Abstract
We describe the histological changes in the collagen fibers of a 50-year-old male who presented keratoconus with secondary corneal amyloidosis. Corneal tissue from the patient was obtained following a penetrating keratoplasty and was subjected to histochemical analysis using Masson’s trichrome staining, Congo red staining, anti-lactoferrin antibody, and anti-transforming growth factor-beta-induced protein (TGFBIp) antibody. A Congo red-positive region was detected in the anterior half of the stroma in the center and inferior cornea. Although hemotoxylin and eosin staining revealed irregularity in the Congo red-positive region, other parts of the stroma did not show any abnormalities. Positive staining both by anti-TGFBIp and anti-lactoferrin antibodies was observed in the Congo red-positive region. Interestingly, all the layers of the corneal stroma, including the peripheral region, were positively stained by anti-TFGBIp antibody, even in the Congo red-negative area. Masson’s trichrome staining also showed irregular staining throughout the corneal stroma, even outside of the Congo red-positive region. Additionally, Bowman’s layer, which consists of collagen type IV, was damaged. TGFBIp was strongly expressed and Masson’s trichrome staining was reduced throughout the entire keratoconic stroma. The constant qualitative changes in keratoconic collagen fibers, along with the observed abnormality in the Bowman’s membrane, might point to the pathogenesis of secondary corneal amyloidosis in keratoconus.
Keywords:
Introduction
The pathogenesis of keratoconus is still unclear. Previous studies using histochemical and biochemical analysis have pointed to a variety of possible causes for keratoconus, such as the upregulation of various enzymes, excessive keratocyte apoptosis induced by the interleukin-1 system, abnormal distribution of collagen orientation, and abnormal cytokine expression.Citation1–Citation3 Recently, collagen abnormalities have been reported to be directly associated with keratoconus by studies using multiple methods.Citation4,Citation5
Clinically, we often observe stromal edema following lamellar keratoplasty in keratoconus patients, and the edema in such cases is more obvious than that observed in leukoma patients. Additionally, we observe that the suture is easy to loosen following keratoplasty for keratoconus. These observations led us to speculate that the stromal component might be qualitatively altered in keratoconus. Thus, the keratoconic corneal stroma might be affected by an abnormality other than a genetic mutation within TGFBI as was reported.Citation6
Secondary corneal amyloidosis (SCA) occurs with progressive keratoconus at a frequency ranging from 3.5% to 17.6%.Citation7,Citation8 This indicates that protein aggregation tends to increase in the keratoconic corneal stroma. Previously, we reported that SCA in trichiasis is predominantly induced by lactoferrin Glu561Asp polymorphism.Citation9 A report by Nisson and DobsonCitation10 suggests that Glu561Asp lactoferrin might aggregate and result in amyloidosis. However, we did not observe this polymorphism in SCA in keratoconus.Citation11 Therefore, we speculate that another amyloidogenic protein might play a key role in SCA in keratoconus.
In 2009, Tai et alCitation7 reported that transforming growth factor-beta-induced protein (TGFBIp) is present in the corneal stroma of patients with keratoconus. Subsequently, TGFBIp has been shown to form the core in amyloidosis due to the high number of beta-sheets within the structure of this protein.Citation12–Citation14
In this study, we examined TGFBIp and lactoferrin expression in a keratoconus patient with SCA using anti-TGFBIp and anti-lactoferrin antibodies. We also used Masson’s tri-chrome staining to detect the native collagen, and we observed the affected collagen in the cornea with keratoconus.
Case
The protocol used in this report was approved by the Institutional Review Board of the Japan Health Care Organization Hoshigaoka Medical Center. Written informed consent including publication of the case details and accompanying images was obtained from the patient, and the procedures used conformed to the tenets of the Declaration of Helsinki. A 50-year-old male who had suffered from keratoconus for >40 years complained of progressive opacity in his right cornea. The corrected visual acuity of his right eye was 20/1000. He also presented with atopic skin disease. Slit-lamp examination revealed a milky white soft mass on the top of the keratoconic cornea (). In accordance with our previously published classification method for SCA,Citation15 the mass was classified as having a gelatinous drop-like dystrophy-type appearance. The patient had complained of a foreign body sensation, epiphora, and redness of his right eye. Mild vessel invasion toward the white mass was observed.
Figure 1 Preoperative slit-lamp photographs.
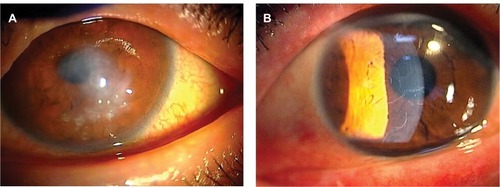
Although the cornea of the fellow eye protruded, which is a typical finding of keratoconus, a milky white mass was not observed. Instead, slit-lamp examination revealed that the fellow cornea showed a linear subepithelial opacity, which was speculated to be an early stage of SCA ().Citation16
To resolve the symptoms and reduced visual acuity caused by the white mass on his cornea, the patient underwent a penetrating keratoplasty (PKP) on his right eye. Histochemical analysis was performed for diagnosis. After the excised specimen of the PKP was fixed with 4% paraformaldehyde at 4°C, 3 μm sections were cut and mounted on slides. After the slides had dried, samples were stained with Congo red, Masson’s trichrome, and hemotoxylin and eosin (H&E). Staining with anti-lactoferrin antibody (Proteintech, Chicago, IL, USA) and anti-TGFBIp/BIG3 antibody (Proteintech) was also performed.
All the sections were incubated with 1% bovine serum albumin in PBS at 24°C for 10 minutes each in order to block any nonspecific binding. Subsequently, the samples were incubated with anti-lactoferrin antibody and anti-TGFBI/BIG3 antibody (described above) for 90 minutes at room temperature. The sections were then washed three times in PBS for 10 minutes, and the binding of the antibodies was followed by a reaction with biotinylated goat anti-rabbit immunoglobulin G and horseradish peroxidase-conjugated streptavidin. The slides were dehydrated using an ethanol series (70%–95%) and xylene, and were then covered with a coverslip using the mounting medium (Malinol; Muto Pure Chemicals Co. Ltd, Tokyo, Japan). All slides were examined by both light and polarizing microscopy (Leica DM4000 B; Leica Microsystems, Wetzlar, Germany).
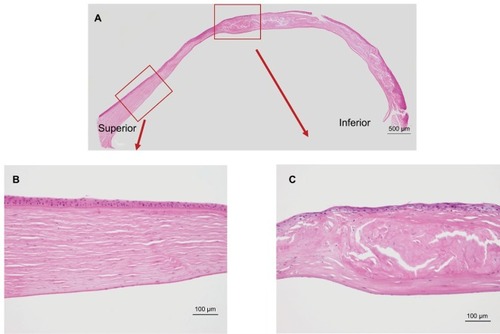
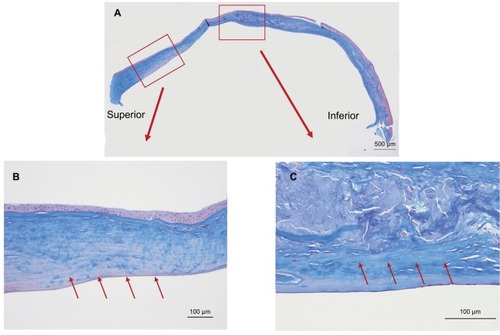
shows the results of Congo red staining of the excised cornea. Although the superior peripheral stroma did not show any marked changes (), Congo red staining was detected in the central and inferior peripheral stroma (), near the limbus. shows the results of H&E staining of the collagen fibers in the cornea. Although the superior peripheral stroma did not show any abnormal staining (), irregularity was observed in the center of the cornea (), in the same region where the amyloid was detected by Congo red staining ().
Figure 2 Congo red staining of the excised cornea.
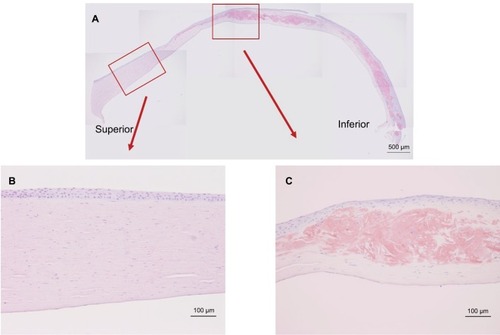
Figure 3 Hemotoxylin and eosin staining of the excised cornea.
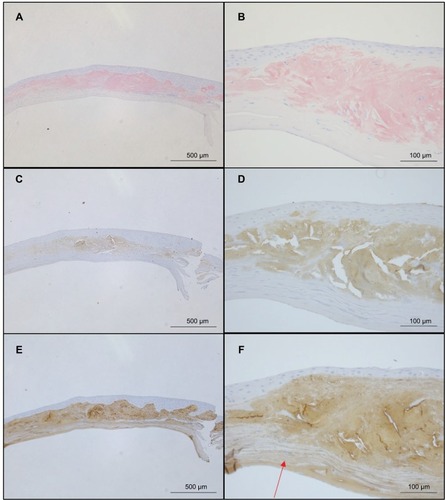
shows the results of Congo red (), anti-lactoferrin antibody (), and anti-TGFBIp antibody () staining. The eosinophilic material was positively stained with Congo red (). This material was mainly located in the stroma as well as in the epithelial layers that showed destruction of Bowman’s layer. The eosinophilic material also showed a positive reaction with the anti-lactoferrin antibody () in almost the same area as that stained positively by Congo red. In contrast, the complete corneal stroma was positively stained with the anti-TGFBIp antibody, including the Congo red-positive and -negative areas. Although staining with anti-TGFBIp antibody was stronger within the Congo red-positive stromal region, this staining was also confirmed within the Congo red-negative region (, arrow).
Figure 4 Comparison of staining by Congo red, anti-lactoferrin antibody, and anti-TGFBIp antibody.
Masson’s trichrome staining () showed irregular staining in the central stroma. The staining was not uniform and was negative in various places in the peripheral cornea (, arrows) where H&E staining showed no marked changes (). The staining was irregular even in the non-amyloid region (, arrows).
Figure 5 Masson’s trichrome staining of the excised cornea.
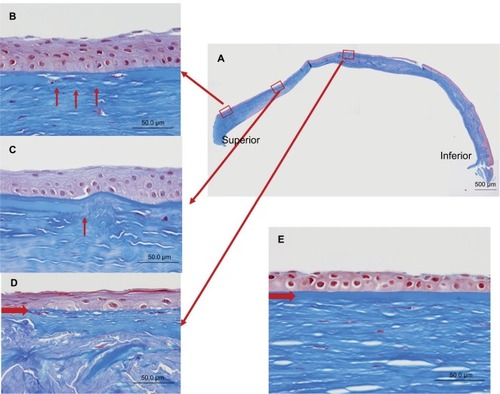
Bowman’s layer, which consists of type IV collagen, was also affected by the keratoconus (). These changes included attenuation (), as well as interruption () and complete disappearance (), when compared to the normal cornea (). One month after undergoing PKP, the corrected visual acuity of the patient’s right eye was limited to 20/200 owing to amblyopia.
Figure 6 Masson’s trichrome staining of the Bowman’s layer.
Discussion
The current report describes the first documented case of abnormal stromal Masson’s trichrome staining in keratoconus with SCA. It has previously been reported that corneal stromal collagen differs in cases of keratoconus compared to normal corneas.Citation1–Citation5 In this study, we observed changes in collagen in keratoconus using the simple method of Masson’s trichrome staining. The mechanism of Masson’s trichrome staining follows a simple procedure, wherein the nucleus is stained purple by iron hematoxylin, the cytoplasm is stained red by acid fuchsin, and the collagen is stained blue by aniline blue. This method is effective even for type I and type IV collagen staining. As can be seen in , normal corneal stroma was stained uniformly blue by Masson’s trichrome staining. In contrast, the keratoconic cornea was not stained with Masson’s trichrome staining, but was uniformly stained with anti-TGFBIp antibody.
Upregulation of various enzymes, such as lysosomal enzymes, and excessive keratocyte apoptosis by several inflammatory cytokines have been reported in keratoconus. Additionally, direct evidence for collagen fiber change has been the differing distribution of collagen orientation as observed by the synchrotron X-ray scattering patternCitation4 and the quantitative analysis of collagen lamellae in keratoconus, as observed by harmonic generation imaging microscopy.Citation5 Marcatelli et alCitation17 reported that the orientation of sutural lamellae close to Bowman’s membrane is significantly different between healthy and keratoconic samples. This suggests that the collagen structure in the keratoconic cornea differs greatly from that in the normal cornea. Our observations strongly support these previous data. In our study, TGFBIp was detected diffusely throughout the whole stromal region, including the non-amyloid region. Our observation of negative Masson’s trichrome staining within the non-amyloidogenic region implies that the corneal collagen in keratoconus might be altered throughout the whole stromal region, rather than in the amyloidogenic region only.
TGFBIp is produced during corneal wound healing, and has been reported to have the ability to form the core of amyloidosis because it has a large number of beta-sheets.Citation12,Citation18 The reason behind the aggregation of excessive amounts of TGFBIp that sometimes occurs in keratoconus remains unknown. Even with the high number of beta-sheets, a biochemical change, such as gene transformation, or changes in proteolytic enzyme levels might be necessary. At present, there is some evidence that proteins are altered in keratoconus. The upregulation of matrix metalloproteinase-9, tumor necrosis factor-α, and interleukin-6 has been well established in keratoconus.Citation19 Temperature and pH can also accelerate protein aggregation,Citation13 and any of these factors could potentially cause excessive TGFBIp aggregation.
It has been previously reported that SCA with trichiasis is predominantly induced by the lactoferrin Glu561Asp polymorphism, which can aggregate easily to form the core of SCA.Citation10 However, this polymorphism is only significant in the secondary amyloidosis in trichiasis, but not in keratoconus.Citation11 The constant expression of TGFBIp and enzymatic changes observed in keratoconus might explain this discrepancy. This further establishes the fact that the pathological mechanism of SCA in keratoconus might differ from that of SCA in trichiasis.
In our case, lactoferrin was positively stained only in the Congo red-positive region of the keratoconic cornea. Previously, we had employed ultrahigh-resolution optical coherence tomography to demonstrate that the amyloidosis in SCA caused by trichiasis deposited above the Bowman’s membrane, without destroying it. Additionally, we had shown the existence of an association between lactoferrin in the tear film and SCA.Citation16 Therefore, in the case of keratoconus, we hypothesize that lactoferrin only deposits on the core of the SCA, which is formed by other amyloidogenic proteins. We have previously proposed a classification method for SCA and have shown that patients with gelatinous drop-like dystrophy-type SCA are younger and predominantly women. Additionally, we have reported that those who suffer from diseases associated with SCA for longer time periods are more likely to develop gelatinous drop-like dystrophy-type SCA.Citation15 In accordance with this finding, the present report implies that SCA of gelatinous drop-like dystrophy-type, as observed in our patient, might result from modification by the gradual deposition of lactoferrin to the core amyloid region over a long period of time.
It would be advantageous to investigate the early stages of SCA in keratoconus in order to determine the mechanism of amyloid formation within the keratoconic cornea. However, histochemical analysis of early-stage keratoconus is usually prohibited by ethical considerations. Interestingly, the linear line observed in the fellow eye of our patient could be an early sign of SCA.Citation20,Citation21 The continuous observation of this clinical phenomenon may, therefore, help to elucidate the mechanism of amyloid formation in keratoconus in the future.
Conclusion
In conclusion, we report a case that suggests that TGFBIp is consistently upregulated and the collagen fibers are qualitatively changed in the complete stroma of the keratoconic cornea. These changes might be a potential biomarker for determining the pathogenesis of SCA in keratoconus.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to thank Editage Co. LTd. for English language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
- DavidsonAEHayesSJardcastleAJTuftSJThe pathogenesis of keratoconusEye201428218919524357835
- GalvisVSherwintTelloAMerayoJBarreraRAceraAKeratoconus: an inflammatory disorder?Eye201529784385925931166
- WisseRPKuiperJJGansRImhofSRadstakeTRVan der LelijACytokine expression in keratoconus and its corneal microenvironment: a systematic ReviewOcul Surf201513427228326235733
- MeekKMTuftSJHuangYChanges in collagen orientation and distribution in keratoconus corneasInvest Ophthalmol Vis Sci20054661948195615914608
- MorishigeNShin-Gyou-UchiRAzumiHQuantitative analysis of collagen lamellae in the normal and keratoconic human cornea by second harmonic generation imaging microscopyInvest Ophthalmol Vis Sci201455128377838525425311
- UdarNKenneyMCCbalukyaMKeratoconus--no association with the transforming growth factor beta-induced gene in a cohort of American patientsCornea2004231131714701952
- TaiTYDamaniMRVoRKeratoconus associated with corneal stromal amyloid deposition containing TGFBIpCornea200928558959319421032
- TrikhaSSahuDJeffryMBoaseDSecondary corneal amyloidosis in keratoconusCornea201130671671721242776
- Araki-SasakiKAndoYKitagawaKLactoferrin Glu561Asp facilitates secondary amyloidosis in the corneaBritish J Ophthalmol2005896684688
- NissonMRDobsonCMIn vitro characterization of lactoferrin aggregation and amyloid formationBiochemistry200342237538212525164
- Araki-SasakiKOsakabeYMiyataKWhat is this thing called “amyloidosis”?Cornea200928suppl808319092411
- SorensenCSRnagerKScaveniusCFibril core of transforming growth factor beta-induced protein (TGFBIp) facilitates aggregation of corneal TGFBIpBiochemistry201554192943295625910219
- LakshminarayananRCahurasiaSSMuruganEBiochemical properties and aggregation propensity of transforming growth factor-induced protein (TGFBIp) and the amyloid forming mutantsOcular Surf2015131925
- SuesskindDAuw-HaedrichCSchorderetDMunierFLLoefflerKUKeratoepithelin in secondary corneal amyloidosisGraefe’s Arch Clin Exp Ophthalmol2006244672573116331487
- Araki-SasakiKHiranoKOsakabeYClassification of secondary corneal amyloidosis and involvement of lactoferrinOphthalmol2013120611661172
- Araki-SasakiKOsakabeYFukuokaHIdetaRHiranoKFindings of secondary corneal amyloidosis with ultrahigh-resolution optical coherence tomographyClin Ophthalmol201482115211925342882
- MercatelliRRattoFRossiFThree-dimentional mapping of the orientation of collagen corneal lamellae in healthy and keratoconic human corneas using SHG microscopyJ Biophotonics2017101758327472438
- KoldsoHAndersenOJNikolajsenCLEarly events in the amyloid formation of the A546T mutant of transforming growth factor beta induced protein (TGFBIp) in corneal dystrophies compared to the non-fibrillating R555W and R555Q mutantsBiochemistry201554365546555626305369
- PahujaNKumarNRShroffRDifferential molecular expression of extracellular matrix and inflammatory genes at the corneal cone apex drives focal weakening in keratoconusInvest Ophthalmol Vis Sci201657135372538227732724
- AldaveAJPrincipeAHLinDYYelloreVSSmallKWLattice dystrophy-like localized amyloidosis of the cornea secondary to trichiasisCornea200524111211515604878
- CortneyDGPoulsenETKennedySProtein composition of TGFBI-R124C- and TGFBI-R555W-associated aggregates suggests multiple mechanisms leading to lattice and granular corneal dystrophyInvest Ophthalmol Vis Sci20155684653466126207300
