Abstract
Melioidosis, an infectious disease caused by Burkholderia pseudomallei, has recently gained importance as an emerging infectious disease in Indonesia. Reports of this infection in Indonesia are limited, although cases have been reported in Makassar, South Sulawesi. We report a case of cutaneous melioidosis caused by pan-drug-resistant, moderate biofilm-producer strain of B. pseudomallei in a diabetic patient. To the best of our knowledge, this is the first case of melioidosis caused by multidrug resistant and biofilm-former strain of B. pseudomallei being reported from Yogyakarta Province, Indonesia. The patient was successfully treated with abscess drainage and debridement, including total contact casting and no antibiotic treatment.
Introduction
Melioidosis, caused by Burkholderia pseudomallei, is an epidemic-prone disease of public health importance in South East Asia and Northern Australia.Citation1 The disease is spread by inhalation, ingestion, and inoculation, and less commonly through person-to-person, sexual, perinatal, vertical, and nosocomial routes.Citation1,Citation2 It has a fascinating pathogenesis, and an extremely diverse clinical manifestation.Citation2 Skin and soft tissue infections are a common manifestation of melioidosis and may be the source of systemic infection, or vice versa. Melioidosis mostly occurs in patients with preexisting conditions such as type-II diabetes, chronic disease of the lung or kidneys, excessive alcohol consumption, viral infection, and use of immunosuppressants, particularly steroids.Citation1,Citation3
The presence of closely related Burkholderia species in clinical specimen poses a major challenge in identifying B. pseudomallei. Rarity of melioidosis beyond endemic cases makes this microorganism difficult to be recognized, particularly by laboratories lacking properly validated diagnostic assay.Citation4
B. pseudomallei naturally resists a large array of antimicrobial agents, such as macrolides, narrow-spectrum cephalosporins, most penicillins, polymyxins, and aminoglycosides. Moreover, clinical evidence indicates that fluoroquinolones are also ineffective.Citation5,Citation6
Bacterial biofilm poses further problems, because it provides the bacteria a means to withstand the host immune system and antimicrobial agents. Formation of biofilm, responsible for dormancy, latency, and relapse of melioidosis, likely allows persistent infection and a greater chance of asymptomatic infection.Citation7,Citation8
We report a case of melioidosis presenting as skin and soft tissue infection, caused by a pan-drug-resistant and biofilm-producer strain of B. pseudomallei in Yogyakarta, Indonesia.
Case report
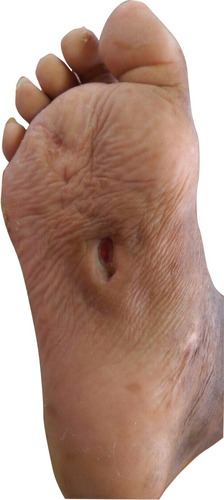
We report a case of a 57-year-old man admitted to Dr Sardjito General Hospital. He complained of recurrent wound infection on his right sole (). He was diagnosed with type-II diabetes 22 years ago and was treated with NovoMix® 30 (Novo Nordisk Indonesia LLC, Jakarta, Indonesia) thrice daily for the last 3 months. His ulcer started spontaneously as a blister 3 months ago. He had a history of similar ulcers on his right foot 7 years earlier. He has a history of hypertension and peripheral artery disease, but none of the following: coronary heart disease, stroke, deep vein thrombosis, or complications of the eye and kidneys. His vital signs were unremarkable. Physical examination on admission revealed an infected ulcer on the sole, 5×4 cm, with depth reaching the muscle layer. Both feet were dry and scaly, lightly hairy, hyperpigmented, and ulcerated. In addition, the right foot had thickened nails and Charcot foot. Laboratory examination found slight anemia (Hb 11.5 g/dL), hyperglycemia (random blood glucose 337 mg/dL), and low high-density lipoprotein (9 mg/dL). Feet and chest X-rays were unremarkable.
The patient refused to be hospitalized; therefore, microbiological examination could not be conducted. The patient was discharged with oral empiric antibiotics: cotrimoxazole 960 mg twice daily and clindamycin 300 mg four times a day. His hypertension was treated with irbesartan 300 mg once daily and his type-II diabetes was managed with Humalog (Eli Lilly Indonesia LLC [company presently closed], Jakarta, Indonesia) 16 units three times a day. Since the wound did not improve, culture and sensitivity tests were conducted, resulting in a scanty growth of Pseudomonas sp., resistant to gentamycin. His antibiotics were adjusted according to the sensitivity pattern. The patient was then discharged and given a 3 week course of oral cefixime and ciprofloxacin, with no improvement.
Since the patient had a history of recurrent abscesses, melioidosis was among the differential diagnoses. The culture and sensitivity tests were repeated. Microbiology examination on a swab sample from the wound base revealed B. pseudomallei. Culture on blood agar, MacConkey agar, and Ashdown agar yielded short Gram-negative rods with bipolar staining. On Ashdown agar, these colonies appeared pale pink, and after prolonged incubation became dark purple, dry, and wrinkled in the center (). A very strong sweetish-putrid odor was noted, resembling stagnant water. These characteristics are suggestive of B. pseudomallei. To confirm our finding, we conducted nested PCR to detect 16S rRNA gene. We used an outer primer specific to the genus Burkholderia sp. and an inner primer specific to B. pseudomallei in the first and second amplification, respectively. Visualization of a 397 bp-sized DNA bands in electrophoresis with 1% agarose gel confirmed the presence of 16S rRNA gene of B. pseudomallei ().Citation9
Figure 2 Morphology of B. pseudomallei colonies on ash down agar after 48 hours incubation at 37°C.
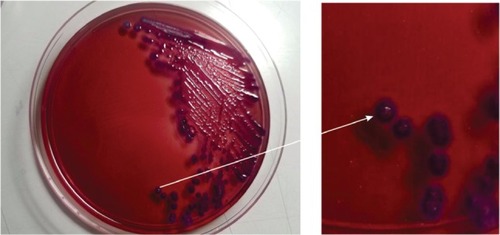
Figure 3 Result of PCR using a 16S rRNA primer.
Antibiotic susceptibility test of B. pseudomallei was done in a biological safety cabinet, by disk diffusion method on Mueller Hinton agar. The resulting zones of inhibition are demonstrated as uniformly circular with confluent lawns of growth. The Clinical and Laboratory Standards Institute has not published the interpretation result for B. pseudomallei. Therefore, we searched the guidelines and reference for the threshold of Burkholderia cepacia, Pseudomonas aeruginosa, or Enterobacteriaceae.Citation10,Citation11 The result revealed resistance to all antibiotics tested ().
Table 1 Antibiotic resistance pattern of B. pseudomallei performed by Kirby–Bauer method of disc diffusion assay
We determined the B. pseudomallei biofilm-forming ability using a modified protocol. We standardized the bacterial density of the spectrophotometer (OD600=1) prior to the test. Then we inoculated the overnight cultures of B. pseudomallei into brain heart infusion broth and incubated it aerobically overnight in a shaking incubator at 37°C. Afterward, we added 200 µL bacterial suspensions to the wells of a 96-well flat bottom plastic tissue culture plate (Nunc, Roskilde, Denmark) and incubated it at 37°C for 48 hours. We used uninoculated Brain Heart Infusion broth as control. Following incubation, we washed the well three times to remove nonadherent bacteria. We then stained the wells with 50 µL filtered 1% crystal violet for 5 minutes. We washed excess stain with water and air dried the wells. Next, we solubilized the crystal violet-bound bacterial cells with 200 µL of 5% acid isopropanol and measured the released stain using a microplate reader at 595 nm. We repeated this procedure eight times and took the average OD595. According to Hassan et al,Citation12 the B. pseudomallei are considered a moderate biofilm-former strain as the OD595 was 0.75±0.03.
The patient underwent surgical abscess-draining, debridement, and application of total contact casting to relieve body weight of the diabetic ulcer. He was followed up until September 2017. At the time of this writing, he was well without evidence of recurrence or relapse, and his ulcer had healed.
Discussion
Definitive diagnosis of melioidosis requires isolation and identification of B. pseudomallei from clinical specimens because its clinical manifestations vary widely. B. pseudomallei is classified as Biosafety Level 3 agent, necessitating the analyzing laboratory to be appropriately equipped. Little clinical experience and lack of pathognomonic symptoms hinder bacterial identification by laboratories faced with an unexpected case of melioidosis. Furthermore, identification of B. pseudomallei necessitates use of intensive microbiological workup, such as confirmatory amplification of 16S rRNA gene.
The patient presented with mild, chronic, and localized skin and soft tissue infection. Based on these clinical manifestations, we considered it as primary cutaneous melioidosis. Skin and soft tissue infection, primary and secondary, account for 13%–24% of melioidosis in published case series.Citation13,Citation14 Primary skin melioidosis is often localized and less severe than other forms of melioidosis. On the other hand, primary skin melioidosis has been reported to be associated with necrotizing fasciitis, sepsis, and internal organ abscesses in Southeast AsiaCitation13,Citation15
We deemed our case to be a pan-resistant melioidosis, likely associated with the biofilm-forming ability of the bacteria. As in many reported cases, the patient had diabetes, which is regarded as a significant risk factor.Citation14,Citation16 Being a farmer with a diabetic foot ulcer, he likely acquired the pathogen through direct contact with contaminated water droplets or soil through a penetrating wound or existing skin abrasion.
The B. pseudomallei we found in this case were moderate biofilm-formers. Chronic infection and colonization of infective biofilms can occur on dead tissue or medical devices.Citation17 Bacterial biofilm can be up to 1,000 times more resistant to antimicrobial agents than their free-living (planktonic) counterpart.Citation18 Biofilms, although not correlated with bacterial virulence,Citation21 present a barrier, limiting diffusion and eventually the activity of the antibiotics.Citation19,Citation20 The full depth of the biofilm is composed of polymeric substances that impede penetration of antimicrobial agent. Moreover, bacterial cells within the biofilm are in a starved state, and are thus slow-growing.Citation18 Slow or nongrowing bacterial cells are less or even unsusceptible to antimicrobial agents.Citation22 Recent studies found that stimulating B. pseudo-mallei biofilm-forming ability resulted in upregulation of some genes responsible for increased resistance toward antimicrobial agents.Citation23 In addition, biofilm formation of B. pseudomallei in vitro is found to be associated with relapse in human melioidosis.Citation8
The current recommendation for management of all forms of melioidosis consists of an intensive phase followed by an eradication phase. In the intensive phase, 2 weeks course of ceftazidim or meropenem is prescribed, followed by 12 weeks course of high dose cotrimoxazole. In our case, however, the patient was not given any antibiotics since none were effective. The patient had his foot abscess surgically drained, wound debrided, and total contact casting applied to relieve any pressure off the ulcer. The outcome was good, and the patient was advised to have routine follow-up to monitor the possibility of relapse of infection.
Despite lacking clinical trials, antibiotics alone – with or without incision and drainage – were reported to have successfully treated patients with localized melioidosis, including those with skin and soft tissue lesions and parotid abscesses.Citation13,Citation24–Citation27 A case series in Malaysia documented recovery without antibiotics in four children with localized melioidosis after surgical drainage of abscess, and in one case even without surgical treatment.Citation28 The current management for biofilm includes sharp debridement; mechanical debridement using curettes, fabric pads, lavage, or ultrasound; and autolytic debridement with moisture-retentive dressings.Citation29,Citation30
To the best of our knowledge, this is probably the first report of pan-drug-resistant B. pseudomallei from Java Island, Indonesia. Along with increasing incidence of melioidosis in Indonesia, this report raises the alarm for the possibility of melioidosis in diabetic foot patients in this area. Microbiologists should be aware of the characteristics of the etiologic agent, and cultures should be handled under laboratory Biosafety Level 3 containment. We recommend that all B. pseudomallei isolates have their antibiotic sensitivity carefully evaluated.
Ethics statement
The study was approved by the Medical and Health Research Ethics Committee of the Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia (KE/FK/0838/EC/2017). Written informed consent was provided by the patient to have the case details and any accompanying images published.
Acknowledgments
The authors give thanks to Ms Lintang for obtaining patient’s data and Mrs Mulyani and Mrs Linda Oktabriana for laboratory assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
- ChengACCurrieBJMelioidosis: epidemiology, pathophysiology, and managementClin Microbiol Rev200518238341615831829
- RedondoMCGómezMLandaetaMEEugeniaMRıHKhalilRMelioidosis presenting as sepsis syndrome: a case reportInt J Infect Dis201115320102011
- ZueterAYeanCYAbumarzouqMRahmanZADerisZZThe epidemiology and clinical spectrum of melioidosis in a teaching hospital in a North-Eastern state of Malaysia: a fifteen- year reviewBMC Infect Dis201611126729246
- HemarajataPBaghdadiJDHoffmanRHumphriesRMBurkholderia pseudomallei: Challenges for the Clinical Microbiology LaboratoryJ Clin Microbiol201654122866287327654336
- SchweizerHPMechanisms of antibiotic resistance in Burkholderia pseudomallei: implications for treatment of melioidosisFuture Microbiol20127121389139923231488
- WuthiekanunVAmornchaiPSaipromNSurvey of antimicrobial resistance in clinical Burkholderia pseudomallei isolates over two decades in Northeast ThailandAntimicrob Agents Chemother201155115388539121876049
- KohSFTaySTPuthuchearySDColonial morphotypes and biofilm forming ability of Burkholderia pseudomalleiTrop Biomed201330342843324189672
- LimmathurotsakulDPaeyaoAWongratanacheewinSRole of Burkholderia pseudomallei biofilm formation and lipopolysaccharide in relapse of melioidosisClin Microbiol Infect20142011O854O85624602145
- DharakulTSongsivilaiSViriyachitraSLuangwedchakarnVTas-saneetritapBChaowagulWDetection of Burkholderia pseudomallei DNA in patients with septicemic melioidosisJ Clin Microbiol19963436096148904424
- DanceDTreatment and prophylaxis of melioidosisInt J Antimicrob Agents201443431031824613038
- Clinical and Laboratory Standards InstituteM100 Performance Standards for Antimicrobial Susceptibility Testing27th informational supplementWayne, PAClinical and Laboratory Standards Institute2017
- HassanAUsmanJKaleemFOmairMKhalidAIqbalMEvaluation of different detection methods of biofilm formation in the clinical isolatesBraz J Infect Dis201115430531121860999
- GibneyKBChengACCurrieBJCutaneous melioidosis in the tropical top end of Australia: a prospective study and review of the literatureClin Infect Dis200847560360918643756
- CurrieBJJacupsSPChengACMelioidosis epidemiology and risk factors from a prospective whole-population study in northern AustraliaTrop Med Int Health20049111167117415548312
- KingsleyPVLeaderMNagodawithanaNSTipreMSathiakumarNMelioidosis in Malaysia: A Review of Case ReportsPLoS Negl Trop Dis20161012118
- BenoitTJBlaneyDDGeeJEMelioidosis Cases and Selected Reports of Occupational Exposures to Burkholderia pseudomallei--United States, 2008–2013MMWR Surveill Summ201564519
- BryersJDMedical biofilmsBiotechnol Bioeng2008100111818366134
- LewisKRiddle of biofilm resistanceAntimicrob Agents Chemother2001454999100711257008
- PibalpakdeePWongratanacheewinSTaweechaisupapongSNiumsupPRDiffusion and activity of antibiotics against Burkholderia pseudomallei biofilmsInt J Antimicrob Agents201239435635922364716
- MongkolrobRTaweechaisupapongSTungpradabkulSCorrelation between biofilm production, antibiotic susceptibility and exopolysaccharide composition in Burkholderia pseudomallei bpsI, ppk, and rpoS mutant strainsMicrobiol Immunol2015591165366326486518
- TaweechaisupapongSKaewpaCArunyanartCVirulence of Burkholderia pseudomallei does not correlate with biofilm formationMicrob Pathog2005393778516084684
- PanomketPBurkholderia pseudomallei and biofilmsAsian Biomed201593285290
- SawasdidolnCTaweechaisupapongSSermswanRWTattawasartUTungpradabkulSWongratanacheewinSGrowing Burkholderia pseudomallei in biofilm stimulating conditions significantly induces antimicrobial resistancePLoS One201052110
- AchappaBMadiDVidyalakshmiKCutaneous melioidosisJ Clin Diagnostic Res2016109WD01WD02
- NgauyVLemeshevYSadkowskiLCrawfordGCutaneous melioidosis in a man who was taken as a prisoner of war by the Japanese during World War IIJ Clin Microbiol200543297097215695721
- BodilsenJLanggaardHNielsenHLCutaneous melioidosis in a healthy Danish man after travelling to South-East AsiaBMJ Case Rep2015201520142016
- MeckenstockRTherbyAMarque-JuilletSCutaneous melioidosis in adolescent returning from GuadeloupeEmerg Infect Dis201218235936022305492
- HowHSNgKHYeoHBTeeHPShahAPediatric melioidosis in Pahang, MalaysiaJ Microbiol Immunol Infect200538531431916211138
- RhoadsDDWolcottRDPercivalSLBiofilms in wounds: management strategiesJ Wound Care2008171150250818978690
- MetcalfDBowlerPBiofilm delays wound healing: A review of the evidenceBurns Trauma201311527574616