reveals a gonadotroph adenomaFertil Steril20027861311131312477530
- SchootDCCoelingh BenninkHJMannaertsBMLambertsSWBouchardPFauserBCHuman recombinant follicle-stimulating hormone induces growth of preovulatory follicle without concomitant increase in androgen and estrogen biosynthesis in a woman with isolated gonadotropin deficiencyJ Clin Endocrinol Metab1992746147114731592896
- MannaertsBde LeeuwRGeelenJComparative in vitro and in vivo studies on the biological characteristics of recombinant human follicle-stimulating hormoneEndocrinology19911295262326301935792
- NeulenJYanZRaczekSHuman chorionic gonadotropin-dependent expression of vascular endothelial growth factor/vascular permeability factor in human granulosa cells: importance in ovarian hyperstimulation syndromeJ Clin Endocrinol Metab1995806196719717775647
- WangTHHorngSGChangCLHuman chorionic gonadotropin-induced ovarian hyperstimulation syndrome is associated with upregulation of vascular endothelial growth factorJ Clin Endocrinol Metab20028773300330812107240