Abstract
Budd–Chiari syndrome has been described as a late complication of Behçet’s disease. Although the mortality rate associated with Behçet’s disease is low, it can escalate in the presence of Budd–Chiari syndrome and may be further complicated by intracardial thrombus formation. It is therefore important to detect and initiate management early in the disease course. The imaging modalities of choice should be minimally invasive as certain procedures may aggravate Behçet’s disease by initiating a thrombosis or aggravating an existing one. In Behçet’s disease-induced Budd–Chiari syndrome, cardiac investigation is crucial in the work-up in order to identify any cardiac involvement and determine the etiology of intracardial thrombus. Furthermore, the treatment should ultimately focus on controlling the activity of Behçet’s disease. We report an unusual case of Behçet’s disease presenting with Budd–Chiari syndrome complicated by intracardial thrombus in a young Korean man.
Introduction
Behçet’s disease is a systemic vasculitis of unknown cause involving veins and arteries of all sizes, first described as ‘Triple Symptom Complex’ by Professor Hulusi Behçet in 1937. He described the association of oral and genital ulceration with uveitis and considered this to be indicative of possible viral etiology.Citation1 Since the original description, these initial three manifestations have been studied and described in much detail, as well as extensive findings in other systemic manifestations of the disease.Citation2,Citation3
In Behçet’s disease, long-term mortality has been reported to be associated with central nervous system involvement and major vessel disease including arterial aneurysm and Budd–Chiari syndrome.Citation4 In one study, Budd–Chiari syndrome accounted for 10% of all deaths in Behçet’s disease. Although the angiographic findings are not specific to Behçet’s disease, the high probability of inferior vena cava (IVC) involvement in Behçet’s disease has meant the necessity of including Behçet’s disease among the differential diagnoses in a case of Budd–Chiari syndrome.Citation5 In extremely rare cases, extensive cardiopulmonary involvement can lead to development of intracardial thrombi, posing further therapeutic challenges.
Behçet’s disease occurs worldwide, but high prevalence along the ancient silk trade routes extending from eastern Asia to the Mediterranean basin, have earned it the name of Silk Road disease. We report a rare case of Behçet’s disease presenting with Budd–Chiari syndrome and an intracardial thrombus in a young Korean man.
Case report
A 31-year-old Korean man presented with a 1-month history of intermittent abdominal discomfort and abdominal pain on walking. He had already been evaluated 1 month earlier at another hospital for similar episodes of abdominal discomfort with diarrhea and indigestion prior to visiting our clinic in February 2011. He was also recently diagnosed with hyperthyroidism associated with symptoms including weight loss, palpitations, and heat intolerance. The patient reported himself as otherwise well with no other significant past medical history or pathological family history.
The abdominal computed tomography (CT) scan performed 1 month ago showed extensive thrombosis in the middle and lower hepatic vein and IVC with inhomogeneous attenuation in the liver. Low attenuation gall bladder wall thickening with subserosal edema was detected. A moderate amount of ascites and a small amount of pleural effusion was seen on the right side ().
Figure 1 Abdominal CT scan showing extensive thrombus formation at presentation. Lack of opacification of the segmental portal vein after contrast injection indicates recent thrombosis.
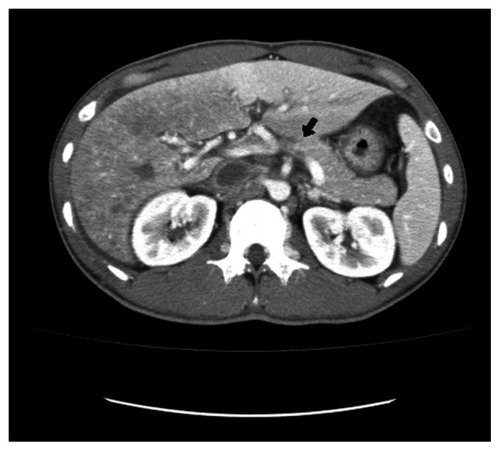
Based on the abdominal CT findings, the patient was evaluated for possible causes, including the diagnosis of myeloproliferative disease, protein C deficiency, protein S deficiency and, given the high geographical incidence, Behçet’s disease. He gave history of recurrent oral and genital ulcerations and follicular skin lesions in the past year. The patient reported nonspecific lethargy and arthralgia in the left elbow and right wrist. There was no evidence of uveitis. Pathergy test was negative.
Prior to his transfer to our hospital for further evaluation, the patient was treated initially with high-dose heparinization, followed by intravenous urokinase infusion with warfarin 5 mg daily. The patient responded partially and the repeat abdominal CT scan performed before his transfer showed resolved thrombus on the right renal vein but persisting thrombi in the hepatic vein and IVC.
On his initial evaluation at our hospital, the examination findings were as follows: a chronically ill looking male patient with blood pressure 115/75 mmHg, pulse 89 beats/minute, respiratory rate 20, and a body temperature of 38.3°C. On cardiac examination, no murmur was audible. Chest auscultation was clear with good air entry bilaterally. The abdomen was soft, nontender, and not distended. There was no hepatomegaly, splenomegaly, or visible abdominal veins. He had a widespread folliculitis on his abdomen, and genital and oral ulcers were present. Neurological examination was normal.
Laboratory investigation showed raised thyroid- stimulating hormone (TSH) and free T4 (thyroxine), undetected rheumatoid factor, and serum complement; liver enzymes, including aspartate aminotransferase (AST) and alanine transaminase (ALT), were increased with levels of 148 and 136 IU/L, respectively; lactate dehydrogenase (LDH) was 575 IU/L; high C-reactive protein (4.39); negative serological findings for viral hepatitis, HIV and syphilis; hypercoagulable work-up revealed normal levels of protein C, S, and anti-thrombin III; and normal indirect binocular ophthalmoscopy. Janus kinase 2 (JAK-2) testing to identify underlying latent myeloproliferative disorder was negative.
No interval change was seen upon repeat abdominal CT scan. A CT chest pulmonary angiogram with 3D contrast showed a wedge-shaped low density perfusion defect in the antero-medial segment of the left lower lobe with reduced pulmonary artery size, suggestive of chronic pulmonary thromboembolism.
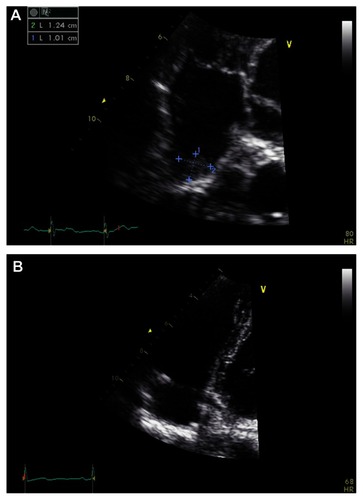
An echocardiogram showed normal left ventricle cavity size and systolic function with calculated ejection fraction of 66%. No abnormalities were seen on the wall or the valves. No other intracardiac shunts, including patent foramen ovale, were identified. However, a mobile thrombus with measured size 10 mm × 13 mm was seen in the right atrium (RA) (). The patient was subsequently commenced on high-dose steroid therapy, in addition to previous anticoagulation.
Figure 2 Echocardiogram showing (A) mobile thrombus in right atrium, and (B) 1 month after methylprednisolone and anticoagulation therapy.
After multidisciplinary discussion with the cardiothoracic surgeons regarding possible surgical management of the thrombus, it was agreed that segmental defect was not an absolute indication for surgery in the asymptomatic patient and a short term follow-up with repeat imaging should be performed. The patient was discharged on 15 mg prednisolone and high-dose warfarin daily with a target international normalized ratio (INR) of 2–3, and an inpatient follow-up was scheduled in 1 month.
A repeat echocardiogram performed 1 month later showed no signs of the previous thrombus in the RA and IVC (). A CT pulmonary angiogram showed no interval change of segmental perfusion defect in the left lower lobe since the previous scan. The patient was discharged from the hospital on a tapering dose of 10 mg prednisolone and 4 mg warfarin per day.
At 4-month re-evaluation, no physical stigmata of chronic liver disease were present, and liver function tests were only mildly deranged at AST 16 IU/L, ALT 14 IU/L, C-reactive protein 1.66, and INR 3. CT chest pulmonary artery and deep vein with contrast showed persisting deep vein thrombosis in the bilateral common iliac vein and IVC but no evidence of pulmonary embolism and significantly decreased extent of perfusion defect.
Discussion
This case demonstrates an unusual case of Behçet’s disease presenting with Budd–Chiari syndrome and an intracardial thrombus formation. Behçet’s disease is known to be a multi- system disorder with vasculitis playing a major role in the disease process. The prevalence of Behçet’s disease in South Korea is higher than most other places in the world, and has an unusual female predominance with a high positive rate of ocular lesions and lower positivity with pathergy test.Citation6
Budd–Chiari syndrome is a condition with obstruction to the venous outflow of the liver due to occlusion of the hepatic vein. It was first described by an internist named George Budd in 1845, who described the classic triad of abdominal pain, hepatomegaly, and ascites; subsequently, the pathologist Hanns Chiari added the first pathologic description of a liver with ‘obliterating endophlebitis of the hepatic veins’ in 1899. The syndrome most often occurs in patients with underlying thrombotic diathesis, including myeloproliferative disorders, tumors, chronic inflammatory disease, clotting disorders, and infection.Citation7 Acutely, it presents with abdominal pain, nausea, vomiting, tender hepatomegaly, and ascites. In chronic cases, there is enlargement of the liver, mild jaundice, ascites, negative hepatojugular reflex, and splenomegaly with portal hypertension. The incidence of Behçet’s disease as a cause of Budd–Chiari syndrome is reported to be between 2% and 5.8%. A study by Sahin et al reported Behçet’s disease as the third most common cause of the Budd–Chiari syndrome.Citation8,Citation9
Budd–Chiari syndrome is often considered to be a late complication of Behçet’s disease, with one study reporting the average delay in diagnosis of Budd–Chiari syndrome from the date of Behçet’s disease diagnosis is 4.5 years.Citation10 Among patients with Behçet’s disease, the vascular complication of Budd–Chiari syndrome is more prevalent and severe in male patients and in patients from the Middle and Far East.Citation11
Although Budd–Chiari syndrome is regarded as a late complication, the clinical course of Budd–Chiari syndrome caused by Behçet’s disease is rapid and is quite different from that of idiopathic Budd–Chiari syndrome. Unlike the idiopathic type, the vessels can become occupied with granulomatous tissue as a result of fibrosis in Behçet’s disease-induced Budd–Chiari syndrome.Citation12–Citation14
Even though the mortality rate from Behçet’s disease is not high, it has been reported that development of Budd– Chiari syndrome in conjunction with Behçet’s disease has a poorer prognosis.Citation8 It is therefore important to diagnose Budd– Chiari syndrome in its early stages and start management immediately. In the past, the main emphasis on treatment of Budd–Chiari syndrome was anticoagulation. More recently, some success has been reported with liver decompression by shunting proceduresCitation15 or, in the case of membranous obstruction, transcardiac membranotomy.Citation16 Thrombolytic agents such as urokinase are superior to heparin in treating deep venous thromboses, particularly in acute setting.Citation17
The radiologic work-up is the key to the diagnosis and monitoring of disease activity as well as assessing the effect of the treatment. Plain radiographic changes may be apparent in the thorax including hilar enlargement and peripheral lung masses secondary to pulmonary artery aneurysm, diaphragmatic elevation due to pulmonary artery occlusion or atelectasis, consolidation due to infarction or hematoma, and pleural effusion.Citation18 Noncontrast CT scans showing diffuse hepatic hypodensity due to edema with global liver enlargement and ascites are characteristic in Budd–Chiari syndrome.Citation19 Furthermore, thrombus in the IVC or in the cardiac chamber can easily be demonstrated by echocardiography.
Conventional angiography is the gold standard method of evaluating pulmonary and coronary arteries, however this procedure may present some risks to Behçet’s disease patients. Venous puncture or rapid injection of a large quantity of contrast medium may initiate a thrombosis or aggravate an existing one.Citation20 The combination of the aforementioned noninvasive procedures are preferred in investigating Behçet’s disease-induced Budd–Chiari syndrome.
In our case, the intracardial thrombus extended into the RA and was detected on echocardiogram. With the absence of baseline echocardiogram, it is difficult to determine whether the development of a thrombus is iatrogenic or related to Behçet’s disease. Previous studies have described that intracaval thrombi can be associated with vasculitis, and cannot currently be controlled well with anticoagulation therapy. A report by McCarthy in 1985 showed that medical management alone resulted in death within 6 months in 12 out of 14 patients. Some clinicians recommend medical therapy alone for patients with fewer symptoms, relatively normal liver markers, and in cases with absent or easily controlled ascites.Citation7,Citation20 On this basis, we did consider surgical management of the thrombus but it resolved spontaneously and surgical intervention was unnecessary in this case.
Once the thrombosis has been managed either surgically or medically, controlling vasculitis becomes particularly important in preventing re-obstruction. When Behçet’s disease causes Budd–Chiari syndrome, patients may suffer from a number of recurring symptoms that lead to clinical progression and increased mortality risk. Comprehensive and aggressive treatment including immunosuppressive therapy targeting Behçet’s disease must be initiated to prevent future events.
Conclusion
Budd–Chiari syndrome is not an uncommon complication of Behçet’s disease. It can be further complicated by intracardial thrombus formation, significantly increasing the mortality. The prime aim of the treatment should be controlling the disease activity of Behçet’s disease, closely followed by anticoagulation therapy and continuous monitoring. Furthermore, noninvasive modalities must be used when evaluating and monitoring Budd–Chiari syndrome to minimize the risks related to Behçet’s disease.
Disclosure
The authors report no conflicts of interest in this work.
References
- BehçetHMattesonELOn relapsing, aphthous ulcers of the mouth, eye and genitalia caused by a virus. 1937Clin Exp Rheumatol2010284 Suppl 60S2S520868561
- KerkeniNZaraaIAyachiJEl EuchDMokniMBen OsmanABehçet’s disease: A profile of mucocutaneous featuresActa Dermatovenerol Alp Panonica Adriat2010192111520664915
- HochbergMCSilmanAJSmolenJSWeinblattMEWeismanMHRheumatology24th edPhiladelphia, PAMosby Elsevier2008
- YaziciHFEsenFMortality in Behçet’s syndromeClin Exp Rheumatol2008265 Suppl 51S138S14019026156
- SaatciIOzmenMBalkanciFAkhanOSenaatiSBehçet’s disease in the etiology of Budd-Chiari diseaseAngiology19934453923988480917
- BangDLeeJHLeeESEpidemiologic and clinical survey of Behçet’s disease in Korea: the first multicenter studyJ Korean Med Sci200116561561811641532
- HortonJDSan MiguelFLMembranoFBudd-Chiari syndrome: illustrated review of current managementLiver Int200828445546618339072
- BayraktarYBalkanciFKansuEBudd-Chiari syndrome: analysis of 30 casesAngiology19934475415518328682
- UskudarOAkdoganMSasmazNYilmazSTolaMSahinBEtiology and portal vein thrombosis in Budd-Chiari syndromeWorld J Gastroenterol200814182858286218473410
- HoumanHLamloumMBen GhorbelIKhiari-Ben SalahIMiledMVena cava thrombosis in Behçet’s disease. Analysis of a series of 10 casesAnn Med Interne (Paris)19991508587590 French10686638
- Ben GhorbelIEnnaiferRLamloumMKhanfirMMiledMHoumanMHBudd-Chiari syndrome associated with Behçet’s diseaseGastroenterol Clin Biol200832331632018400436
- B’chir HamzaouiSHarmelABouslamaKle groupe tunisien d’étude sur la maladie de BehçetBehçet’s disease in Tunisia. Clinical study of 519 casesRev Med Interne20062710742750 French16987570
- DanaciMGülSYazganYHülagüSUskentNBudd-Chiari syndrome as a complication of Behçet’s syndrome. A case reportAngiology199647193958546354
- KorkmazCKasifogluTKebapciMBudd-Chiari syndrome in the course of Behçet’s disease: clinical and laboratory analysis of four casesJoint Bone Spine200774324524817369069
- OrloffMJJohansenKHTreatment of Budd-Chiari syndrome by side-to-side protocaval shunt: experimental and clinical resultsAnn Surg19781884494512697434
- TakeuchiJTakadaAHasumuraYMatsudaYIkegamiFBudd-Chiari syndrome associated with obstruction of the inferior vena cava. A report of seven casesAm J Med197151111205570315
- ElliotMSImmelmanEJJefferyPA comparative randomised trial of heparin versus streptokinase in the treatment of acute proximal venous thrombosis: an interim report of a prospective tiralBr J Surg19796612838843389338
- YakutZIOdevKPulmonary and cardiac involvement in Behcet disease: 3 case reportsClin Appl Thromb Hemost200713331832217636195
- LimJHParkJHAuhYHMembranous obstruction of the inferior vena cava: comparison of findings at sonography, CT, and venographyAJR Am J Roentgenol199215935155201503015
- McCarthyPMvan HeerdenJAAdsonMASchaferLWWiesnerRHThe Budd-Chiari syndrome. Medical and surgical management of 30 patientsArch Surg198512066576624004551