Abstract
Introduction
Cervical cancer screening has demonstrated high efficacy in reducing cervical cancer mortality worldwide. However, clinician sampling is often perceived as an uncomfortable procedure that could reduce screening uptake. Self-sampling methods for HPV diagnosis have shown high sensitivity, which could increase acceptance and screening rates among women.
Purpose
This study aims to identify the perceived barriers and advantages of self-sampling methods versus clinician sampling for cervical cancer screening in a rural setting in Ecuador.
Patients and Methods
A qualitative study was conducted. Seven focus group discussions took place in the rural Parish of El Valle in Azuay Province, Cuenca, Ecuador. Women native to this rural area were included in the study. FGDs were recorded and transcribed, and content analysis was performed to categorize and analyze the data.
Results
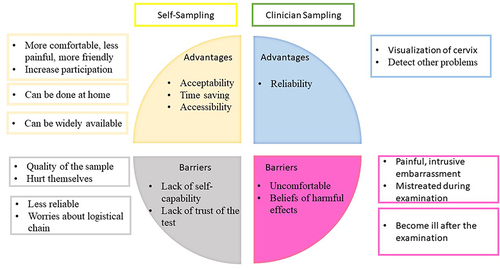
A total of 45 women participated in the study. Clinician sampling was perceived as a painful and intrusive method. However, participants believed that it is more reliable compared to self-sampling methods, attributing this to the direct visualization of the cervix, which facilitates the detection of cervical pathologies. The perceived advantages of self-sampling included increased comfort, pain reduction, time savings, the ability to perform the test at home, and the potential for widespread availability through pharmacies or local traditional healers. Nevertheless, doubts about the test’s reliability as well as the user’s proficiency in self-testing posed barriers to the adoption of this technique.
Conclusion
Self-sampling methods offer several advantages over clinician sampling, such as enhanced privacy, comfort, and accessibility to cancer screening. Barriers primarily revolved around users’ proficiency in performing the test and the reliability of the results. Providing training for using self-sampling tests could address these barriers.
Introduction
Since 1970, mortality from cervical cancer has witnessed a 70% reduction in developed countries. This remarkable decline can be attributed to the widespread adoption of large-scale population screenings, vaccination against the human papillomavirus (HPV), and the precise monitoring and treatment of premalignant lesions.Citation1 Around 80% of cervical cancer deaths occur in low- and middle-income countries (LMICs);Citation2 the disparities in cervical cancer prevention policies and the low coverage of screening could explain the higher rates observed in those countries.Citation3,Citation4
In Ecuador, cervical cancer (CC) ranks as the second most common form of cancer in women. In 2020 alone, 1534 new cases were identified, leading to the unfortunate loss of 813 women to CC.Citation5 Regrettably, the number of deaths and new cases in the country has not exhibited a discernible reduction over the past decade.Citation6,Citation7
Ecuador has a national strategy to prevent CC, primarily centered on cervical screening and vaccination against HPV (human papillomavirus).Citation8–10 The public health system in Ecuador provides free access to screening, primarily utilizing cytology, for individuals aged 21 to 65 years, with screening intervals of 3 years. Additionally, the system offers vaccination for girls aged 9 to 14 years, as part of the strategy to combat cervical cancer.Citation11 Despite these governmental efforts, 41.6% of women in Ecuador have never been screened.Citation12
Several barriers to CC screening, described in the international literature, are prevalent in Ecuador. The most common obstacles include extended waiting times, lack of privacy, feelings of embarrassment, and individual factors such as anxiety, discomfort, etc.Citation13,Citation14 Additionally, the lack of risk perception and knowledge about CC could pose obstacles for women residing in rural areas.Citation15,Citation16
In 2021, the World Health Organization (WHO) launched The Cervical Cancer Elimination Initiative.Citation4 The strategy of this initiative, named 90-70-90, promotes that 90% of the target population is vaccinated against HPV, 70% of the population is screened with highly sensitive HPV diagnostic tests, at least twice during their lifetime (at 35 and 45 years), and addressing 90% of abnormal results are addressed with appropriate treatment and follow-up.Citation17,Citation18 Primary HPV screening has proven to be more effective than the Pap smear in detecting early cervical premalignant lesions. Individual and organizational barriers could also diminish screening uptake and adherence and decrease follow-up.Citation19–21
The utilization of self-sampling methods as the primary screening approach has demonstrated an increase in adherence among under-screened women.Citation22,Citation23 Their sensitivity and specificity are comparable to those of clinician sampling.Citation24 Several publications highlighted the high sensitivity of self-sampling methods. Urine sampling and vaginal sampling have demonstrated a sensitivity of 90.5% and 98.9%, respectively.Citation25,Citation26 Urine sampling demonstrated a specificity of 82.8%, while vaginal self-sampling showed specificity ranging from 73.9% to 100% in the diagnosis of High-Risk HPV.Citation26,Citation27 Recent studies have demonstrated a high concordance for HPV diagnosis between clinician sampling and vaginal self-sampling, ranging from 89.2% to 97.5% and 87.6% to 91.1% for urine sampling.Citation28 This contrasts with the traditional cervical Pap smear, which reported sensitivity and specificity of 55% and 75%, respectively.Citation29,Citation30 In addition, self-sampling methods have shown high acceptance rates ranging from 90.5% to 97.3%.Citation28 The acceptability among indigenous populations and in rural areas enhances adherence to cervical cancer screening.Citation31,Citation32 Self-sampling represents a potentially cost-effective strategy capable of surmounting barriers in settings with low coverage and acceptance of cervical screening.Citation33–35
In Ecuador, no strategies involving self-sampling or urine sampling for HPV detection have been implemented. This study precedes the launch of a pilot study centered on a self-sampling initiative known as the CAMIE project. Moreover, this initial exploration of self-sampling perceptions for HPV diagnosis aims to offer clinicians and policymakers insights into both the advantages and barriers associated with its implementation at the community level.Citation36 This study aims to identify the possible barriers and advantages of the implementation of self-sampling methods versus clinician sampling for cervical cancer screening in the rural setting of Cuenca, Ecuador.
Materials and Methods
Research Design
This qualitative study was conducted from January to March 2021 in El Valle Parish of Cuenca, Ecuador, as a component of the qualitative phase of the CAMIE project (Making Cervical Cancer Screening Accessible Through Self-Sampling: A Step Towards Health Equality by Empowering Women in an Intercultural Context). Focus group discussions (FGDs) were selected as the method to facilitate participant interaction and share their ideas within this context. The phenomenological approach served as the framework for understanding the diverse perceptions of the participants. Reporting was based on the SRQR guidelines for qualitative research.
Recruitment and Setting
A convenience sampling recruitment method was employed for this study. Women from the Parish were recruited using a snowball technique, facilitated by collaboration with the two primary health centers in the community and the local government of El Valle Parish.
To ensure diversity, women from different levels of educational backgrounds, ages, and economic activities were included. The inclusion criteria were as follows: being able to provide consent to participate in the FGDs; residing in the Parish area of El Valle; possessing the mental capacity to comprehend the project’s objectives and provide informed consent; being of legal age (18 years or older) to sign the informed consent form; having initiated sexual activity; and having undergone at least one Pap smear during their lifetime, administered by a physician. The last criteria were considered to enable comparison between experiences in clinician sampling and self-sampling.
Face-to-face FGDs took place in community settings and places provided by health centers, the local government, and the community. All FGDs were led by the principal investigator and a researcher from the project.
The number of focus groups was determined based on data saturation, achieved when no new information was obtained from FGDs or when no new knowledge on the proposed topic emerged during the discussions. This criteria was used to determine sample size.Citation37,Citation38
Data Collection
Initially, a thematic guide for the focus groups was developed through a comprehensive literature review and insights from previous research. This guide included questions designed to address the following objectives: (a) understanding knowledge and perceptions regarding cervical cancer, (b) assessing knowledge about cervical cancer screening, and (c) exploring perceptions and acceptability related to self-sampling. For this research, only responses related to the latter question were considered. Additional topics covered in the guide have been previously published elsewhere.Citation16
Questions related to the advantages and barriers of each sampling method were used to motivate participation and ascertain opinions on each technique (eg, clinician sampling “What are the advantages and barriers of this method?”). , displays the key questions utilized during the FGDs.
Box 1 Content of Focus Group Guide
The researchers provided explanations regarding the instruments (ie, the Evalyn® Brush from Rovers Medical Devices, and a urine collector) used for collecting the urine samples and for vaginal self-testing due to participants’ lack of prior experience with the devices; supplementary clarifications related to the application of the instruments were provided. No information about the reliability of self-sampling methods was provided to the participants to avoid suggestions about their preferences. These methods were presented as potential alternatives aimed at reaching women in a rural context.
The focus groups were initiated with an introduction outlining the study’s objectives and by expressing commitment to the participants. Before the discussions, participants provided signed informed consent and granted permission for audio recording.
The participants were assured of the confidentiality of their contributions. The FGDs were conducted by a moderator, and an observer responsible for field notes was also present in the session. All members of the research team possess experience in the biomedical health system. Each focus group lasted between 1 and 1.5 hours. At the end of each session, both the moderator and the observer identified any necessary clarifications. The research team consisted of two members — one female and one male — both with expertise in qualitative research.
Data Analysis
The data collected in the FGDs were audio-recorded and transcribed. The research team ensured the accuracy of the transcriptions by verifying and correcting any errors. All field notes and the transcriptions of the FGDs were thoroughly reviewed for a compressive understanding of their contents.
Thematic content analysis was performed based on a narrative aiming to explore the participants’ perceptions, opinions, and interpretations of the research questions. It also involved collecting data from different perspectives and developing categories of meanings.Citation39 All transcripts were uploaded to N-Vivo 12 for Windows. Data analysis and coding were conducted by the first author (B.V.) and two experienced researchers (G.G. and A.N.).
Coding was defined based on the literature review and organized according to the participants’ perceptions of the sampling methods. The main codes were the advantages and barriers of each sampling method to the analysis. Opinions were categorized as an advantage when participants expressed a favourable view of the sampling test, whereas barriers encompassed negative perceptions or identified difficulties in the application of the sampling method.
Sub-categories emerged through inductive analysis and were subsequently organized and interconnected based on the main categories associated with each sampling test according to the main categories of each sampling test.Citation40,Citation41 The most important quotations related to advantages and barriers were carefully chosen and translated into English to enrich the results.
To ensure the quality of the qualitative studies, constant observation, well-structured and -executed fieldwork, reflection, and triangulation among the observers were performed.
Ethical Statement
This study was approved under the guidance of the Declaration of Helsinki and the Council for International Organizations of Medical Sciences (CIOMS). All procedures involving human participants were approved by the bioethical committee of the University of Cuenca (approval code UC-COBIAS-2020-26). All participants were informed about the purpose of the study and signed an informed consent form before the start of FGDs.
Results
Overall, 45 women participated in seven FGDs conducted between January 2020 and March 2021. The average duration of the FGDs was 1.2 hours. All FGDs were held in person, adhering to COVID-19 safety measures in Ecuador. and present the general characteristics and locations of the FGDs.
Table 1 Sociodemographic characteristics of the participants
Table 2 Focus Group
The results are presented based on participants’ perceptions of the advantages and barriers associated with each sampling method. Both positive factors (advantages) and negative perceptions (barriers) are highlighted. summarizes the advantages and barriers of sampling methods.
Clinician Sampling
Advantages
Reliability of the test: All women considered the Pap smear (clinician sampling) to be the gold standard for the prevention and detection of cervical cancer. They felt that it was more effective, safer, precise, and secure for diagnosis. Participants emphasized that the direct inspection of the vagina enables health professionals to have a direct view and access to the cervix, which is an advantage over self-sampling
Because it seems to me that it is safer. (Participant 43 years FGD 7)
Confidence that it (the speculum) goes where it should go. (Participant 35 years FGD 1)
Moreover, some women believe that the examination presents an opportunity to detect and treat other infections simultaneously.
That is why I do a Pap smear (clinician sampling) in that sense because there are infections too, that is why I also do a Pap smear to receive other kinds of treatment for infections. (Participant 45 years FGD 7)
Barriers
Uncomfortable procedure: Nearly all participants concluded that the Pap smear (clinician sampling) was an uncomfortable examination. The majority of the women expressed feelings of pain, embarrassment, or discomfort during this procedure. This discomfort emerges as a primary reason for some women to avoid cervical cancer screening.
A nightmare is how it used to be. I tell you, years ago, I did not do the pap smear at all. (Participant 56 years FGD 5)
Of course, discomfort, shame, embarrassment practically. Yes, it is a bit fastidious. (Participant 38 years FGD 4)
Some of the women expressed a sense of mistreatment during the examination, viewing this type of procedure as a breach of their intimate privacy. These sentiments were reported to be heightened, particularly when the health professional conducting the examination is male.
It’s such a thing… how to say it? A violation! Participant 58 years FGD 5)
Taking off (the clothes) and getting naked, and sitting back and spreading your legs. So that, all of that process is traumatic. (Participant 35 years FGD 1)
But I think that shame is a more, shame when a male gynaecologist comes. Participant 38 years FGD 4)
Harmful effects: Due to cultural patterns, women held the view that the use of a speculum could potentially cause harm to the genital tract, leading to injuries that might result in infections and even cervical cancer.
That is very important to know because it is scary because you know that it can hurt (the speculum) and when it already hurts (cervix), cancer hits there. That is what our elderly said, why would they go for it, if they get sicker (after the procedure). (Participant 45 years FGD 7)
I did the exam, and after 3 days a breakout of pimples was appearing down here (in the vagina) and I was already going crazy, I had to hurry back to the health center to see why. (Participant 56 years FGD 5)
The majority of participants perceived the vaginal examination as invasive. Some believe that a portion of the cervix is removed during the examination. In two FGDs, women thought that the sound heard when the speculum opens is due to the extraction of a piece of the cervix.
I speak for myself, for me, it was something new and that bothered me like a troc!(feel and hear a sound similar to troc) It did something like that. I don’t know if something was cut, pulled, I don’t know what it was. (Participant 40 years FGD 6)
Self-Sampling (Vaginal Self-Sampling)
Advantages
Acceptability: All participants from all FGDs identified positive aspects of self-sampling. Thus, they perceived it as a novel and unfamiliar method, and all expressed a willingness to undergo testing and experience it independently. Furthermore, there was a unanimous agreement among women that this method has the potential to reach under-screened women and contribute to saving lives.
I would be willing to do the test, and see if it is reliable. (Participant 30 years FGD 1)
There would be more women (who can have access) and many deaths would be avoided because there are still, there are women who die from uterine cancer. (Participant 57 FGD 3)
Time-saving: All women in the FGDs shared the belief that the waiting time to secure an appointment and receive attention at the health center would be reduced. Moreover, they considered that self-sampling might alleviate or prevent work absences.
That it is no longer necessary to spend time in the health center, you can take the sample and only leave it there. (Participant 35 years FGD 5)
Time, time is the worst enemy, how I already tell you, many (don’t go) for work or due to other things. and even if you have an appointment, it is not that you arrive and they examine you, you have to be 20 minutes before, and if there was an emergency, you have bad luck! The emergency is given priority and you keep waiting for a turn. (Participant 40 years FGD 5)
Comfortability: All women unanimously agreed that self-sampling could offer increased privacy, and an additional advantage is the flexibility it provides, allowing the test to be performed at home when women have ample time. Moreover, the women believed that the procedure would be less painful.
I can do it at home, by myself, I lock myself in the bathroom, rather than lie down in the room or bed. (Participant 40 years FGD 5)
If it is like this, I would have done it sooner, and so it could avoid the pain when it is done (Clinician sampling). (Participant 40 years FGD 4)
Accessibility: An additional benefit is that during the COVID-19 pandemic, self-sampling eliminates the need to visit a healthcare center, thereby reducing the risk of infection.
With this virus, that you can catch anywhere, (COVID-19) better to send it to the house and that’s it, I think I could do it by myself. (Participant 58 years FGD 2)
Some women in the focus group practice ancestral medicine and believe they could share insights with other women in the community, providing them with the device to encourage participation in cervical screening. Additionally, another perceived advantage is the availability of the device in pharmacies, allowing women to collect samples and bring them to the laboratory.
Because we as ancestral medicine people, can talk and offer to our women, clients, communities. (Participant 52 years FGD 2)
And if you can buy it in the pharmacy? …. you can take it yourself… the sample, they give it to you and you can detect if there is a virus or disease. That it is something very practical and it will help and benefit a lot of women here in the parish. (Participant 38 years FGD 4)
Barriers
Lack of self-capability: The women expressed concerns about potential discomfort or injury during the application of the device and anticipated experiencing pain or shame during the sampling process.
I mean, it is ugly inside what comes out (comes out the brushes) and scratches what it is delicate! Inside Oh my God, it is stiff! When I saw it first, I thought it was cotton. (Participant 58 years FGD 5)
I say I am ashamed. So that, it is more that if people should be trained; train, how they have to use it. (Participant 64 years FGD 2)
All groups of women acknowledge the effectiveness of self-sampling but anticipate challenges in its application. They expressed concerns that an incorrect procedure might yield inaccurate results, leading some to prefer clinician sampling. The belief was that healthcare professionals possess the expertise needed for proper sample collection.
One of the disadvantages, as I say, maybe when taking, not knowing or taking the sample for the first time, maybe as soon as something starts you can get hurt or you don’t like it, or you take it wrong, if it doesn’t go well. It was practically a waste. (Participant 40 years FGD 6)
It would be much better for me. But now my question is if it would be okay. So, I don’t know how what the method would be like… Because you suppose, the doctor knows how he takes it, where he takes it, what to take out, and I imagine it is more effective than one (self-sampling). (Participant 57 years FGD 3)
Lack of trust: Some women considered self-sampling to be an incomplete examination. They believed that during a vaginal examination, health professionals can detect other pathologies, such as infections.
Because I would not only like to know if I have the papillomavirus, but also anything that may be happening inside, and the doctor, apart from taking the sample, will see if there are injuries. So, I do prefer to go to the doctor. (Participant 35 years FGD 5)
Urine Sampling
Advantages
Acceptability: The effectiveness of this technique raises more doubts compared to clinician sampling and self-sampling methods. However, women perceived it as the most acceptable and user-friendly option. The technique offers increased privacy, making it more comfortable Since almost all of the women have provided a urine sample at least once in their lifetime, they found it easy to draw comparisons with the process of vaginal sampling.
Time too. With the urine test it would also be much more comfortable, easier. (Participant 35 years FGD 1)
If with this, cancer is detected in the uterus, that is, I prefer urine because I go to the bathroom, do urine…. More relaxed. (Participant 43 years FGD 6)
In other words, we have already done the urine. so we used to do it. (Participant 43 years FGD 6)
All women recognized an advantage in urine sampling: it eliminates the need for introducing devices into the vagina. They believed this method to be more comfortable, reducing feelings of shame, and enhancing privacy. The women considered the simplicity and acceptability of this approach as a factor that could engage under-screened women.
Sure, urine would be more feasible for me. In the urine I have no longer to be showing, nothing is going to be introduced. (Participant 58 years FGD 2)
And even an easier way and therefore if all the people will come. I swear that all the women will uptake it. (Participant 57 years FGD 3)
Barriers
Lack of trust: Nearly all participants expressed doubts regarding the effectiveness of the method. This approach avoids a clinical examination and could potentially miss various cervical pathologies. Additionally, the results may be less reliable compared to clinician sampling.
But if, for example, if in the cervix there were something. they detected me, I don’t remember what the name was like, like some pimples. they wouldn’t see that, therefore (prefer) the traditional one. No, no, the one from urine, that would not detect. (Participant 51 years FGD 1)
Participants are concerned that the technique of urine sample collection and/or the method of collection could elevate the contamination rates, potentially leading to inaccurate results
Well, you also run a risk of contamination no?. well, I don’t know, I believe that at some point we can mishandle or contaminate the sample in one way or another. (Participant 59 years FGD 7)
Some of the participants believed that urine samples should be collected under the same circumstances as a Pap smear (considering an abstinence period and absence of menstrual bleeding). Ordinarily, health professionals control these factors before the examination; without such control, urine sampling might yield erroneous results.
They always give recommendations to get a pap smear, do not have intercourse. 72 hours in advance. (Urine) That is not safe, (and needs) hygiene care. (Participant 30 years FGD 1)
Concerns emerged about the storage and proper transportation of the samples, to prevent any spilling. Extra efforts should be made to avoid this circumstance.
Be careful to bring urine, all of that would also be a bit of a disadvantage because you take this (self-intake device), put it there, cover It, and…. maybe it (the sample) will be spreading, it will happen. On the other hand, in urine you do have to be very careful when bringing…, I think. (Participant 40 years FGD 6)
Discussion
Cervical cancer screening is crucial for reducing cervical cancer mortalityCitation4 by detecting premalignant lesions at early stages, thus improving survival rates and quality of life for affected women.Citation42–44 However, screening coverage can vary due to factors such as affordability, accessibility, availability, and acceptability of sampling methods.Citation45 Self-sampling offers a potential solution to these barriers,Citation22 with high accuracy and favorable acceptability across different settings.Citation28 The community-specific perceptions of these methods could significantly influence the success of their implementation.
This study explores the perceived barriers and advantages of self-sampling methods at the community level among a population previously unexposed to this method, traditionally undergoing cervical cancer screening via Pap smear performed by a clinician.
Understanding the perceptions of women in rural communities regarding different sampling methods can inform strategies to increase screening rates, starting with effective patient-physician communication and offering various sampling options.
This research revealed that self-sampling methods are highly acceptable among rural women. However, concerns regarding accuracy and the ability to perform the test correctly emerged during FDGs.
Clinician sampling is frequently perceived as uncomfortable and even painful. Moreover, gynaecological examination may induce embarrassment and a compromised sense of privacy due to the exposure of private parts. The combination of fear, pain, and embarrassment has the potential to compromise participation rates in cervical cancer screening. Similar findings are documented in the literature. Yeo Mun in Singapore and Grigore M in Romania reported that the discomfort experienced during a gynaecological examination with a speculum could diminish the future uptake of cervical cancer screening among women.Citation46,Citation47
Due to the sense of vulnerability and anxiety during a pelvic examination, along with the lack of knowledge about cervical cancer prevention, some women in our study considered gynaecological examination to be harmful. They believed that diseases could result from the examination, such as infection of the genital tract, or even cervical cancer. Similar results were found by Celal B. in Turkey, where in certain cases, the lack of information about the procedure could lead to an increased sense of discomfort.Citation48 False beliefs that cervical cancer can be caused by cancer screening could also contribute to resistance toward the examination. Lack of knowledge about cervical cancer, mixed with cultural beliefs, could explain this phenomenon.Citation16,Citation47,Citation49
Nevertheless, the women in our study considered traditional clinician sampling (ie, Pap smear) with a speculum to be the gold standard for cervical cancer screening. Some of them expressed a preference for this examination over self-sampling. Similar results were found by Morgan et al, this preference could be attributed to a lack of knowledge about the efficacy, accuracy, and advantages of the latter, along with the respondents’ self-perceptions of their proficiency to perform the test by themselves.Citation50 However, evidence indicates a high level of acceptance and proficiency when self-sampling methods are introduced to communities.Citation23,Citation51
Furthermore, since HPV testing is currently performed similarly to Pap smears, women are often unaware of the differences in sensitivity and specificity between cytological and primary HPV screening, as well as their respective advantages. This situation reinforces the belief among women that clinician sampling is the gold standard for screening, contributing to their hesitancy in adopting alternative forms of early cancer detection.Citation33,Citation52–54
Regarding self-sampling methods (ie, urine and vaginal self-sampling), almost all participants in this study perceived self-sampling as a more comfortable, less painful, and useful way to overcome barriers to routine cancer prevention screening. Similar results were found by Shin et al in Korea, where women accepted HPV self-sampling at a higher rate than clinician sampling (urine sampling OR 2.47; self-sampling OR 2.01).Citation55 Women consider that self-sampling methods (eg, urine sampling and vaginal self-sampling) offer more privacy, induce less embarrassment, and are less painful and easier to use.Citation51,Citation56
An additional advantage, as highlighted by our participants, is the potential for self-sampling methods to be administrated by midwives, community workers, and community ancestral medicine healers. These professionals can act as vital links between the community and the health centers, playing a crucial role in rural healthcare in Ecuador. Such initiatives have proven effective in engaging underserved women in rural communities.Citation33,Citation57
Despite the favorable perceptions of self-sampling methods, women in our study expressed doubts about their ability to perform the test correctly. A similar phenomenon was found by Sy et al, in Micronesia, where women preferred to have a trained worker instead of taking the sample themselves.Citation58 In a study conducted in the United States by Jeronimo et al, women believed that self-sampling methods could not be a first-line screening method due to their low perceived effectiveness.Citation59 These perceptions may stem from a low level of familiarity with self-sampling techniques and a lack of knowledge about their high sensitivity compared to the Pap smear.Citation52 Education plays a crucial role; with adequate training and information, women often find that self-sampling methods are easier and safer to perform.Citation51 Such awareness can enhance their willingness to adopt these methods.Citation60 Promotional and educational initiatives led by health personnel, including nurses and midwives in community settings, have the potential to dismantle barriers to self-sampling methods for cervical cancer screening.Citation61,Citation62
Women in our FGDs perceived self-sampling in their households as a potential solution for the prolonged traveling and waiting times at the healthcare centers. However, concerns arose regarding the transportation of samples to health centers. Firstly, there was apprehension about samples being damaged during transit, and secondly, the possibility of samples arriving too late at the laboratory, rendering them useless. These issues could be mitigated by implementing an organized program that includes efficient sample transportation, thereby enhancing satisfaction and adoption of self-sampling methods.Citation63 Additionally, dry storage of vaginal samples does not compromise the integrity of the specimens.Citation64
Self-sampling has proven to be a cost-effective testing method in diverse settings; additional research is essential to assess its practical acceptance among women in rural areas. The insights into barriers and perceived advantages, as documented in our study, can inform the development of targeted educational modules for both community women and healthcare professionals in the field.
Several implications for further research emerged from participants’ reflections. Before the introduction of self-sampling methods at the community level, an educational initiative should be conducted within the community. Participants should be allowed to decide between clinician sampling and self-sampling following a decision-making process.
At the community level, traditional medicine healers could play an active role in the cancer screening process. Following a brief training and the establishment of the sample transport mechanism, their involvement could facilitate screening for women who have never been screened or are under-screened, particularly in areas lacking medical facilities or health professionals.
Moreover, enlisting traditional healers or health promoters within the community could help overcome language or cultural barriers, thereby increasing screening uptake and adherence to follow-up, ultimately contributing to a reduction in cervical cancer mortality.
Strength and Limitations
One limitation of this study is that self-sampling methods are currently unavailable in Ecuador. Despite presenting the device during FGDs, the women we interviewed could only imagine the process of performing self-sampling methods.
Due to the specific number and characteristics of the participants, the findings of this study cannot be directly extrapolated to other settings.
Nevertheless, one of the strengths of this research is its provision of valuable insights into the barriers and facilitators that must be taken into account before implementing self-sampling screening at the community level. Additionally, it sheds light on methods to enhance the clinician sampling uptake by identifying barriers and dispelling false beliefs among women at the community level.
Furthermore, this paper offers insights into strategies at the community level aimed at increasing cervical cancer screening uptake. This includes involving traditional healers or health promoters in settings where health facilities are not readily available.
Conclusion
This study represents the initial endeavor in Ecuador to evaluate the acceptance of self-sampling methods. The findings offer valuable insight for clinicians and policymakers, aiding in understanding the advantages and barriers associated with different sampling methods. This insight can inform precautions and strategic planning before the widespread implementation of self-sampling testing at the community level. Additionally, our results can assist clinicians in identifying existing sampling barriers contributing to low cervical cancer screening coverage in Ecuador.
This study successfully achieved its objectives, confirming that women in rural settings perceived self-sampling methods as advantageous compared to clinician sampling. These advantages include reduced waiting times at healthcare centers, along with the perceived benefits of being less painful and easier to use. Furthermore, the availability of self-sampling test facilities has the potential to increase cervical cancer screening uptake, particularly among under-screened women. An additional benefit is the active involvement of community and traditional healers in screening programs, where they can distribute sample devices at the community level and encourage women to undergo screening.
Barriers to the implementation of self-sampling methods are linked to individual self-perceived proficiency in performing and concerns about the accuracy of the results. These barriers mostly stem from a lack of knowledge about these techniques, which could be addressed through sufficient patient training and information.
Further studies should be conducted to evaluate the acceptability of self-sampling methods in the field by offering those methods to participants.
Ethical Approval and Consent to Participate
This study was approved under the guidelines of the Declaration of Helsinki and the Council for International Organizations of Medical Sciences (CIOMS). All procedures involving human participants were approved by the Bioethical Committee of the University of Cuenca (approval code: UC-COBIAS-2020-262). A signed informed consent was obtained from each participant. The informed consent included the participants authorization for the publication of their anonymized responses. All of the personal information was encoded and treated confidentially.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
Funding was provided by VLIR-UOS as part of the project entitled “Making cervix cancer screening accessible through self-sampling: a step towards health equality by empowering women in an intercultural context (CAMIE)”. We are grateful to all of the participants who provided their collaboration. Our sincere thanks go to the people who helped us with the sample collection and collaborated on this project: Lorena Mora and Miguel Castro. We also thank the institutions that have supported us: the University of Antwerp, the Universidad de Cuenca, the Vicerrectorado de investigación de la Universidad de Cuenca (VIUC), the Universidad Técnica Particular de Loja, the Ministry of Public Health of Ecuador, primary health centers from El Valle, and the autonomous government of El Valle Parish). This paper has been uploaded to Research Square as a preprint: https://www.researchsquare.com/article/rs-944198/v1.
Additional information
Funding
References
- Buskwofie A, David-West G, Clare CA. A review of cervical cancer: incidence and disparities. J Nat Med Associa. 2020;112(2):229–232. doi:10.1016/j.jnma.2020.03.002
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Perehudoff K, Vermandere H, Williams A, et al. Universal cervical cancer control through a right to health lens: refocusing national policy and programmes on underserved women. BMC Int Health Hum Rights. 2020;20(1):21. doi:10.1186/s12914-020-00237-9
- World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem. World Health Organization; 2020. Available from: https://www.who.int/publications/i/item/9789240014107. Accessed May 24, 2024.
- International Agency for Research in Cance. International agency for research in cancer. Ecuador fact sheets 2020. IARC; 2021. Available from: https://gco.iarc.fr/today/data/factsheets/populations/218-ecuador-fact-sheets.pdf. Accessed May 24, 2024.
- Bruni L, Albero G, Serrano B, et al. Human papillomavirus and related diseases report Ecuador. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre); 2019. Available from: https://hpvcentre.net/statistics/reports/ECU.pdf. Accessed May 24, 2024.
- Vega B, Sacoto C Prevalencia de cáncer de cuello uterino en el Ecuador y estrategias para su reducción. Facultad de Ciencias Médicas Universidad de Cuenca; 2012. Available from: https://dspace.ucuenca.edu.ec/bitstream/123456789/20387/1/Bernardo%20Vega%20C.%20y%20Catalina%20Sacoto.pdf. Accessed May 24, 2024.
- Ministerio de Salud Pública del Ecuador M. Estrategia Nacional para la prevención del Cáncer en el Ecuador 2017. MSP; 2017. Available from: https://aplicaciones.msp.gob.ec/salud/archivosdigitales/documentosDirecciones/dnn/archivos/ac_0059_2017.pdf. Accessed May 24, 2024.
- Ministerio de Salud Pública del Ecuador M. Plan Nacional de salud sexual y salud repoductiva 2017–2021. MSP; 2017. Available from: https://ecuador.unfpa.org/sites/default/files/pub-pdf/PLAN%20NACIONAL%20DE%20SS%20Y%20SR%202017-2021.pdf. Accessed May 24, 2024.
- Ministerio de Salud Pública del Ecuador M. Esquema de vacunación Ecuador; 2019. Available from: https://www.salud.gob.ec/wp-content/uploads/2020/01/ESQUEMA-DE-VACUNACIO%CC%81N.DIC_.2019.ok_.pdf. Accessed May 24, 2024.
- de Salud Pública Del Ecuador M M. Protocolos con evidencia para la detección oportuna del cáncer de cuello uterino; 2015. Available from: https://webcache.googleusercontent.com/search?q=cache:FxdiESuIbPYJ:https://aplicaciones.msp.gob.ec/salud/archivosdigitales/sigobito/tareas_seguimiento/1614/protocolos_cancer_c%25C3%2589rvico_uterino._13_revision__borrador.-1.doc+&cd=1&hl=es&ct=clnk&gl=ec. Accessed May 24, 2024.
- Instituto Nacional de Estadísticas y Censos. Encuesta Nacional de Salud y Nutrición. INEC; 2018. Available from: https://www.ecuadorencifras.gob.ec/documentos/web-inec/Estadisticas_Sociales/ENSANUT/ENSANUT_2018/Principales%20resultados%20ENSANUT_2018.pdf. Accessed May 24, 2024.
- Agurto I, Bishop A, Sánchez G, Betancourt Z, Robles S. Perceived barriers and benefits to cervical cancer screening in Latin America. Preventive Med. 2004;39(1):91–98. doi:10.1016/j.ypmed.2004.03.040
- Zorogastua K, Erwin D, Thelemaque L, Pulley L, Jandorf L. Intrinsic factors of non-adherence to breast and cervical cancer screenings among latinas. J Racial Ethnic Health Disparit. 2016;3(4):658–666. doi:10.1007/s40615-015-0184-x
- Vega Crespo B, Neira VA, Ortíz Segarra J, et al. Barriers and facilitators to cervical cancer screening among under-screened women in Cuenca, Ecuador: the perspectives of women and health professionals. BMC Public Health. 2022;22(1):2144. doi:10.1186/s12889-022-14601-y
- Bautista-Valarezo E, Vega Crespo B, Maldonado-Rengel R, Espinosa M, Neira V, Verhoeven V. Knowledge and perceptions about cervical cancer and HPV screening in women in rural areas of Ecuador: a qualitative research study. IJERPH. 2022;19(17):11053. doi:10.3390/ijerph191711053
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. Geneva: World Health Organization; 2021. Available from: https://apps.who.int/iris/handle/10665/342365. Accessed May 24, 2024.
- Giannini A, Di Donato V, Sopracordevole F, et al. Outcomes of high-grade cervical dysplasia with positive margins and HPV persistence after cervical conization. Vaccines. 2023;11(3):698. doi:10.3390/vaccines11030698
- Baezconde-Garbanati L, Agurto I, Gravitt PE, et al. Barriers and innovative interventions for early detection of cervical cancer. Salud Publica Mex. 2019;61(4):456. doi:10.21149/10425
- Kang M, Ha SY, Cho HY, et al. Comparison of papanicolaou smear and human papillomavirus (HPV) test as cervical screening tools: can we rely on HPV test alone as a screening method? An 11-year retrospective experience at a single institution. J Pathol Transl Med. 2020;54(1):112–118. doi:10.4132/jptm.2019.11.29
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. Obstetrical Gynecol Surv. 2018;73(11):632–634. doi:10.1097/OGX.0000000000000608
- Nelson EJ, Maynard BR, Loux T, Fatla J, Gordon R, Arnold LD. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sex Transm Infect. 2017;93(1):56–61. doi:10.1136/sextrans-2016-052609
- Madzima TR, Rn MV, Ccfp AL Emerging role of HPV self-sampling in cervical cancer screening for hard-to-reach women; 2017:5.
- Asare M, Abah E, Obiri-Yeboah D, Lowenstein L, Lanning B. HPV self-sampling for cervical cancer screening among women living with HIV in low- and middle-income countries: what do we know and what can be done? Healthcare. 2022;10(7):1270. doi:10.3390/healthcare10071270
- Cómbita AL, Gheit T, González P, et al. Comparison between urine and cervical samples for HPV DNA detection and typing in young women in Colombia. Cancer Prev Res. 2016;9(9):766–771. doi:10.1158/1940-6207.CAPR-16-0038
- Kuriakose S, Sabeena S, Binesh D, et al. Diagnostic accuracy of self‐collected vaginal samples for HPV DNAdetection in women from South India. Int J Gynecol Obstet. 2020;149(2):219–224. doi:10.1002/ijgo.13116
- Asciutto KC, Ernstson A, Forslund O, Borgfeldt C. Self-sampling with HPV mRNA analyses from vagina and urine compared with cervical samples. J Clin Virol. 2018;101:69–73. doi:10.1016/j.jcv.2018.02.002
- Aimagambetova G, Atageldiyeva K, Marat A, et al. Comparison of diagnostic accuracy and acceptability of self-sampling devices for human Papillomavirus detection: a systematic review. Prevent Med Rep. 2024;38:102590. doi:10.1016/j.pmedr.2024.102590
- DrS J, Saini DS. A Comparison of 3 ways of conventional pap smear, liquid- based cytology and colposcopy vs cervical biopsy for early diagnosis of premalignant lesions or cervical cancer in women with abnormal conventional pap test. Int J Clin Obstet Gynaecol. 2020;4(3):68–71. doi:10.33545/gynae.2020.v4.i3b.576
- Nkwabong E, Laure Bessi Badjan I, Sando Z. Pap smear accuracy for the diagnosis of cervical precancerous lesions. Trop Doct. 2019;49(1):34–39. doi:10.1177/0049475518798532
- Murchland AR, Gottschlich A, Bevilacqua K, et al. HPV self-sampling acceptability in rural and indigenous communities in Guatemala: a cross-sectional study. BMJ Open. 2019;9(10):e029158. doi:10.1136/bmjopen-2019-029158
- Léniz J, Barriga MI, Lagos M, Ibáñez C, Puschel K, Ferreccio C. HPV vaginal self-sampling among women non-adherent to Papanicolaou screening in Chile. Salud pública Méx. 2013;55(2):162–169. doi:10.1590/S0036-36342013000200007
- Agorastos T, Chatzistamatiou K, Tsertanidou A, et al. Implementation of HPV-based cervical cancer screening combined with self-sampling using a midwifery network across rural Greece: the GRECOSELF study. Cancer Prev Res. 2019;12(10):701–710. doi:10.1158/1940-6207.CAPR-19-0192
- Malone C, Barnabas RV, Buist DSM, Tiro JA, Winer RL. Cost-effectiveness studies of HPV self-sampling: a systematic review. Preventive Med. 2020;132:105953. doi:10.1016/j.ypmed.2019.105953
- Surriabre P, Allende G, Prado M, et al. Self-sampling for human papillomavirus DNA detection: a preliminary study of compliance and feasibility in Bolivia. BMC Women’s Health. 2017;17(1):135. doi:10.1186/s12905-017-0490-z
- Aftab Hussain Talpur M, Napiah M, Ahmed Chandio I, Ahmed Qureshi T. A comprehensive evaluation of basic health facilities according to local standards and demographic features of Umarkot Sub-region, Pakistan. RJASET. 2014;7(22):4754–4762. doi:10.19026/rjaset.7.862
- Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52(4):1893–1907. doi:10.1007/s11135-017-0574-8
- Vasileiou K, Barnett J, Thorpe S, Young T. Characterising and justifying sample size sufficiency in interview-based studies: systematic analysis of qualitative health research over a 15-year period. BMC Med Res Methodol. 2018;18(1):148. doi:10.1186/s12874-018-0594-7
- Lindgren BM, Lundman B, Graneheim UH. Abstraction and interpretation during the qualitative content analysis process. Int J Nurs Stud. 2020;108:103632. doi:10.1016/j.ijnurstu.2020.103632
- Colorafi KJ, Evans B. Qualitative descriptive methods in health science research. HERD. 2016;9(4):16–25. doi:10.1177/1937586715614171
- Azungah T. Qualitative research: deductive and inductive approaches to data analysis. QRJ. 2018;18(4):383–400. doi:10.1108/QRJ-D-18-00035
- Bogani G, Sopracordevole F, Ciavattini A, et al. Duration of human papillomavirus persistence and its relationship with recurrent cervical dysplasia. Eur J Cancer Prev. 2023;32(6):525–532. doi:10.1097/CEJ.0000000000000822
- Balasubramaniam G, Gaidhani RH, Khan A, Saoba S, Mahantshetty U, Maheshwari A. Survival rate of cervical cancer from a study conducted in India. IJMS. 2020;73:203–211. doi:10.25259/IJMS_140_2020
- Jentschke M, Lehmann R, Drews N, Hansel A, Schmitz M, Hillemanns P. Psychological distress in cervical cancer screening: results from a German online survey. Arch Gynecol Obstet. 2020;302(3):699–705. doi:10.1007/s00404-020-05661-9
- Akinyemiju TF, McDonald JA, Lantz PM. Health care access dimensions and cervical cancer screening in South Africa: analysis of the world health survey. BMC Public Health. 2015;15(1):382. doi:10.1186/s12889-015-1686-5
- Yeo C, Fang H, Thilagamangai KSSL, Shorey S, Shorey S. Factors affecting Pap smear uptake in a maternity hospital: a descriptive cross-sectional study. J Adv Nurs. 2018;74(11):2533–2543. doi:10.1111/jan.13769
- Grigore M, Popovici R, Pristavu A, Grigore AM, Matei M, Gafitanu D. Perception and use of Pap smear screening among rural and urban women in Romania. European Journal of Public Health. 2017;27(6):1084–1088. doi:10.1093/eurpub/ckx112
- Yanikkerem E, Özdemir M, Bingol H, Tatar A, Karadeniz G. Women’s attitudes and expectations regarding gynaecological examination. Midwifery. 2009;25(5):500–508. doi:10.1016/j.midw.2007.08.006
- Kabalika C, Mulenga D, Mazaba ML, Siziya, PhD S. Acceptance of cervical cancer screening and its correlates among women of a Peri-Urban high-density residential area in Ndola, Zambia. Int J MCH AIDS. 2018;7(1):17–27. doi:10.21106/ijma.223
- Morgan K, Azzani M, Khaing SL, Wong YL, Su TT. Acceptability of women self-sampling versus clinician-collected samples for HPV DNA testing: a systematic review. J Low Genit Tract Dis. 2019;23(3):193–199. doi:10.1097/LGT.0000000000000476
- Vega Crespo B, Neira VA, J OS, et al. Evaluation of urine and vaginal self-sampling versus clinician-based sampling for cervical cancer screening: a field comparison of the acceptability of three sampling tests in a rural community of Cuenca, Ecuador. Healthcare. 2022;10(9):1614. doi:10.3390/healthcare10091614
- Yeh PT, Kennedy CE, de Vuyst H, Narasimhan M. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Glob Health. 2019;4(3):e001351. doi:10.1136/bmjgh-2018-001351
- Skroumpelos A, Agorastos T, Constantinidis T, Chatzistamatiou K, Kyriopoulos J. Economic evaluation of HPV DNA test as primary screening method for cervical cancer: A health policy discussion in Greece. PLoS One. 2019. Vol. 14. (12). e0226335.
- Bhatla N, Singhal S. Primary HPV screening for cervical cancer. Best Pract Res Clin Obstet Gynaecol. 2020;65:98–108. doi:10.1016/j.bpobgyn.2020.02.008
- Shin HY, Lee B, Hwang SH, et al. Evaluation of satisfaction with three different cervical cancer screening modalities: clinician-collected Pap test vs. HPV test by self-sampling vs. HPV test by urine sampling. J Gynecol Oncol. 2019;30(5):e76. doi:10.3802/jgo.2019.30.e76
- Rosenbaum AJ, Gage JC, Alfaro KM, et al. Acceptability of self-collected versus provider-collected sampling for HPV DNA testing among women in rural El Salvador. Int J Gynecol Obstet. 2014;126(2):156–160. doi:10.1016/j.ijgo.2014.02.026
- Dutton T, Marjoram J, Burgess S, et al. Uptake and acceptability of human papillomavirus self-sampling in rural and remote aboriginal communities: evaluation of a nurse-led community engagement model. BMC Health Serv Res. 2020;20(1):398. doi:10.1186/s12913-020-05214-5
- Sy AU, Hernandez BY, Tareg A, Reichhardt M, Buenconsejo-Lum L. Acceptability and feasibility of a community based participatory research project comparing cytology and urine HPV DNA testing for cervical cancer screening in Yap, Federated States of Micronesia. Cancer Epidemiol. 2017;50:283–288. doi:10.1016/j.canep.2017.07.008
- Jeronimo J, Perkins RB, Scalici J, Pierce JY. Should self-sampling be an option for women in the United States? J Low Genit Tract Dis. 2019;23(1):54–57. doi:10.1097/LGT.0000000000000453
- Ørnskov D, Jochumsen K, Steiner PH, Grunnet IM, Lykkebo AW, Waldstrøm M. Clinical performance and acceptability of self-collected vaginal and urine samples compared with clinician-taken cervical samples for HPV testing among women referred for colposcopy. A cross-sectional study. BMJ Open. 2021;11(3):e041512. doi:10.1136/bmjopen-2020-041512
- Ruddies F, Gizaw M, Teka B, et al. Cervical cancer screening in rural Ethiopia: a cross- sectional knowledge, attitude and practice study. BMC Cancer. 2020;20(1):563. doi:10.1186/s12885-020-07060-4
- Saei Ghare Naz M, Kariman N, Ebadi A, Ozgoli G, Ghasemi V, Rashidi Fakari F. Educational interventions for cervical cancer screening behavior of women: a systematic review. Asian Pac J Cancer Prev. 2018;19(4):1.
- Austad K, Chary A, Xocop SM, et al. Barriers to cervical cancer screening and the cervical cancer care continuum in rural Guatemala: a mixed-method analysis. JGO. 2018;4(4):1–10. doi:10.1200/JGO.17.00228
- Eperon I, Vassilakos P, Navarria I, et al. Randomized comparison of vaginal self-sampling by standard vs. dry swabs for Human papillomavirus testing. BMC Cancer. 2013;13(1):353. doi:10.1186/1471-2407-13-353