Abstract
Background
The purpose of this work was to investigate whether clinical cytology could be useful in the preoperative diagnosis of pelvic actinomycosis.
Methods
This study involved the prospective collection of samples derived from the endometrium and the uterine cervix, and retrospective data analysis. Nine patients with clinically diagnosed pelvic actinomycosis were enrolled. The clinical and hematological characteristics of patients were recorded, and detection of actinomyces was performed by cytology, pathology, and bacteriological culture of samples and by imprint intrauterine contraceptive device (IUD) cytology.
Results
The detection rate of actinomyces was 77.7% by combined cervical and endometrial cytology, 50.0% by pathology, and 11.1% by bacterial culture.
Conclusion
The higher detection rate of actinomyces by cytology than by pathology or bacteriology suggests that careful cytological examination may be clinically useful in the preoperative diagnosis of pelvic actinomycosis.
Introduction
Actinomycosis is a chronic suppurative, granulomatous inflammatory condition with abscess and fistula formation caused by anaerobic Gram-positive bacilli (Actinomyces species) commonly found within the oral cavity and intestinal tract of healthy individuals.Citation1 It has been reported that pelvic actinomycosis is associated with chronic insertion of an intrauterine contraceptive device (IUD).Citation2,Citation3 A 20-year study in Japan of 122 women with pelvic actinomycosis revealed that 90.7% of them were IUD users.Citation4 It is difficult to diagnose an actinomycotic adnexal mass by imaging modalities before surgery. Even with clinical features of actinomycosis, a pelvic mass containing a solid or cystic component is mostly diagnosed as suspected malignancy by either magnetic resonance imaging, computer tomography imaging, or ultrasonography.Citation5 Most cases of actinomyces are diagnosed by histopathological examination after surgery. Furthermore, actinomycotic adnexal abscesses progress slowly, with local invasion to adjacent organs. Thus, it is difficult to make a differential diagnosis of malignancy.Citation6–Citation8 Therefore, it is important to find some clinical modality to diagnose this slowly progressive disease preoperatively. Here we report nine cases of pelvic actinomycosis at our hospital along with an analysis of their clinicopathologic, cytologic, and bacteriologic characteristics. Our aim was to identify a single diagnostic intervention for pelvic actinomycosis before undertaking any operative procedure.
Materials and methods
During the period 2005–2010, nine patients diagnosed with pelvic actinomycosis were recruited for this study from the Department of Obstetrics and Gynecology, Nagasaki University. Papanicolaou smears and tissue samples were collected prospectively from the uterine cervix and endometrium, and data analysis was done retrospectively. Undertaking a transvaginal approach, cervical cytology by Hatta brushing (Soft Medical Co, Tokyo, Japan) and endometrial cytology using a spatula (Softcyte, Soft Medical Co, Tokyo, Japan) were performed by three operators (TT, KH, and MK). IUDs removed during the first visit or at surgery were used for imprint cytology by making a smear after direct touching on a slide glass. Papanicolaou staining was performed after alcohol fixation. Gomori’s methenamine silver stain was performed using the Papanicolaou-stained specimens. Endometrial tissue samples were collected by endometrial suction curette (Pipet Curet, MX140, Cooper Surgical, Trumbull, CT). We prospectively evaluated for the presence of actinomycosis in samples by cytological, pathological, and bacteriological examination. A bacterial culture system was used to cultivate vaginal discharge, IUD-adherent materials, and abscess contents derived from each patient using an Arginine glycerin salt agar plate. All prospective cytology, pathology, and bacteriology findings were retrospectively reanalyzed and the validity of the results was confirmed independently by experts in clinical cytology (KM) and in gynecological cytopathology and microbiology (HN and SM). We could remove only two IUDs before surgery in our study, and failed to remove the remaining seven IUDs.
Clinical features recorded from patient files included age, chief complaint, gravidity, parity, duration of IUD use, type of IUD inserted, patients with or without atypical genital bleeding and/or discharge, and operative procedure. Hematology (white cell count, red cell count, platelet count, C-reactive protein level) was analyzed and three tumor makers (CA125, CA19-9, CA54/61) were measured in the sera collected from these patients.
Results
Clinical findings and the operative methods used in our nine cases are described in . The mean patient age was 49.2 (range 32–57) years. All women were parous and had an IUD inserted. The median duration of IUD use was 15.3 (range 7–25) years. All women complained of lower abdominal pain and fever as the main clinical manifestation, and were diagnosed as having pelvic inflammatory disease during their first hospital visit. All cases except one had had an IUD for more than 10 years. Information regarding the type of IUD inserted was unknown in three cases. Four women (44.4%) complained of atypical genital bleeding, with vaginal discharge of varying color noted in five cases (55.5%). Eight cases underwent hysterectomy and adnexectomy, and one case underwent segmental sigmoid colon resection.
Table 1 Clinical findings in patients with pelvic actinomycosis
The peripheral blood picture and serum tumor markers of our nine cases are shown in . All cases showed a remarkable increase in white cell count and C-reactive protein, indicating severe inflammatory changes. Seven of nine cases (77.8%) had increased serum platelet levels. Three serum tumor markers were measured to exclude malignancy, with some cases showing abnormal levels. Four of seven cases (57.1%) had abnormal serum tumor markers ().
Table 2 Laboratory findings in patients with pelvic actinomycosis
The actinomyces detection rate as evaluated by cytology, pathology, and bacteriology is shown in . Actinomyces colonies were present in five of nine cases (55.6%) on cervical and/or endometrial cytology performed before surgery.
Table 3 Detection rate of actinomyces on cytology, pathology, and bacterial cultivation
We detected actinomyces in four of nine patients (44.4%) by cervical cytology and three of eight cases (37.5%) by endometrial cytology (). Actinomyces were detected in a total of six patients (66.7%) by cytology of either the uterine cervix and/or endometrium or imprint IUD cytology before surgery. All three cases with imprint IUD cytology showed actinomyces colonies and bacterial threads which were confirmed by Gomori’s methenamine silver stain (). Imprint IUD cytology was done in three cases after IUD removal before surgery or from an IUD retained in the uterus after surgery. One patient (case 6 in ) with positive imprint IUD cytology before surgery showed negative findings on both cervical and endometrial cytology. Actinomyces colonies were detected in two of four patients (50%) by Papanicolaou smear of abscess contents obtained during surgery. Case 5 in showed a 2–3 mm sized yellowish-white granulated discharge present in the vagina and many actinomyces colonies on squash preparation (). Case 8 in showed the presence of actinomyces colonies only in abscess contents of the right adnexa ().
Figure 1 (A) Cytology of the endometrium, with basophilic granules of actinomyces seen with an inflammatory background (Papanicolaou stain, object lens magnification 10×). (B) Magnified image of (A) (Papanicolaou stain, 40×). Thin filamentous mycelia are seen spreading outwards. (C) Imprint cytology of intrauterine contraceptive device. Many actinomyces colonies are seen within severe inflammatory background (Papanicolaou stain, 10×). (D) Gomori methenamine-silver stain of (C) showing presence of numerous blackish fine mycelia (40×).
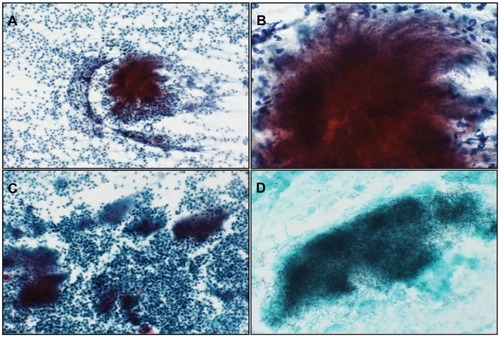
Figure 2 Brush cytology of granular discharge containing many actinomyces colonies (A) (Papanicolaou stain, object lens magnification 4×). (B) Magnified image of (A) (Papanicolaou stain, 40×) showing actinomyces strangles with abundant mycelia radiating outwards. (C) Gomori methenamine silver stain of (B) showing presence of blackish thin filamentous mycelia radiating outwards (40×).
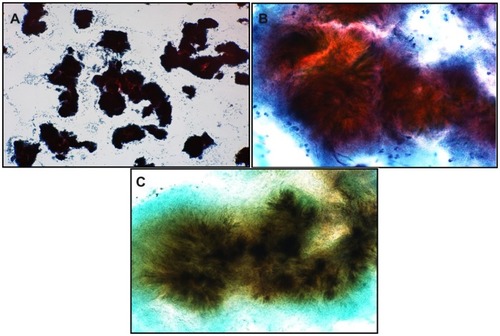
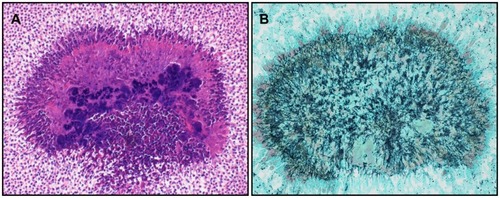
Figure 3 Hematoxylin and eosin stain of sample derived from right adnexal abscess. (A) Basophilic actinomyces colonies are seen in the central hematoxylin-stained area and eosinophilic colonies are seen in the periphery (sulfur granules). Splendore-Hoeppli materials are also seen in the outermost layer (object lens magnification 20×). (B) Gomori methenamine silver stain of (A) showing abundant mycelia radiating outwards (20×).
We could identify actinomyces colonies in all cases either by cytology or histology with a clinical diagnosis of pelvic actinomycosis. However, bacteriological examination of vaginal discharge, IUD, and abscess content specimens obtained before surgery showed colony formation of actinomyces in only one patient (case 1 in ), and was not detected after surgery. For four patients in whom pathological and bacteriological examination was unable to detect actinomyces, cervical or endometrial cytology was able to detect actinomyces in three cases.
Discussion
We demonstrate here that about 70% of pelvic actinomyces can be diagnosed by cervical and/or endometrial cytology or imprint IUD cytology before surgery, and the detection rate is even better when compared with pathological diagnosis after surgery. We found that three of four cases with a negative pathological diagnosis of pelvic actinomyces had actinomyces colonies on cervical or endometrial cytology. This result is clinically significant, even though we enrolled only a small number of patients in this study.
Information is limited in the literature regarding the preoperative diagnosis of pelvic actinomycosis using noninvasive procedures such as cervical or endometrial cytology. Abdominal actinomycosis develops most frequently in the gastrointestinal tract, especially around the ileocecal junction, but pelvic actinomycosis in women is rare. Brenner and Gehring reported an increased incidence of pelvic actinomycosis in IUD users,Citation2 and subsequent studies have found a close relationship between pelvic actinomycosis and prolonged IUD use.Citation3,Citation6–Citation10 This is consistent with the findings of our study, in which all of our nine women had used an IUD for periods ranging from 7 to 20 years.
Keebler et alCitation11 reported that the risk of actinomyces infection increased if an IUD is used for longer than 2 years, with an infection rate of 8.4% (9/107) in women using an IUD for 1–2 years and 19% (16/84) for 2–3 years. Aoki and ImamuraCitation12 reported a similar infection rate in women who had used an IUD for a mean period of 3.5 years. Fujiwara and KoumotoCitation4 reviewed 112 women with pelvic actinomycosis who had used an IUD for a period of two decades. In their study, the actinomycosis infection rate was 90.7% for a mean period of IUD use of 9.8 (range 1.7–30) years. The average duration of IUD use in our cases was 15.3 (range 7–25) years, which is longer than in any of the previous studies. The results of our study further strengthen the association between prolonged IUD use and actinomyces infection. Although the type of IUD was unknown in three of our cases, most of the cases positive for actinomyces infection used an FD-1 device. The link between occurrence of infection and this particular type of IUD needs to be investigated further.
Clinical features of pelvic inflammatory disease are abdominal pain, fever, and an increase in serum white blood cell and/or C-reactive protein levels. All of our cases attended hospital because of abdominal pain and fever, showed a marked increase in white blood cell and C-reactive protein levels, and had a clinical diagnosis of pelvic inflammatory disease (abscess in the pouch of Douglas or adnexa) before surgery. Cedermark et alCitation13 reported that a raised platelet count might be useful to differentiate actinomycosis from other infections, and there have been similar reports from Japan.Citation14,Citation15 Seven cases (77.8%) in our study showed a marked increase in platelet count (≥40 × 104/μL). An elevated platelet count with symptoms of pelvic inflammatory disease might be helpful in isolating cases with actinomycosis. From the bacteriological point of view, it is necessary to confirm a diagnosis of actinomycosis by bacterial culture; however, the detection rate by bacterial culture has been reported to be low. Hager et alCitation16 reported a detection rate of 2% by bacterial culture and 8% by cytology. Similarly, Fujiwara and KoumotoCitation4 reported that none of their 10 cases with actinomyces were able to be detected by bacterial culture. Therefore, actinomyces colonies are unlikely to be detected by bacteriological examination.
Actinomyces are fastidious bacteria that are difficult to isolate by culture. During bioculture of actinomyces, it is necessary to maintain strict anaerobic conditions in an environment containing 6%–10% carbon dioxide. In addition, detection by bacterial culture may be difficult on overnight incubation due to slow growth of actinomyces.Citation17 This might explain why we could isolate actinomyces in only one sample by bacterial culture. The amount sampled, collection site, or timing of sample collection could also be important. In our study, samples from only one case showed actinomyces species (11.1%) by bacterial culture before surgery and not postoperatively.
Gupta et alCitation18 reported the presence of actinomyces in the cervical smears of women using an IUD, which corresponded with subsequently published reports. The actinomyces detection rate by cytology was found to be as high as 8%–44% in IUD users, but as low as 0%–2.8% in nonusers.Citation16,Citation18,Citation19 All of our patients used an IUD and our actinomyces detection rate by cervical cytology (44.4%) was similar to that in the published reports. We did not investigate the incidence of actinomyces in women who were not IUD users. Instead, we performed double cytology (cervical and endometrial) before surgery and found that combining these two cytological procedures with imprint IUD cytology before surgery may increase the detection rate to 66.7% when compared with cervical (44.4%) or endometrial (37.5%) cytology. Actinomycosis was detected in two of nine cases by imprint IUD cytology before surgery. One case had parallel positive findings by cervical cytology and another case had negative findings by cervical and endometrial cytology. This finding may be clinically important, favoring imprint IUD cytology before surgery in patients with clinically suspected actinomycosis presenting with positive findings for pelvic inflammatory disease and negative findings on cervical or endometrial cytology.
We suspected actinomycosis clinically because of the presence of granulated vaginal discharge and a history of prolonged IUD use, with subsequent confirmation on cytology and histology. The presence of sulfur granules is a definite marker for a pathological diagnosis. We could detect sulfur granules on postoperative histopathology in four of our eight patients (50%, ). However, abundant sulfur granules are rarely visible on cytology without close examination of large numbers of samples.Citation20,Citation21
Diagnosis of pelvic actinomycosis is not possible by imaging. The image findings of actinomycotic adnexal abscesses are similar to that of malignancy, because actinomycotic adnexal abscess progresses with invagination deep into the peritoneum and fascia. Thus, it is difficult to differentiate malignancy from actinomycotic abscess by diagnostic imaging. Most cases of actinomyces are diagnosed postoperatively, with less chance (<20%) of a diagnosis before surgery.Citation22 Although one of our cases was suspected to be malignant, all patients visited our hospital with the findings of a pelvic mass, a diagnosis of pelvic inflammatory disease, and with a history of prolonged IUD use before surgery. Our cases were compatible with a diagnosis of pelvic actinomycosis.
In conclusion, our higher detection rate of actinomyces by cytology than by pathology or bacteriology suggests that careful cytological examination may be clinically useful in the preoperative diagnosis of pelvic actinomycosis. Clinical features suggestive of pelvic actinomycosis and a prolonged history of IUD use require further investigation in a larger number of patients.
Disclosure
This study was presented in part at the 52nd Annual Spring Meeting of the Japanese Society of Clinical Cytology, May 20–22, 2011, Fukuoka, Japan. Otherwise, the authors report no conflicts of interest in this work.
References
- ReichenbachJLopatinUMahlaouiNActinomyces in chronic granulomatous disease: an emerging and unanticipated pathogenClin Infect Dis2009491703171019874205
- BrennerRWGehringSW2ndPelvic actinomycosis in the presence of an endocervical contraceptive deviceObstet Gynecol19672971736017947
- HendersonSRPelvic actinomycosis associated with an intrauterine deviceObstet Gynecol1973417267324696986
- FujiwaraMKoumotoYClinical investigation of 10 cases of pelvic actinomycosis and literature reviewMod Trends Obstet Gynecol2010591521 Japanese
- HaHLeeHKimHAbdominal actinomycosis: CT findings in 10 patientsAJR Am J Roentgenol19931617917948372760
- ShiraiAMachidaTSuzukiRTwo cases of actinomycosis manifesting as a pelvic massObstet Gynecol200067817821 Japanese
- SaijoYKobayashiTTerasawaKA case report of actinomycosis of ovaryObstet Gynecol199865815818 Japanese
- AlfuhaisTReinholdCPelvic actinomycosis associated with intrauterine device use: case reportCan Assoc Radiol J20035416016212866241
- HamaguchiDMatsudaKKitajimaMA case of pelvic actinomycosis after prolonged intrauterine contraception diagnosed by postoperative cytologyJpn J Clin Cytol200948381385 Japanese
- MatsudaKMoriyamaSKoteraKA case report of pelvic actinomycosis recognized with prolonged use of an intrauterine contraceptive deviceKyushu Bran Jpn Clin Cytol2007386368 Japanese
- KeeblerCChatwaniASchwartzRActinomycosis infection associated with intrauterine contraceptive devicesAm J Obstet Gynecol19831455965996829637
- AokiTImamuraKActinomycosis in wearers of intrauterine deviceJpn J Clin Cytol198221535540 Japanese
- CedermarkBSundbladRWillemsJSuspected neoplasm of the liver with pulmonary metastases cured by surgery and penicillin: disseminated actinomycosis revisitedAm J Surg19811413843867212189
- MinaguchiTSugaseMPelvic actinomycosis associated with prolonged use of an intrauterine contraceptive deviceObstet Gynecol19966315891591 Japanese
- KurodaUMatsubaraKKusanagiYA case of pelvic actinomycosis caused by intra uterine deviceClin All Round19984724252427 Japanese
- HagerWDDouglasBMajmudarBPelvic colonization with actinomyces in women using intrauterine contraceptive devicesAm J Obstet Gynecol1979135680684507119
- MabezaGFMacFarlaneJPulmonary actinomycosisEur Respir J20032154555112662015
- GuptaPKHollanderDHFrostJKActinomycetes in cervicovaginal smears: an association with IUD usageActa Cytol1976202952971066933
- CurtisEMPineLActinomuces in the vaginas of women with and without intrauterine contraceptive devicesAm J Obstet Gynecol19821408808847023240
- McCormicJFScorgieRDUnilateral tuboovarian actinomycosis in the presence of an intrauterine deviceAm J Clin Pathol197768622626920661
- LuffRDGuptaPKSpenceMRPelvic actinomycosis and the intrauterine contraceptive device. A cytohistomorphologic studyAm J Clin Pathol197869581586665579
- FiorinoASIntrauterine contraceptive device-associated actinomycotic abscess and actinomyces detection on cervical smearObstet Gynecol1996871421498532252