Abstract
Dramatic advances have been made in brachytherapy for cervical cancer. Radiation treatment planning has evolved from two-dimensional to three-dimensional, incorporating magnetic resonance imaging and/or computed tomography into the treatment paradigm. This allows for better delineation and coverage of the tumor, as well as improved avoidance of surrounding organs. Consequently, advanced brachytherapy can achieve very high rates of local control with a reduction in morbidity, compared with historic approaches. This review provides an overview of state-of-the-art gynecologic brachytherapy, with a focus on recent advances and their implications for women with cervical cancer.
Introduction
Cervical cancer is the third-most common cancer among women worldwide, with an annual incidence of 530,000 cases and 250,000 deaths. In the developing world, it is the second leading cause of cancer death among women.Citation1,Citation2 Its incidence in developed countries has (fortunately) decreased by 70% over the past 50 years, with the adoption of improved screening methods in cervical cytology.Citation3 Additional reduction in its incidence is anticipated with the implementation of human papilloma virus vaccine, which targets the high-risk subtypes most responsible for cervical cancers.Citation4
For women who develop locally advanced cervical cancer, the standard of care has evolved from external beam radiation therapy (EBRT) alone, to EBRT plus brachytherapy, to combined EBRT plus brachytherapy with concurrent chemotherapy.Citation5,Citation6 The external beam portion of treatment encompasses treatment to the pelvic lymph nodes, parametria, and primary tumor, to a dose adequate to control microscopic disease. The addition of brachytherapy serves to boost the gross tumor, and improves disease control and survival.Citation7–Citation11 The addition of chemotherapy serves predominantly as a radiosensitizer, resulting in improvements of about 5% in overall survival.Citation5
Brachytherapy involves the application of a radioactive source in close proximity to the tumor. It takes advantage of the inverse-square law, whereby radiation dose is inversely proportional to the square of the distance from the source. In practical terms, this allows for a very high dose to the tumor with relative sparing of the surrounding normal structures. Brachytherapy is the only demonstrated method of providing the high dose required to control cervical cancer (>80 Gray [Gy]), without causing undue side effects.
The aim of this review is to explore current best practice and state-of-the-art developments in cervical cancer brachytherapy, including patient selection, applicator selection, operative technique, and radiation treatment planning. It is intended for those with a general interest in the treatment of cervical cancer. A detailed analysis of historical approaches to brachytherapy and treatment planning is beyond the scope of this review.
Patient evaluation and staging
In general, all women with locally advanced cervical cancer – International Federation of Gynecology and Obstetrics (FIGO) stage IB2-IVA – should be considered for brachytherapy as part of their definitive treatment management. The initial work-up should include a cervical biopsy for histopathologic diagnosis. As per FIGO recommendations, an examination under anesthesia should be performed to determine clinical stage. This includes a pelvic exam, sigmoidoscopy, and cystoscopy. Initial bloodwork should include a complete blood count, basic metabolic panel, liver function tests, and evaluation of renal function. In cases of hydroureter and/or hydronephrosis from direct tumor extension toward the pelvic sidewall or ureteral obstruction from metastatic lymphadenopathy, ureteral stents or nephrostomy tube placement may be indicated, to relieve significant obstruction prior to therapy.
Although not included as part of routine FIGO staging, additional imaging at diagnosis can provide valuable information to guide prognosis, determine management, and assist in treatment planning.Citation12 For locally advanced disease, pelvic magnetic resonance imaging (MRI) and positron emission tomography-(PET) computed tomography (CT) should be obtained at diagnosis when possible. T2-weighted pelvic MRI is superior to clinical exam or CT, for determining the initial disease extent, with overall staging accuracy estimated between 75%–96%.Citation13–Citation15 Compared with CT, MRI has superior soft tissue resolution. It has been shown to better detect tumor extent, parametrial involvement, and invasion of surrounding organs. In a series of patients, staged with MRI, followed by surgery, MRI staging accuracy was 81%.Citation16 Obtaining an MRI at diagnosis also aids the radiation oncologist in treatment planning, for both EBRT and brachytherapy.
PET-CT provides complementary information at the time of diagnosis. It is the superior imaging method for lymph node detection, with a sensitivity and specificity of >99% for metastatic lymph nodes of >5 mm.Citation17 Kidd et al have shown PET-staged lymph nodes to be highly prognostic of progression-free survival, independent of FIGO stage.Citation18
Following staging evaluation, patients who proceed with definitive radiation treatment will have a planning CT simulation prior to the start of their EBRT. This is a simulation of how the patient will be set up and treated daily during the course of their EBRT. Patients are typically simulated in the supine position, with some type of immobilization device for the pelvis and/or lower extremities. Often, intravenous contrast will be used to help better delineate the pelvic vasculature, and some physicians also use oral contrast to improve delineation of the bowel. Patients are commonly asked to have a comfortably full bladder for the simulation and daily treatments, as this helps displace the bowel from the pelvis, thereby reducing dose and (ultimately) toxicity to the bowel. Prior to the simulation, patients should also have marker seeds implanted into the lowest extent of disease, to ensure that the inferior field border of the external beam radiation field adequately encompasses the most distal extent of disease. A discussion of the pros and cons of using a three-dimensional (3D) conformal “4-field box” technique (versus using intensity modulated radiation therapy) for treating the pelvis is beyond the scope of this review.
EBRT to the pelvis is generally delivered daily, Monday to Friday, for 5 weeks (25–28 treatments). Concurrent chemotherapy (most commonly, weekly bolus cisplatin 40 mg/m2) is begun at Day 1 of radiotherapy. Cisplatin should be given before radiation, on the day the patient receives both treatments. Ideally, chemotherapy is given at the beginning of the week, rather than the end. It should not be given on the same days as brachytherapy. EBRT, also, should not be given on the same day as brachytherapy.
Brachytherapy treatments are either interdigitated with EBRT (generally, starting no earlier than week 3 of treatment) or are given after EBRT is completed. Starting brachytherapy later in the treatment course allows for maximal tumor shrinkage, thus allowing for smaller brachytherapy treatment volumes. Ultimately, the most critical part of deciding when to start brachytherapy is the monitoring of treatment response, through regular pelvic examinations during the course of EBRT. At each visit, the remaining disease extent should be clearly documented and, ideally, drawn on a diagram. Each individual’s treatment response will determine the appropriate time and method to initiate brachytherapy. Patients undergoing concurrent chemotherapy should also have bloodwork measured weekly, especially prior to brachytherapy implants. In the setting of neutropenia, brachytherapy implants should be delayed until it has resolved.Citation19
It is well established that the overall treatment time of EBRT and brachytherapy should be less than 8 weeks, for patients treated with radiotherapy alone. Beyond this duration, local control and survival has been shown to decrease by ~1% per day.Citation20–Citation23
High dose rate brachytherapy versus low dose rate brachytherapy
When starting the brachytherapy component of treatment, one must first decide on whether to use high dose rate (HDR) or low dose rate (LDR) brachytherapy. Historically, cervical brachytherapy used exclusively LDR sources. Treatments were delivered over 1–2 fractions, with treatment times of (typically) 1–3 days, requiring prolonged patient immobilization. LDR brachytherapy is delivered at a point A dose rate of <0.4 Gy/hour, typically using the cesium-137 isotope.
Since the early 2000s, there has been increasing adoption and utilization of HDR, as opposed to LDR. Eighty-five percent of respondents to a recent American Brachytherapy Society (ABS) survey reported having HDR at their institution.Citation24
With HDR, a remote afterloading technology allows a small iridium source attached to the end of a cable to be robotically driven through multiple channels, stopping at predetermined points (dwell positions) for varied lengths of time.
HDR brachytherapy is delivered at a point A dose rate of >12 Gy/hour, primarily using the iridium-192 isotope. The advantages of HDR include the precise positioning of the source, infinitely variable dwell times and dwell positions – allowing for “dose sculpting” – shorter treatment times (minutes versus days), and the protection of health care personnel from radiation exposure.Citation25–Citation27 Overall clinical outcomes and toxicities are felt to be similar with both HDR and LDR.Citation25
A third type of treatment, not commonly used in the United States, is known as pulsed-dose rate (PDR) brachytherapy. This hybrid form of treatment uses an HDR source and remote afterloader to mimic the radiobiologic effects of LDR treatment,Citation28,Citation29 which is accomplished by deploying the source for a brief period of time, hourly, over a prolonged treatment delivery period (2–3 days). PDR has potential radiobiologic advantages, but it requires prolonged patient immobilization and hospitalization. It remains in use at several key academic centers, but patients treated with PDR represent only a small proportion of all cervical cancer patients who are treated with brachytherapy.
Applicator selection
Brachytherapy for cervical cancer can be performed using an intracavitary, interstitial, or combination approach. Intracavitary brachytherapy involves placing the radioactive source using an applicator, through the vaginal cavity, and can treat the upper vagina, cervix, and uterus. In interstitial brachytherapy, catheters (small tubes) are placed in and around residual disease, using a transperineal/vaginal approach.
The choice of technique depends primarily on disease extent and anatomy (). It is imperative to consider which approach should be used, starting at the time of diagnosis. There are few centers with true expertise in interstitial brachytherapy and the early recognition of a patient who needs this type of treatment allows for appropriate coordination of care.Citation19
Figure 1 Selecting intracavitary versus interstitial brachytherapy.
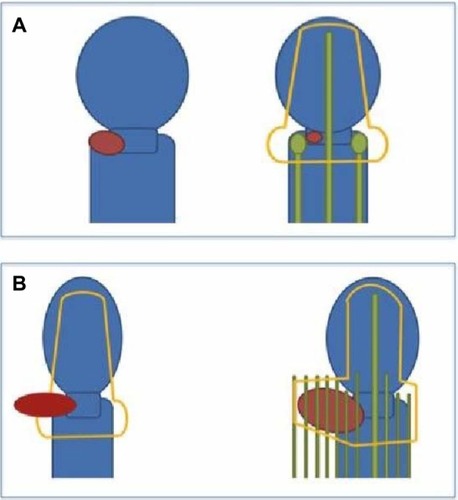
Intracavitary brachytherapy
Intracavitary brachytherapy remains the most commonly practiced form of brachytherapy for cervical cancer.Citation30 A wide variety of commercially available applicators exists for intracavitary brachytherapy. Two of the most common include variations on the Tandem and Ovoid (T&O) or Tandem and Ring (T&R) design (). The T&O consists of a tandem, an intrauterine tube placed through the cervix to the level of the uterine fundus, and two ovoids (colpostats) that are placed on either side of the cervix in the lateral vaginal fornices. The T&R also consists of a tandem, and utilizes a ring that sits on either side of the cervix. There are some differences in ease of use and distribution of radiation dose between these two applicators. However, both result in similar outcomes. So, the decision to use one over the other is mostly user-dependent.
Figure 2 Examples of an intracavitary implant.
Abbreviation: CT, computed tomography.
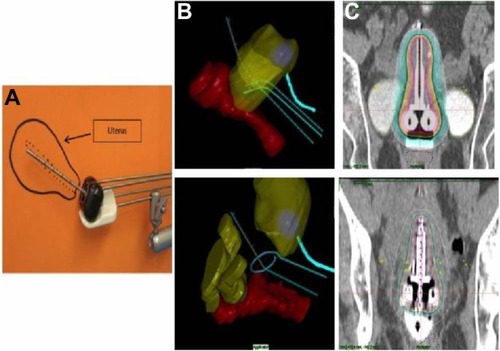
These brachytherapy procedures are performed as outpatients, when using HDR, and as inpatients, when using LDR.
Interstitial brachytherapy
Interstitial brachytherapy utilizes a transperineal template, through which several hollow tubes are inserted directly into tissues (). A tandem and central vaginal cylinder are incorporated into the template. Commonly used templates include the Syed-Neblett template and the Martinez Universal Perineal Interstitial Template.
Figure 3 Examples of an interstitial implant.
Abbreviation: CT, computed tomography.
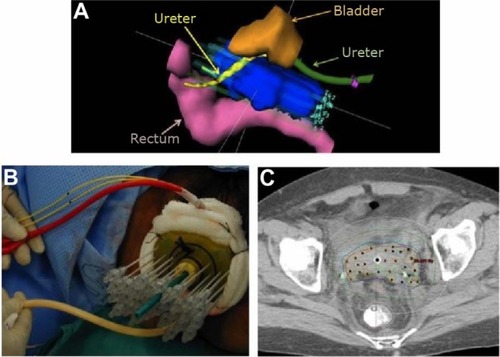
Interstitial brachytherapy has been characterized as the treatment of choice when intracavitary applicators are deemed unsuitable. Recognized indications for interstitial brachytherapy include large tumors, lower vaginal involvement, lateral extension of disease (beyond the reach of intracavitary applicators), and ill-fitting intracavitary applicators (which may result from an effaced cervix or narrow vaginal fornices).Citation19,Citation26 Patients who have had a supracervical hysterectomy and who develop cancer in the cervical stump should also be considered for interstitial implants. Template-based interstitial methods are (ultimately) the most flexible form of gynecologic brachytherapy, and can be used for the widest variety of clinical scenarios.
Hybrid applicators have also been developed that allow for several interstitial catheters to be placed in combination with a T&R or T&O applicator.Citation31,Citation32 These applicators allow for many fewer needles to be placed, but offer a much more restricted spatial distribution, compared with a template. Conversely, these applicators are potentially less user-dependent, and are more amenable to day procedures than are template-based approaches.
Applicator placement
The placing of an intracavitary applicator can be performed either in the operating room or a procedure suite at a clinic. Some practitioners prefer the patient to be kept under general anesthesia for the insertion process. Other practitioners perform the procedure under either conscious sedation or local anesthesia. Some physicians place a Smit sleeve into the cervical os at the time of the first applicator insertion. This allows the cervical os to be easily identified and dilated, allowing easier insertion of the tandem at subsequent treatments.Citation33,Citation34
For the procedure itself, a Foley catheter is placed in the bladder. A speculum is then placed, to visualize the cervix. If marker seeds were not placed at the time of EBRT, then seeds should be placed in at least two locations in the cervix (eg, 11:00 and 2:00). A single tooth tenaculum can then be used on the anterior lip of the cervix, to provide counter traction when trying to sound the uterus, and later for inserting the tandem. It is important that this be done carefully, so as not to perforate the uterus. Based on the length and curvature of the uterus, one then selects an appropriate tandem to insert. Many physicians now use either transabdominal or transrectal ultrasound to ensure appropriate placement of the tandem, rather than relying on fluoroscopy alone.Citation35
After the tandem has been inserted, the ovoids/ring can be placed. It is important to select the largest ovoids/ring that can fit in the fornices, to ensure the best dose differential between the tumor and normal tissues. After the applicator is in place, it must be stabilized, and the rectum and bladder should be displaced from the applicator as much as possible. Two-inch gauze packing, with a radiopaque lining, is commonly used to accomplish this. The radiopaque lining allows one to view the packing using X-ray. One must be cautious to not displace the ovoids/ring inferiorly from the os during this process. This is the most technique-dependent portion of the procedure, and tends to be the most uncomfortable part for the patient. An alternative to using gauze packing – which appears to be more consistent between insertions and more comfortable – is using balloon-type vaginal packing.Citation36 After packing is complete, it is important to verify that the applicator is in the proper position. Most commonly, an anteroposterior and lateral X-ray are taken, to ensure the appropriate positioning of the tandem relative to the ovoids/ring, cervical os, and packing.
The technique for interstitial brachytherapy is more involved, as it requires the insertion of multiple small, hollow tubes to encompass the residual disease. The procedure is done in the operating room, with either general anesthesia or some combination of spinal/epidural and sedation. There are multiple techniques available to guide the placement of the catheters, including laparoscopic assistance (to ensure the bowel is not perforated), fluoroscopic guidance, ultrasound guidance, CT guidance, and MRI guidance. A discussion of the pros and cons of each of these techniques is beyond the scope of this review.
Performing brachytherapy implants is a user-dependent skill. Like other surgical procedures, high-volume centers demonstrate superior outcomes, and poor quality implants result in less-desirable patient outcomes.Citation37–Citation39 It is alarming that reductions in cause-specific and overall survival have been noted with decreasing utilization of brachytherapy for cervical cancer patients in the United States.Citation40 As cervical cancer is a rare disease in the United States, it is important that teams taking care of these women maintain an adequate volume of cases, as brachytherapy technique is a critical part of ensuring ideal outcomes for these patients.
Treatment planning/dosimetry
Determining the appropriate treatment target volume
Ideally, following the completion of applicator insertion, a simulation is performed using an MRI or CT scan. Traditionally, this has been done using plain film X-rays only, but one can quickly appreciate the limitations of this. On X-rays, you have limited ability to see the actual tumor and the organs at risk (rectum, bladder, and sigmoid). This necessitates prescribing to points in two dimensions rather than being able to prescribe to 3D volumes, as can be done with CT and MRI images ().
Figure 4 Examples of brachytherapy.
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging.
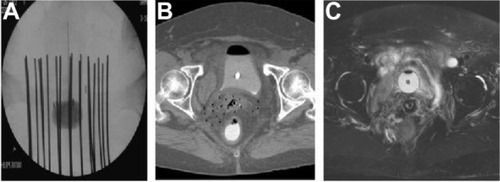
The International Committee on Radiation Units and Measurements’ Report 38 (ICRU38) outlines the most commonly applied conventions for prescribing dose to points.Citation41 The radiation dose should be prescribed to point A – a point located 2 cm superior to the cervical os, and 2 cm lateral, along the plane perpendicular to the intrauterine tandem.Citation19,Citation42 Point A derives from the Manchester system, and was defined by Tod and Meredith in a paper that detailed the nature and course of radiation necrosis following radium-based brachytherapy.Citation43 They noted that, following radiation, there was necrosis at the paracervical vessels. They defined point A within this paracervical triangle (commonly thought of as where the ureter crosses the uterine artery) as a point of limiting tolerance. As improvements in imaging from X-ray to CT/MRI occurred, studies by Potter et al and Narayan et al demonstrated that defining point A using plain films often results in point A lying inside or outside the uterus, thereby under- or overdosing the true volume of interest.Citation44,Citation45 This discrepancy could contribute to local failures and/or complications.
Beyond just adequately dosing the tumor, the limitations of a two-dimensional (2D) approach also extend to constraining the dose to normal tissues (bladder, rectum, sigmoid). Based on ICRU38, organs at risk doses are calculated based on surrogate points. The bladder point is defined as the surface of the foley catheter balloon (filled with 7 cc contrast medium), to represent the maximal dose to the bladder. The rectal point is specified as 0.5 cm posterior to the vaginal wall, directly posterior to the center of the ovoids or the ring applicator. Studies using 3D imaging demonstrate that the ICRU38 organs at risk points do not always accurately estimate the true highest dose to the organ at risk, and that using 3D contouring of the entire organ at risk is necessary to accurately determine maximum doses.Citation46
While the use of ICRU38 reference points provides a useful standard practice for cervical brachytherapy, the incorporation of 3D imaging into brachytherapy practice has clear advantages. The GEC-ESTRO working group released a series of recommendations, beginning in 2005, which sought to establish a common language and set of parameters for 3D image-(MRI) based treatment planning.Citation12,Citation47–Citation49 These recommendations introduced new fundamental concepts to gynecologic brachytherapy. While the key concepts from these recommendations are presented here, practitioners are referred directly to these papers for reference.
Rather than prescribing to a point, the working group suggested prescribing to a high-risk clinical target volume (HR-CTV). They define this volume as the area of gross residual disease at the time of brachytherapy. It includes the gross disease at the time of implant (determined on T2-weighted MRI), the entire cervix, and any areas clinically suspicious for residual disease (palpable abnormalities or indurations) or suspicious on imaging (residual “gray zones” on T2 MRI). The optimal dose to the HR-CTV remains undefined, though it is commonly prescribed to the same dose as would have been prescribed to point A (generally, >80 Gy).
They also define a second volume called the intermediate risk clinical target volume (IR-CTV). It includes the HR-CTV, the gross disease at diagnosis, plus an additional margin of 5–15 mm, based on the clinical situation. The IR-CTV should receive more than 60 Gy, through dose contributed by EBRT and brachytherapy.
The GEC-ESTRO recommendations promote an adaptive brachytherapy strategy; that is, a volume-based treatment approach, whereby the target is modified with each brachytherapy fraction, based on response to treatment. Ideally, an MRI is obtained (with the applicator in place) with each fraction. Where logistic and financial challenges prohibit this approach, an alternative strategy is to obtain an MRI with the first fraction, and CT for subsequent fractions.Citation50 CT-based determination of cervical volumes is known to be inferior to MRI-based determinations, and so using MRI as much as possible is thought to be best.Citation51
The GEC-ESTRO guidelines also suggest several parameters to describe target dose coverage. These include D90 and D100 (the dose delivered to 90% and 100% of the volume), as well as V100 (the volume receiving 100% of the prescription dose). The combined total dose from EBRT and brachytherapy can be calculated using a linear quadratic model to determine the equivalent dose in 2 Gy fractions (EQD2). It is important to understand that the doses from EBRT and brachytherapy using HDR are not additive. For example, a patient who receives 45 Gy EBRT, followed by 6 Gy × 5 fractions of HDR, does not receive a total dose of 75 Gy but an EQD2 of 84.3 Gy. Sample worksheets to conduct these calculations are available from the ABS website.Citation52
For organs at risk, GEC-ESTRO recommendations suggest reporting the dose for the most exposed 0.1 cc, 1.0 cc, and 2.0 cc (D0.1cc, D1cc, and D2cc), of the rectum, sigmoid, and bladder.
As data regarding optimal treatment volumes evolve, recording the dose to point A (and taking care not to under dose point A) is still recommended. Internationally, prescription to point A remains the norm. But practice is rapidly shifting away from prescriptions based solely on ICRU reference points, to volume-based prescriptions.Citation30
Determining the appropriate dose and fractionation scheme
After determining what volume or point to prescribe to, one must select a dose fraction scheme (how many treatments, and the size of the dose, per treatment). A variety of dose/fractionation schedules are used in clinical practice for HDR brachytherapy. The most common fractionation schedules in the United States are 5–6 Gy × 5 fractions. However, shorter regimens are also in use, including 7 Gy × 4, 8 Gy × 3, and 10 Gy × 2.Citation53,Citation54 Hesitation for adopting higher doses per fraction comes from studies which suggest higher rates of complications with doses >7 Gy per fraction.Citation55 Intracavitary brachytherapy fractions are typically delivered 1–2 times per week. For template-based interstitial implants, the entire treatment is commonly done during one insertion, given the complexities involved with proper placement of the brachytherapy catheters. Fractions may be delivered twice-daily, separated by 6 hours, with the total brachytherapy course delivered over 2–3 days.
Direct comparisons between dose/fractionation schedules for cervical brachytherapy are limited.Citation56–Citation58 The European study on MRI-guided brachytherapy (EMBRACE) is a multicenter trial (sponsored by GEC-ESTRO) that will prospectively assess MRI-guided cervical brachytherapy. A variety of brachytherapy prescriptions will be used at the discretion of individual institutions. Therefore, the results of this study will provide the best information yet on the merits of different fractionation options, in the era of adaptive, image-guided brachytherapy.
In the absence of convincing outcomes data, most fractionation schemes are influenced by practicalities and resource constraints. Fewer insertions are associated with less anesthesia, less chance for operative complications, less demand for operating room time, shorter overall treatment time, with the potential for less repeat imaging and lower use of treatment planning resources. The potential disadvantages include requirements for inpatient care, prolonged patient immobility and its attendant risks, and uncertainty associated with applying a single treatment plan for multiple fractions.
Finally, interstitial and intracavitary brachytherapy have not been compared using similar fractionation schedules; however, slightly lower doses are recommended by the ABS, when using interstitial approaches.Citation53 This is mostly related to issues with meeting rectal constraints when treating larger volumes, as is often the case with interstitial brachytherapy.
Clinical outcomes using image-guided brachytherapy
Early series using advanced, image-guided adaptive brachytherapy have shown major improvements in both local control and reduced normal tissue toxicity.Citation59,Citation60 One of the first studies to compare 2D versus 3D planning was the French STIC study.Citation61 In the cohort of patients treated with EBRT plus chemotherapy followed by brachytherapy, at 24 months, local relapse-free survival was 74% and 79%, while grade 3–4 toxicity rates were 23% versus 3% in the 2D versus 3D groups, respectively. There was also an improvement seen in overall survival: 65% versus 74%. Similar improvements with image-guided brachytherapy were presented in a series of 156 patients from Vienna.Citation60 At 3 years, overall local control was 95% (98% for tumors 2–5 cm, and 92% for tumors >5 cm). Cancer-specific survival rates at 3 years were 83% for stage IB, 84% for stage IIB, and 52% for stage IIIB. Grade 3–4 toxicities were low, at around 8%. The same group also showed a strong dose–effect relationship for local control, with local control rates >90% being associated with HR-CTV D90 of >86 Gy.Citation62 Similar importance of the D90 was presented at ESTRO 2013, on retro-EMBRACE data (patients with MRI- or CT-guided 3D treatment prior to the start of the EMBRACE study).Citation63 It was demonstrated that in 592 patients (with a median follow-up of 31 months) that a D90 to the HR-CTV of >92 Gy resulted in an overall local control rate of 95%. Ultimately, the EMBRACE study will provide us with definitive data regarding the true value of image-guided brachytherapy.
Outcomes for template-based interstitial brachytherapy cannot be directly compared to intracavitary or hybrid intracavitary/interstitial treatments, because of differences in patient selection. Moreover, individual interstitial series are highly varied, in terms of patient selection, size, and treatment technique. Pinn-Bingham et al reported the largest series of HDR interstitial brachytherapy for cervical cancer.Citation64 One hundred and sixteen women were treated with 50.4 Gy EBRT and two applications of HDR to a dose of 36 Gy. About 60% of patients also received interstitial hyperthermia. At a median follow-up of 35.1 months, the clinical locoregional control rate was 85%, and the 5-year disease survival rate was 60%. Approximately 13% of patients had late grade 3 toxicities. While this study utilized CT-based planning, it did not use the GEC-ESTRO definitions of HR-CTV, nor did it report D2cc doses to organs at risk. In addition the use of hyperthermia and the timing of the implants make generalization and comparison of these results challenging.
Toxicity
Despite the limitations in 2D brachytherapy planning, toxicity can be reliably correlated to dose based on ICRU points.Citation65–Citation69 With the introduction of 3D-based planning, volume-based dose parameters have been investigated, and also appear to provide clinically meaningful correlation with toxicities.
Early investigations demonstrated a clinical correlation between rectal dose volume and toxicity.Citation70,Citation71 The most consistently validated organ at risk parameter has been the D2cc rectum. Georg et al prospectively studied 141 cervical cancer patients treated with image-guided adaptive brachytherapy in combination with EBRT, with or without chemotherapy.Citation59,Citation72 At a median follow-up of 51 months, the 5-year actuarial side effect rate for the rectum was 12%, using the LENT-SOMA scoring system. Using a cut-off D2cc of >75 Gy, overall late rectal side effects were 20% versus 5%, and 12% versus 4%, for grade ≥2 rectal toxicity. A 10% rate of grade ≥2 rectal toxicity was associated with a D2cc of >78 Gy. A study of 51 women with a variety of gynecologic malignancies (including recurrences), treated with interstitial brachyther-apy at Brigham and Women’s Hospital, demonstrated that a 10% risk of grade ≥2 rectal toxicity correlated with a D2cc of >62 Gy.Citation73 These studies had important differences in design, population, toxicity scoring, follow-up, and treatment method, but nonetheless, illustrate a clear correlation between rectal D2cc and toxicity.
Dose–volume relationships have also been shown to correlate with late bladder toxicities. Georg et al showed a correlation for all dose volume histogram points (D0.1cc, D1cc, and D2cc) and ultimately suggested a cut-off of D2cc >100 Gy, which corresponded to 9% versus 13% rates of grade 0–1 versus grade 2–4 toxicity.Citation59
No clear correlation between dose–volume and toxicity has been determined for the vaginal mucosa, sigmoid, urethra, or ureters.
Overall, in the era of image-guided brachytherapy, later grade ≥3 rectal, bowel, and bladder toxicity rates are typically in the ≤10% range. Ongoing prospective trials (and the ABS) suggest limiting the D2cc rectum to <70–75 Gy, D2cc sigmoid to <75 Gy, and D2cc bladder to <90 Gy.Citation53 Moving forward, consistency in toxicity scales is required, for comparison between series as well as for improved reporting of patient-reported morbidity.
Alternatives to brachytherapy
The possible disadvantages of brachytherapy include that it is invasive, resource-intensive, can be technically challenging, and is ideally performed in women who have a good performance status. Investigators have started to publish early experiences of using an EBRT technique called stereotactic body radiation therapy (SBRT), as a substitute for brachytherapy in patients who are not deemed appropriate brachytherapy candidates. SBRT still delivers radiation from outside, as in standard EBRT, but at a much higher dose per fraction. It is typically completed in five or fewer sessions. Its goal is to noninvasively mimic the dose distribution that can be achieved with HDR brachytherapy. Dosimetric studies have shown these methods to provide good target coverage, and doses to organs at risk similar to brachytherapy, but clinical data is very limited.Citation74–Citation77 At the current time, techniques such as SBRT, in place of brachytherapy, should only be performed in clinical trials.
Conclusion
Progress from 2D- to 3D-based imaging and treatment planning for cervical cancer brachytherapy has improved local control, reduced toxicity, and improved overall survival for women. Further data from 3D-based techniques is accumulating, and results from the EMBRACE trial will provide strong evidence regarding the true merits of this approach. This is indeed an exciting time for brachytherapy.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
- ParkinDMBrayFFerlayJPisaniPGlobal cancer statistics, 2002CA Cancer J Clin20055527410815761078
- KamangarFDoresGMAndersonWFPatterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the worldJ Clin Oncol200624142137215016682732
- GustafssonLPontenJZackMAdamiHOInternational incidence rates of invasive cervical cancer after introduction of cytological screeningCancer Causes Control1997857557639328198
- KoutskyLAAultKAWheelerCMA controlled trial of a human papillomavirus type 16 vaccineN Engl J Med2002347211645165112444178
- GreenJAKirwanJMTierneyJFSurvival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysisLancet2001358928478178611564482
- NCCN clinical practice guidelines in oncology: Cervical cancer [Internet]2011 [cited February 27, 2011]National Comprehensive Cancer Network Available from: http://www.nccn.org
- LancianoRMWonMCoiaLRHanksGEPretreatment and treatment factors associated with improved outcome in squamous cell carcinoma of the uterine cervix: A final report of the 1973 and 1978 patterns of care studiesInt J Radiat Oncol Biol Phys19912046676762004942
- HanksGEHerringDFKramerSPatterns of care outcome studies. Results of the national practice in cancer of the cervixCancer19835159599676821861
- CoiaLWonMLancianoRMarcialVAMartzKHanksGThe patterns of care outcome study for cancer of the uterine cervix. Results of the second national practice surveyCancer19906612245124562249184
- MontanaGSMartzKLHanksGEPatterns and sites of failure in cervix cancer treated in the USA in 1978Int J Radiat Oncol Biol Phys199120187931993634
- LogsdonMDEifelPJFigo IIIB squamous cell carcinoma of the cervix: An analysis of prognostic factors emphasizing the balance between external beam and intracavitary radiation therapyInt J Radiat Oncol Biol Phys199943476377510098431
- Haie-MederCPotterRVan LimbergenERecommendations from gynaecological (GYN) GEC-ESTRO working group (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTVRadiother Oncol200574323524515763303
- SalaEWakelySSeniorELomasDMRI of malignant neoplasms of the uterine corpus and cervixAJR Am J Roentgenol200718861577158717515380
- KapurTEggerJDamatoASchmidtEJViswanathanAN3-T MR-guided brachytherapy for gynecologic malignanciesMagn Reson Imaging20123091279129022898699
- HricakHGatsonisCCoakleyFVEarly invasive cervical cancer: CT and MR imaging in preoperative evaluation – ACRIN/GOG comparative study of diagnostic performance and interobserver variabilityRadiology2007245249149817940305
- HricakHLaceyCGSandlesLGChangYCWinklerMLSternJLInvasive cervical carcinoma: Comparison of MR imaging and surgical findingsRadiology198816636236313340756
- SironiSBudaAPicchioMLymph node metastasis in patients with clinical early-stage cervical cancer: Detection with integrated FDG PET/CTRadiology2006238127227916304090
- KiddEASiegelBADehdashtiFLymph node staging by positron emission tomography in cervical cancer: Relationship to prognosisJ Clin Oncol201028122108211320308664
- ViswanathanANThomadsenBAmerican Brachytherapy Society Cervical Cancer Recommendations CommitteeAmerican Brachytherapy SocietyAmerican Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. part I: General principlesBrachytherapy2012111334622265436
- GirinskyTReyARocheBOverall treatment time in advanced cervical carcinomas: A critical parameter in treatment outcomeInt J Radiat Oncol Biol Phys1993275105110568262826
- PerezCAGrigsbyPWCastro-VitaHLockettMACarcinoma of the uterine cervix. I. impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapyInt J Radiat Oncol Biol Phys1995325127512887635767
- PetereitDGSarkariaJNChappellRThe adverse effect of treatment prolongation in cervical carcinomaInt J Radiat Oncol Biol Phys1995325130113077635769
- LancianoRMPajakTFMartzKHanksGEThe influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation: A patterns-of-care studyInt J Radiat Oncol Biol Phys19932533913978436516
- ViswanathanANEricksonBAThree-dimensional imaging in gynecologic brachytherapy: A survey of the American Brachytherapy SocietyInt J Radiat Oncol Biol Phys201076110410919619956
- WangXLiuRMaBYangKTianJJiangLHigh dose rate versus low dose rate intracavity brachytherapy for locally advanced uterine cervix cancerCochrane Database Syst Rev20107CD00756320614461
- DemanesDJRodriguezRRBendreDDEwingTLHigh dose rate transperineal interstitial brachytherapy for cervical cancer: High pelvic control and low complication ratesInt J Radiat Oncol Biol Phys199945110511210477013
- ParkHCSuhCOKimGEFractionated high-dose-rate brachytherapy in the management of uterine cervical cancerYonsei Med J200243673774812497657
- BrennerDJHallEJFractionated high dose rate versus low dose rate regimens for intracavitary brachytherapy of the cervix. I. general considerations based on radiobiologyBr J Radiol1991647581331412004204
- BrennerDJHallEJConditions for the equivalence of continuous to pulsed low dose rate brachytherapyInt J Radiat Oncol Biol Phys19912011811901993627
- ViswanathanANCreutzbergCLCraigheadPInternational brachytherapy practice patterns: A survey of the gynecologic cancer intergroup (GCIG)Int J Radiat Oncol Biol Phys201282125025521183288
- DimopoulosJCKirisitsCPetricPThe vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: Clinical feasibility and preliminary resultsInt J Radiat Oncol Biol Phys2006661839016839702
- KirisitsCLangSDimopoulosJBergerDGeorgDPotterRThe vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: Design, application, treatment planning, and dosimetric resultsInt J Radiat Oncol Biol Phys200665262463016690444
- SmitBJvan WijkALAn improved, disposable indwelling intrauterine tube (“smit sleeve”) not requiring retaining stitches for brachy-radiotherapy for carcinoma of the cervixEur J Gynaecol Oncol201334428929024020130
- SmitBJdu ToitJPGroenewaldWAAn indwelling intrauterine tube to facilitate intracavitary radiotherapy of carcinoma of the cervixBr J Radiol19896273368692914194
- EricksonBFoleyWGillinMUltrasound-guided transperineal interstitial implantation of pelvic malignancies: Description of the techniqueEndocurie Hypertherm Oncol199511107113
- WelliverMLinLEvaluation of a balloon-based vaginal packing system and patient-controlled analgesia for patients with cervical cancer undergoing high-dose-rate intracavitary brachytherapyPractical Radiation Oncology20123426326824674396
- ViswanathanANMoughanJSmallWThe quality of cervical cancer brachytherapy implantation and the impact on local recurrence and disease-free survival in radiation therapy oncology group prospective trials 0116 and 0128Int J Gynecol Cancer201222112313122193645
- EricksonBEifelPMoughanJRowndJIarocciTOwenJPatterns of brachytherapy practice for patients with carcinoma of the cervix (1996–1999): A patterns of care studyInt J Radiat Oncol Biol Phys20056341083109216099599
- CornBWHanlonALPajakTFOwenJHanksGETechnically accurate intracavitary insertions improve pelvic control and survival among patients with locally advanced carcinoma of the uterine cervixGynecol Oncol19945332943008206401
- HanKMilosevicMFylesAPintilieMViswanathanANTrends in the utilization of brachytherapy in cervical cancer in the United StatesInt J Radiat Oncol Biol Phys201387111111923849695
- International Commission of Radiation Units and MeasurementsDose and Volume Specification for Reporting Intracavitary Therapy in GynaecologyBethesda, MDICRU1985Report No: 38
- PotishRGerbiBEnglerGDose Prescription, Dose Specification, and Applicator Geometry in Intracavitary TherapyMadison, WIMedical Physics Publishing1995
- TodMMeredithWJTreatment of cancer of the cervix uteri, a revised manchester methodBr J Radiol19532630525225713042092
- PotterRKirisitsCFidarovaEFPresent status and future of high-precision image guided adaptive brachytherapy for cervix carcinomaActa Oncol20084771325133618661430
- NarayanKBarkatiMvan DykSBernshawDImage-guided brachytherapy for cervix cancer: From Manchester to MelbourneExpert Rev Anticancer Ther2010101414620014884
- PelloskiCEPalmerMChronowskiGMJhingranAHortonJEifelPJComparison between CT-based volumetric calculations and ICRU reference-point estimates of radiation doses delivered to bladder and rectum during intracavitary radiotherapy for cervical cancerInt J Radiat Oncol Biol Phys200562113113715850913
- PotterRHaie-MederCVan LimbergenERecommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachyther-apy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiologyRadiother Oncol2006781677716403584
- HellebustTPKirisitsCBergerDRecommendations from gynaecological (GYN) GEC-ESTRO working group: Considerations and pitfalls in commissioning and applicator reconstruction in 3D image-based treatment planning of cervix cancer brachytherapyRadio-ther Oncol2010962153160
- DimopoulosJCPetrowPTanderupKRecommendations from gynaecological (GYN) GEC-ESTRO working group (IV): Basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapyRadiother Oncol2012103111312222296748
- RowndJEricksonBUSA: Medical College of Wisconsin, MilwaukeeViswanathanAKirisitisCBeaEricksonGynecologic Radiation Therapy: Novel Approaches to Image-Guidance and ManagementNew YorkSpringer2011231238
- ViswanathanANDimopoulosJKirisitsCBergerDPotterRComputed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: Results of a prospective trial and preliminary guidelines for standardized contoursInt J Radiat Oncol Biol Phys200768249149817331668
- American Brachytherapy Society: Brachytherapy Guidelines [Internet]2014 [cited November 1, 2013]Reston, VAAmerican Brachytherapy Society Available from: http://www.americanbrachytherapy.org/guidelines/index.cfm
- ViswanathanANBeriwalSDe Los SantosJFAmerican Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. part II: High-dose-rate brachytherapyBrachytherapy2012111475222265437
- SharmaDNRathGKThulkarSKumarSSubramaniVJulkaPKHigh-dose rate interstitial brachytherapy using two weekly sessions of 10 gy each for patients with locally advanced cervical carcinomaBrachytherapy201110324224820932811
- OrtonCGSeyedsadrMSomnayAComparison of high and low dose rate remote afterloading for cervix cancer and the importance of fractionationInt J Radiat Oncol Biol Phys1991216142514341938550
- HuangEYSunLMLinHA prospective cohort study to compare treatment results between 2 fractionation schedules of high-dose-rate intracavitary brachytherapy (HDR-ICBT) in patients with cervical cancerInt J Radiat Oncol Biol Phys201385112312822672751
- PetereitDGPearceyRLiterature analysis of high dose rate brachytherapy fractionation schedules in the treatment of cervical cancer: Is there an optimal fractionation schedule? Int J Radiat Oncol Biol Phys199943235936610030262
- ToitaTKakinohanaYOgawaKCombination external beam radiotherapy and high-dose-rate intracavitary brachytherapy for uterine cervical cancer: Analysis of dose and fractionation scheduleInt J Radiat Oncol Biol Phys20035651344135312873679
- GeorgPLangSDimopoulosJCDose-volume histogram parameters and late side effects in magnetic resonance image-guided adaptive cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys12011792356362
- PotterRGeorgPDimopoulosJCClinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancerRadiother Oncol2011100111612321821305
- Charra-BrunaudCLevitchiMDelannesMDosimetric. Clinical results of a french prospective study of 3D brachytherapy for cervix carcinomaRadiother Oncol201199S57
- DimopoulosJCPotterRLangSDose-effect relationship for local control of cervical cancer by magnetic resonance image-guided brachytherapyRadiother Oncol200993231131519679365
- TanderupKFokdalLSturdzaADose-response for local control in image guided cervix brachytherapy in the retroEMBRACE studyPaper presented at ESTRO 2013April 19–23, 2013Geneva, Switzerland
- Pinn-BinghamMPuthawalaAASyedAMOutcomes of high-dose-rate interstitial brachytherapy in the treatment of locally advanced cervical cancer: Long-term resultsInt J Radiat Oncol Biol Phys20138537142022763030
- PerezCABreauxSBedwinekJMRadiation therapy alone in the treatment of carcinoma of the uterine cervix. II. analysis of complicationsCancer19845422352466722748
- PerezCAGrigsbyPWLockettMAChaoKSWilliamsonJRadiation therapy morbidity in carcinoma of the uterine cervix: Dosimetric and clinical correlationInt J Radiat Oncol Biol Phys199944485586610386643
- BarillotIHoriotJCMaingonPImpact on treatment outcome and late effects of customized treatment planning in cervix carcinomas: Baseline results to compare new strategiesInt J Radiat Oncol Biol Phys200048118920010924989
- ClarkBGSouhamiLRomanTNChappellREvansMDFowlerJFThe prediction of late rectal complications in patients treated with high dose-rate brachytherapy for carcinoma of the cervixInt J Radiat Oncol Biol Phys19973859899939276363
- ChenSWLiangJAYangSNLiuRTLinFJThe prediction of late rectal complications following the treatment of uterine cervical cancer by high-dose-rate brachytherapyInt J Radiat Oncol Biol Phys200047495596110863065
- GeorgPKirisitsCGoldnerGCorrelation of dose-volume parameters, endoscopic and clinical rectal side effects in cervix cancer patients treated with definitive radiotherapy including MRI-based brachytherapyRadiother Oncol200991217318019243846
- KoomWSSohnDKKimJYComputed tomography-based high-dose-rate intracavitary brachytherapy for uterine cervical cancer: Preliminary demonstration of correlation between dose-volume parameters and rectal mucosal changes observed by flexible sigmoidoscopyInt J Radiat Oncol Biol Phys20076851446145417482766
- GeorgPPotterRGeorgDDose effect relationship for late side effects of the rectum and urinary bladder in magnetic resonance image-guided adaptive cervix cancer brachytherapyInt J Radiat Oncol Biol Phys201282265365721345618
- LeeLJViswanathanANPredictors of toxicity after image-guided high-dose-rate interstitial brachytherapy for gynecologic cancerInt J Radiat Oncol Biol Phys20128451192119722592049
- SethiRAJozsefGGrewDIs there a role for an external beam boost in cervical cancer radiotherapy? Front Oncol20133323386995
- HsiehCHWeiMCHsuYPShould helical tomotherapy replace brachytherapy for cervical cancer? Case ReportBMC Cancer20101063721092235
- MarnitzSKohlerCBudachVRobotic radiosurgery: Emulating brachytherapy in patients with locally advanced cervical carcinoma. Technique, feasibility and acute toxicityRadiat Oncol20138110923638755
- HaasJAWittenMRClanceyOEpiscopiaKAccordinoDChalasECyberKnife boost for patients with cervical cancer unable to undergo brachytherapyFront Oncol201222522655266
