Abstract
Objective
To investigate the potential protective impact of miR-10a-modified HUMSCs-derived exosomes on both premature ovarian failure and the functionality of ovarian granulosa cells in a POF model.
Methods
KGN cells were co-cultured with cisplatin-diaminedichloroplatinum (II) (10 μM) for 24 h to establish an in vitro POF model. The cells were distributed into three distinct groups: the control group, the POF group, and the POF + HUCMSC group. The plasmid sh-NC, sh-miR-10 a and miR-10 a mimic were transfected into KGN cells. After co-cultured with HUCMSC-EVs for 48 h, they were divided into HUCMSC group, sh-miR-10 a-HUMSCs-exosomes group and miR-10 a-HUMSCs-exosomes group. Flow cytometry was adopted to assess the impact of HUMSCs surface immune antigens and miR-10a-HUCMSCs-exosomes on KGN cell apoptosis. Additionally, the evaluation of cell proliferation was carried out through CCK-8 and EDU assays. Western blot analysis was utilized to detect the Caspase-3, Bax, and Bcl-2 proteins levels. Furthermore, the levels of TNF-α, IL-6, IL-10, MDA, SOD, and CAT were quantified using ELISA.
Results
Compared with the Control group, the POF group inhibited the growth of ovarian granulosa cells (P<0.01), reduced the number of EDU cells (P<0.01), and increased the protein expression of Caspase-3 (P<0.05) and Bax (P<0.01). HUMSCs treatment significantly down-regulated the expression of IL-6, TNF-α and MDA, while up-regulating the expression of IL-10, SOD and CAT (P<0.01); the overexpression of miR-10a promoted cell growth, besides, the introduction of miR-10a-HUMSCs-derived exosomes led to an elevation in the proliferation rate of OGCs affected by POF and concurrently suppressed the apoptosis rate.
Conclusion
HUMSCs-derived exosomes modified by miR-10a have protective effects on premature ovarian failure and ovarian granulosa cell function in POF model.
Premature ovarian failure (POF) is a significant concern within the field of female reproductive health. The past few years have witnessed a discernible upswing in the POF incidences.Citation1 Characterized by an absence of mature ovarian follicles in women younger than 40, POF is concomitant with reduced estrogen and elevated gonadotropin levels. The clinical manifestation commonly involves symptoms like hot flashes, excessive perspiration, sleep disturbances, absence of menstruation, and sexual dysfunction.Citation2,Citation3 Various etiological factors contribute to POF, encompassing genetic abnormalities, autoimmune conditions, and environmental influences.Citation4 Given the suboptimal outcomes associated with conventional therapeutic interventions, the use of Human Umbilical Cord Mesenchymal Stem Cells (HUMSCs) for the purpose of ovarian regeneration represents an innovative and promising treatment strategy. The human umbilical cord is like a robust reservoir of mesenchymal stem cells. Differing from stem cells derived from bone marrow, those obtained from the human umbilical cord offer the advantages of expedited procurement with minimal discomfort. Furthermore, these cells exhibit a similar triad of capabilities to bone marrow cells, encompassing tissue repair, modulation of immune responses, and anti-cancer properties.Citation5
Similar to MSCs originating from different sources, HUMSCs exhibit a unique ability for self-renewal while retaining their potential for differentiation into various cell types including bone cells, adipocytes, chondrocytes, hepatocytes, and neurons. The enhancement of ovarian function through stem cells is primarily attributed to their paracrine effects, rather than their direct commitment to specific cellular lineages.Citation6 Vesicles released by Mesenchymal Stem Cells (MSCs) encompass an array of cytokines, growth factors, regulatory miRNAs, mRNAs, and signaling lipids. These components collectively influence cellular communication, intercellular signaling, and alterations in cell or tissue metabolism, thereby imparting considerable impacts.Citation7 Exosomes, tiny vesicles spanning 10 to 100 nm in diameter, are actively secreted by various cellular origins. Their essential role lies in enabling cellular communication through the conveyance of bioactive lipids, nucleic acids, and proteins.Citation8 Exosomes hold significant importance in the context of the paracrine functionality of MSCs. An increasing body of research underscores the potential of MSC-exosomes as an alternate therapeutic avenue to harness MSC paracrine effects. Specifically, HUMSCs exhibit the capability to safeguard ovarian granulosa cells (OGCs) from apoptotic processes and regulate immune factor actions in the context of POF.Citation9 Despite these insights, the comprehensive mechanisms through which exosomes counterbalance ovarian functionality remain incompletely demonstrated.
The primary goal in this study was to explore the potential protective effects of miR-10a-modified exosomes derived from HUMSCs on both premature ovarian aging and the function of ovarian granulosa cells within a POF model.
Materials and Methods
HUCMSC Isolation and Identification
HUCMSCs were purchased from Zhongyuan Xiehe Stem Cell Bioengineering Co., Ltd. In this study, the biological characteristics of HUCMSC s were used to establish a separation method based on cell-attached slide, and the serum-free medium of umbilical cord mesenchymal stem cells was selected. The umbilical cord tissue was broken into particles the size of rice grains. The umbilical cord particles were inoculated into the culture medium and left to stand at 37°C for 48 hours. The culture medium was replaced every 48 hours and cytokines were added. On the 7th day of culture, the cells were harvested and frozen for later use.
The immunophenotypic characteristics of HUCMSCs were evaluated via flow cytometry analysis. To mitigate non-specific binding, the cells underwent pre-treatment with 10% normal goat serum. Following this step, the cells were subjected to incubation with a series of FITC dye-labeled human monoclonal primary antibodies, including CD44, CD73, CD90, CD105, CD19, CD34, and HLA-DR (at a dilution ratio of 1:100) for a duration of 30 minutes. Subsequently, the cells were re-suspended in 10% normal goat serum and subjected to cellular analysis using a CyAn ADP analyzer.
Establishment and Grouping of POF Models
The human ovarian granulosa cell tumor cell line KGN was obtained from the American Type Culture Collection (ATCC, Manassas, USA), a cell line derived from human granulosa tumors, were cultivated in RPMI 1640 medium enriched with 10% fetal bovine serum, maintaining a controlled environment at 37°C with 5% CO2 to ensure optimal growth conditions. To simulate an in vitro POF model, Cis-diamine dichloroplatinum (II) (10 μM) was co-cultured with KGN cells for 24 hours. For experimental setup, KGN cells (5×103 cells per well) were seeded in 96-well plates and incubated overnight. Subsequently, the culture medium was replaced with 100 μL of HUCMSCs (at a concentration of 1×109 cells per milliliter) in serum-free medium for a 48-hour incubation period. The samples were divided into two groups: the Control group (KGN cells without treatment) and the Treatment group; POF group: KGN cells were treated with cis-diaminedichloroplatinum (II) (10μM) for 24 hours; POF+HUCMSC group: KGN cells were subjected to a 24-hour treatment with cis-diaminedichloroplatinum (II) at a concentration of 10 μM.
Plasmids sh-NC, sh-miR-10a, and miR-10a mimic were transfected into KGN cells via Lipoamine 2000 transfection. Following a 24-hour transfection period, KGN cells (5 × 103 cells per well) were seeded into 96-well plates and subjected to an overnight incubation. Subsequently, the culture medium was replaced with 100 μL of hUCMSC-derived extracellular vesicles (hUCMSC-EVs) at a concentration of 1×109 per milliliter, and the cells were cultured in serum-free medium for a duration of 48 hours. The cells were randomly divided into HUCMSC group: treat KGN cells with cis-diamine dichloroplatinum (II) (10 μM) for 24 hours, transfect sh-NC plasmid into KGN cells and co-culture with HUCMSCs for 48 hours; sh-miR-10a-HUMSCs-exosomes group: KGN cells were exposed to cisplatin (II) (10μM) for 24 hours, and then the sh-miR-10a plasmid was transfected into KGN cells and co-cultured with HUCMSC for 48 hours; miR-10a-HUMSCs-exosomes group: KGN cells were exposed to cis-diaminedichloroplatinum (II) (10 μM) for 24 hours, and sh-miR-10a mimic plasmid was transfected into KGN cells and co-cultured with HUCMSCs for 48 hours.
Extraction of HUCMSC Exosomes
HUMSCs were cultured in a serum-deprived medium for a duration of 72 hours. Afterward, the cells were subjected to centrifugation at a speed of 1200 × g for a duration of 25 minutes at a temperature of 4°C, aimed at removing cellular debris and nonviable cells. The resulting supernatant underwent further purification through filtration using a 0.2 mm filter. Subsequently, the cellular suspension was subjected to ultracentrifugation at the speed of 120,000×g for 2.5 hours at 4°C, followed by an additional ultracentrifugation step at 120,000 × g for 2 hours. Finally, the resulting pellet was resuspended in PBS.
Western Blot
Cellular proteins were extracted utilizing radioimmunoprecipitation assay (RIPA) cell lysis buffer. The protein concentration of each sample was subsequently assessed using a BCA kit. Following separation by polyacrylamide gel electrophoresis, the proteins were transferred onto polyvinylidene fluoride membranes using the wet transfer method. The membranes were subsequently exposed to 5% bovine serum albumin (BSA) for a duration of 1 hour to mitigate nonspecific binding. The subsequent step involved incubating the membrane with a combination of primary antibodies, which encompassed mouse anti-human Bcl-2 (diluted at 1:2000), Bcl-2-associated X protein (Bax) (diluted at 1:5000), Caspase-3 (diluted at 1:1000), and GAPDH (diluted at 1:5000), at a temperature of 4°C overnight. Subsequently, conduct three washes with TBST (each rinse lasting 5 minutes) and then proceed to incubation with a diluted solution of horse red peroxidase (HRP)-labeled goat anti-rabbit IgG at a dilution ratio of 1:20,000 and react at room temperature for 1 hour. Then perform another round of TBST washing, and add chromogenic solution for coloring. Lastly, utilizing the gray value ratio of each protein to GAPDH, we conducted protein quantitative analysis using ImageJ 1.48u software.
Cell Viability Assay
Cell proliferation was assessed by quantifying the cellular growth rate. The cells were plated in 96-well plates at a density of 2×103 cells per well and allowed to incubate for 0, 12, 24, and 48 hours. Subsequently, CCK-8 reagent was added to each well with a volume of 10μL, and a subsequent incubation of 2 hours was carried out. The optical density at 450 nm was then quantified using a microplate reader.
Flow Cytometry and EDU Detection
Apoptosis was evaluated using flow cytometry by employing the Annexin V-FITC/PI Apoptosis Detection Kit. Briefly, cells were collected, washed, and then labeled with Annexin V-FITC and PI in a controlled lighting setting. The stained cells were subsequently subjected to analysis using flow cytometry, and the acquired data were subjected to processing using Flow Jo 7.6 software.
Cell proliferation was quantified utilizing the CellTiter-Glo Luminescent Cell Viability Assay. Each well was supplemented with EdU (10 mM), followed by fixation with 4% formaldehyde for a duration of 30 minutes. Post-fixation, EdU was detected employing the Click-iTR EdU kit, and resulting images were observed utilizing a fluorescence microscope.
ELISA
The levels of TNF-α, IL-6, IL-10, MDA, SOD, and CAT in the cell culture supernatant were quantified according to the instructions provided with the respective assay kits.
Statistical Analysis
Statistical analysis was performed using GraphPad v7.0 software, and the data were expressed as (). ANOVA was employed to compare variations among multiple groups, with a significance level of P < 0.05 representing statistical significance.
Results
Immunophenotypic Analysis of HUMSCs
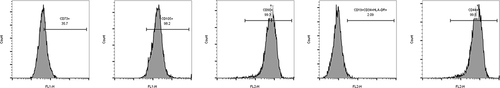
The utilization of flow cytometry enabled the assessment of surface marker expression. illustrates that the expression percentages of CD44, CD73, CD90, and CD105, which are HUMSCs surface antigens, exceeded 95%. Conversely, the expression percentages of CD19, CD34, and HLA-DR were below 5%. This signifies that HUMSCs exhibit negativity for CD19, CD34, and HLA-DR, and positivity for CD44, CD73, CD90, and CD105, known as recognized surface markers for HUMSCs.
HUMSCs Promote the Proliferation of Ovarian Granulosa Cells
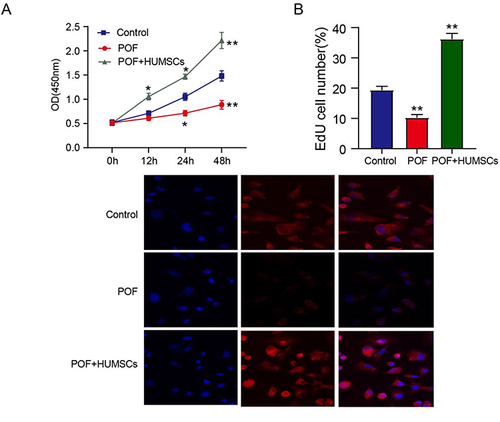
The results shown in and reveal that, when contrasted with the Control group, the POF group exhibited an inhibitory effect on the growth of ovarian granulosa cells (P < 0.01) and a reduction in the count of EDU-labeled cells (P < 0.01). Conversely, HUMSCs promoted cell proliferation, leading to an increase in the number of EdU-labeled cells, cellular metastasis, and ultimately ameliorating the suppressed cell proliferation observed in POF conditions.
Figure 2 HUMSCs increases cell proliferation. (A) CCK-8 was used to detect cell proliferation; (B) The effect of HUMSCs on cell proliferation was analyzed by EDU assay, The nucleus is colored blue by DAPI and the cytoplasm is colored red by EDU.
HUMSCs Inhibit the Apoptosis of Ovarian Granulosa Cells
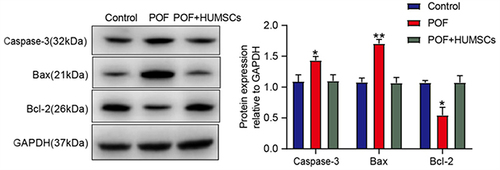
Ovarian granulosa cells regulate oocyte and follicle development and have been shown to be targets for cytotoxic drugs. In order to further study the possible pathways through which HUMSCs regulate the apoptosis of ovarian granulosa cells, we detected the expression levels of apoptosis-related pathways in ovarian tissues of Control, POF and POF+HUMSCs groups. illustrates that the levels of Caspase-3 (P < 0.05) and Bax (P < 0.01) showed elevated expression within the POF group, whereas they demonstrated reduced expression in both the Control group and the POF+HUMSCs group. Conversely, the anti-apoptotic protein Bcl-2 displayed contrasting expression.
HUMSCs Can Inhibit Ovarian Inflammation and Oxidative Stress Caused by POF
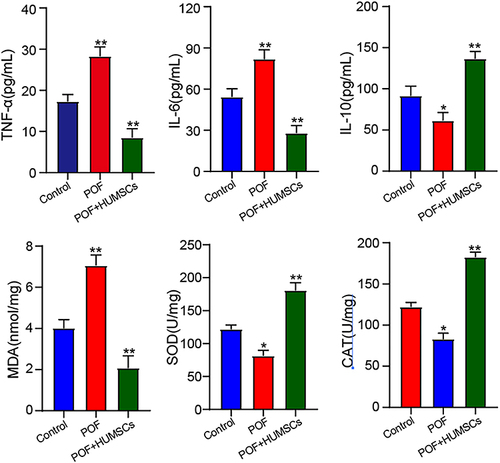
To investigate the impact of HUMSCs on ovarian inflammation induced by cyclophosphamide, illustrates that, in contrast to the Control group, a significant increase was observed in the levels of IL-6, TNF-α, and MDA. Concurrently, a marked elevation was noted in the levels of IL-10, SOD, and CAT. After HUMSCs treatment, a notable decrease in the expression of IL-6, TNF-α, and MDA was observed, alongside an increase in the expression of IL-10, SOD, and CAT (P < 0.01). This observed difference is of significance.
MiR-10a Increased the Cell Proliferation Rate of POF OGCs in vitro
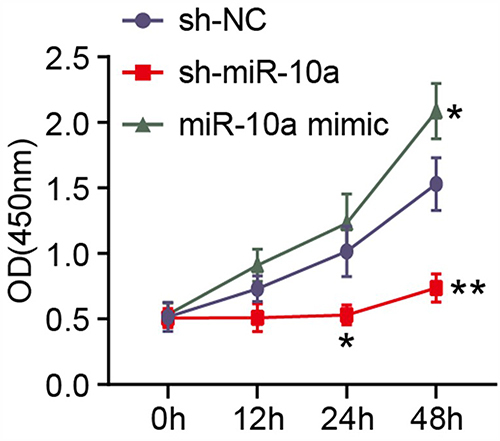
The objective of this experimental session was to ascertain the impact of miR-10a on premature ovarian failure in an in vitro model. illustrates that transfecting miR-10a resulted in an elevation of miR-10a expression, while introducing sh-miR-10a led to a reduction in miR-10a expression in the in vitro model. Notably, the heightened expression of miR-10a promoted cell proliferation, whereas the down-regulation of miR-10a was associated with a diminished cell growth rate. These findings underscore the pivotal role of miR-10a regulation in influencing the outcomes of the in vitro model for premature ovarian failure.
MiR-10a-HUMSCs-Exosomes Can Be Internalized into OGCs
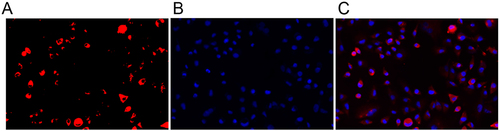
Primary rat OGCs were obtained from ovarian tissue and then cultured. Subsequently, our focus was to investigate the internalization of miR-10a-HUMSCs-exosomes into OGCs. These exosomes were labeled using the fluorescent reagent PKH126 and were subjected to co-culturing with OGCs for 48 hours. illustrates that the distinct red fluorescence emitted by the labeled exosomes was prominently observed in the perinuclear region of the OGCs. This prominent fluorescence signifies the successful internalization of PKH26-labeled exosomes into the cytoplasm of the OGCs, indicative of a phagocytic event. This phenomenon establishes a fundamental premise for the plausible functional impact associated with miR-10a-HUMSCs-exosomes on OGCs.
MiR-10a-Modified Exosomes Derived from HUMSCs Exhibited an Enhanced Proliferation Rate in POF OGCs and Effectively Suppressed the Apoptosis Rate
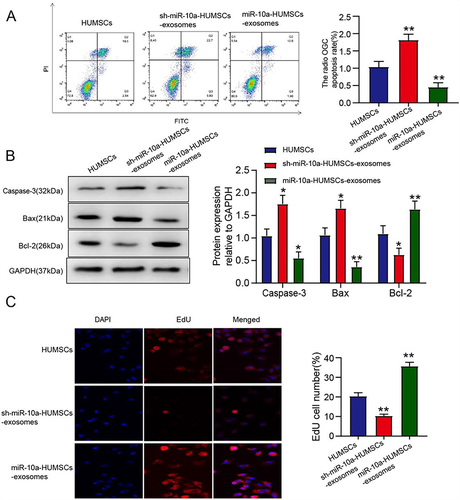
To explore the potential impact of miR-10a modulation in HUMSCs on OGC apoptosis, we established a POF cell model to induce apoptosis in OGCs. Through annexin V-FITC/PI double staining and subsequent flow cytometry analysis, we evaluated the apoptotic status. As illustrated in , the apoptotic proportion of OGCs administered with miR-10a-HUMSCs-exosomes was considerably decreased compared to the HUMSCs-exosomes group, and this observation underscores the inhibitory effect of miR-10a-HUMSCs-exosomes on OGC apoptosis in vitro. Furthermore, as demonstrated in through Western blot analysis, miR-10a-HUMSCs-exosomes induced a reduction in Caspase-3 (P < 0.05) and Bax (P < 0.01) protein expressions, while concurrently elevating the expression level of Bcl-2 protein (P < 0.01). Subsequently, our investigation extended to the impact of miR-10a-HUMSCs-exosomes on OGC proliferation, and the EDU assay as shown in demonstrated a significant promotion of GC proliferation by miR-10a-HUMSCs-exosomes.
Figure 7 miR-10a-HUMSCs-exosomes increased the proliferation rate of POF OGCs and inhibited the apoptosis rate. (A) The impact of miR-10a-HUCMSCs-exosomes on OGC apoptosis was evaluated through flow cytometry analysis. (B) Protein expression was assessed through Western blot analysis; (C) The impact of miR-10a-HUCMSCs-exosomes on OGC proliferation was assessed using the EDU assay, The nucleus is colored blue by DAPI and the cytoplasm is colored red by EDU.
Discussion
POF results from either follicular depletion or dysfunction, resulting in substantial consequences for female fertility and overall well-being.Citation10,Citation11 The incidence of POF is intricately intertwined with the health and state of ovarian granulosa cells. Situated as an encompassing layer of parietal cells surrounding follicles, granulosa cells assume a critical role in fostering follicle growth and maturation, while also contributing to the production of vital glandular hormones necessary for the maintenance of ovarian function. The occurrence of apoptosis among granulosa cells precipitates follicular atresia, culminating in a reduction of follicle count. The diminishing ovarian reserve function serves as a foundational factor in premature ovarian failure, with the aging and apoptosis of ovarian granulosa cells being identified as the key contributors to the decline in ovarian reserve. Therefore, the inhibition of apoptosis in granulosa cells holds a pivotal role in enhancing POF mitigation.Citation12 HUMSCs are classified as non-hematopoietic stem cells, possessing the remarkable abilities of self-renewal and regeneration. These inherent qualities render them a promising avenue for therapeutic intervention in addressing POF. Wang et al have demonstrated that umbilical cord stromal stem cells contribute to the secretion of diverse cytokines and growth factors, acting as essential nutritional contributors. Furthermore, researchers have documented that the transplantation of HUMSCs leads to a reduction in apoptosis, an elevation in sex hormone levels, and the restoration of ovarian function.Citation13
Inflammation response takes on a critical role in the depletion of ovarian follicles.Citation14 An increasing body of evidence underscores the influence of abnormal inflammation in disrupting the normal dynamics of ovarian follicles, ultimately leading to infertility.Citation15 In our study, we observed that HUMSCs exerted a suppressive effect on TNF-α and IL-6 levels, while concurrently elevating IL-10 levels in POF model mice, and this observation suggests that HUMSCs alleviated the effective expression of inflammatory markers within mouse ovaries induced by cyclophosphamide. Furthermore, oxidative stress contributes to the reduction in both follicle count and oocytes.Citation16 In this study, we observed that cyclophosphamide induction enhanced MDA levels, but reduced SOD and CAT activities. In the POF model of HUMSCs transplantation, HUMSCs transplantation exerts a protective effect by inhibiting oxidative stress.
Exosomes, membrane-bound vesicles ranging in diameter from 40 to 100 nm, are ubiquitously found in diverse biological fluids. They are secreted into the extracellular space by various cell types through fusion with the plasma membrane.Citation17 The exosome membrane primarily consists of lipids and proteins. Alongside proteins, exosomes also encapsulate various nucleic acids, such as miRNAs and other ncRNAs, which have been newly identified within the exosome lumen.Citation18 As exosomes circulate, the exosomal RNAs they carry can be internalized by neighboring or remote cells, thereby modulating the biological activity of these recipient cells. This modulation occurs through the transport of lipids, proteins, and nucleic acids.Citation19 Current findings indicate that exosome-borne microRNA (miRNA) serves as a conduit for signal transduction between cells, exerting substantial influence on the biological functions of donor cells on recipient cells in various disease.Citation20 Recent researches have indicated the involvement of exosome-mediated miRNAs in the progression of POF. Take a MSC-derived exosome namely miR-644-5p as an example, which has been identified for its capacity to hinder GC apoptosis, thereby holding potential for restoring ovarian function.Citation12 Moreover, exosomes exhibit the capability to mitigate GC apoptosis through the transfer of miR-1246.Citation21 Furthermore, exosomes carrying miR-146a and miR-10a have been demonstrated protective effects against ovarian follicular atresia in chemotherapy-induced mice.Citation22
Researches have demonstrated that miR-10 has been identified as a distinctive marker of mouse granulosa cells, moreover, it was found that miR-10a inhibited the proliferation of human, mouse and rat granule cells by targeting BDNF.Citation23 This is not consistent with our results, which may be caused by differences in sample processing and experimental conditions. Our experiments found that miR-10 can promote the proliferation of granulosa cells. To further confirm the role of exosomal miR-10, we conducted experiments about HUMSC-derived exosomes. We performed knockdown and overexpression studies, and the outcomes revealed that HUMSC-derived overexpressed miR-10 could remarkably increase the expression of Bcl-2 and decrease the expression of Bax and Caspase-3, which signifies the protective influence of HUMSC-derived miR-10 overexpression against cisplatin-induced apoptosis in ovarian granulosa cells, thereby safeguarding these cells from the damaging effects of cisplatin.
In conclusion, miR-10a-modified exosomes derived from HUMSCs demonstrate a protective effect against premature ovarian aging and ovarian granulosa cell dysfunction in the context of POF models. However, it is crucial to acknowledge that this investigation is still in its preclinical stage, highlighting the need for additional research to unveil the underlying mechanisms involved.
Ethics Approval and Consent to Participate
The study was reviewed and approved by the Ethics Committee of Zhangzhou Affiliated Hospital of Fujian Medical University, Approval No. 2022KYB064.
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
This study was supported by Doctoral Studio Climbing project of Zhangzhou Municipal Hospital Affiliated to Fujian Medical University in 2021 (project No. PDB202107).
Data Sharing Statement
The simulation experiment data used to support the findings of this study are available from the corresponding author upon request.
References
- Shareghi-Oskoue O, Aghebati-Maleki L, Yousefi M. Transplantation of human umbilical cord mesenchymal stem cells to treat premature ovarian failure. Stem Cell Res Ther. 2021;12(1):454. doi:10.1186/s13287-021-02529-w
- Chen L, Guo S, Wei C, Li H, Wang H, Xu Y. Effect of stem cell transplantation of premature ovarian failure in animal models and patients: a meta-analysis and case report. Exp Ther Med. 2018;15(5):4105–4118. doi:10.3892/etm.2018.5970
- Lew R. Natural history of ovarian function including assessment of ovarian reserve and premature ovarian failure. Best Pract Res Clin Obstet Gynaecol. 2019;55:2–13. doi:10.1016/j.bpobgyn.2018.05.005
- Umer A, Khan N, Greene DL, Habiba UE, Shamim S, Khayam AU. The therapeutic potential of human umbilical cord derived mesenchymal stem cells for the treatment of premature ovarian failure. Stem Cell Rev Rep. 2023;19(3):651–666. doi:10.1007/s12015-022-10493-y
- Thaweesapphithak S, Tantrawatpan C, Kheolamai P, Tantikanlayaporn D, Roytrakul S, Manochantr S. Human serum enhances the proliferative capacity and immunomodulatory property of MSCs derived from human placenta and umbilical cord. Stem Cell Res Ther. 2019;10(1):79. doi:10.1186/s13287-019-1175-3
- Wang Z, Wang Y, Yang T, Li J, Yang X. Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Res Ther. 2017;8(1):11. doi:10.1186/s13287-016-0458-1
- Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851–858. doi:10.1002/stem.2575
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi:10.1126/science.aau6977
- Fu YX, Ji J, Shan F, Li J, Hu R. Human mesenchymal stem cell treatment of premature ovarian failure: new challenges and opportunities. Stem Cell Res Ther. 2021;12(1):161. doi:10.1186/s13287-021-02212-0
- Liu T, Liu Y, Huang Y, et al. miR-15b induces premature ovarian failure in mice via inhibition of α-Klotho expression in ovarian granulosa cells. Free Radic Biol Med. 2019;141:383–392. doi:10.1016/j.freeradbiomed.2019.07.010
- Liu T, Li Q, Wang S, Chen C, Zheng J. Transplantation of ovarian granulosa‑like cells derived from human induced pluripotent stem cells for the treatment of murine premature ovarian failure. Mol Med Rep. 2016;13(6):5053–5058. doi:10.3892/mmr.2016.5191
- Sun B, Ma Y, Wang F, Hu L, Sun Y. miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res Ther. 2019;10(1):360. doi:10.1186/s13287-019-1442-3
- Ghadami M, El-Demerdash E, Zhang D, et al. Bone marrow transplantation restores follicular maturation and steroid hormones production in a mouse model for primary ovarian failure. PLoS One. 2012;7(3):e32462. doi:10.1371/journal.pone.0032462
- Mantawy EM, Said RS, Abdel-Aziz AK. Mechanistic approach of the inhibitory effect of chrysin on inflammatory and apoptotic events implicated in radiation-induced premature ovarian failure: emphasis on TGF-β/MAPKs signaling pathway. Biomed Pharmacother. 2019;109:293–303. doi:10.1016/j.biopha.2018.10.092
- Boots CE, Jungheim ES. Inflammation and human ovarian follicular dynamics. Semin Reprod Med. 2015;33(4):270–275. doi:10.1055/s-0035-1554928
- Wei JH, Yuan XY, Zhang JM, Wei JQ. Caspase activity and oxidative stress of granulosa cells are associated with the viability and developmental potential of vitrified immature oocytes. Eur J Obstet Gynecol Reprod Biol. 2016;198:22–26. doi:10.1016/j.ejogrb.2015.12.010
- Wang S, Yu L, Sun M, et al. The therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failure. Biomed Res Int. 2013;2013:690491. doi:10.1155/2013/690491
- Sato-Kuwabara Y, Melo SA, Soares FA, Calin GA. The fusion of two worlds: non-coding RNAs and extracellular vesicles--diagnostic and therapeutic implications (Review). Int J Oncol. 2015;46(1):17–27. doi:10.3892/ijo.2014.2712
- Zhang J, Li S, Li L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13(1):17–24. doi:10.1016/j.gpb.2015.02.001
- Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014;3(1). doi:10.3402/jev.v3.23743
- Yang M, Lin L, Sha C, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab Invest. 2020;100(3):342–352. doi:10.1038/s41374-019-0321-y
- Xiao GY, Cheng CC, Chiang YS, Cheng WT, Liu IH, Wu SC. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci Rep. 2016;6(1):23120. doi:10.1038/srep23120
- Jiajie T, Yanzhou Y, Hoi-Hung AC, Zi-Jiang C, Wai-Yee C. Conserved miR-10 family represses proliferation and induces apoptosis in ovarian granulosa cells. Sci Rep. 2017;7(1):41304. doi:10.1038/srep41304