Abstract
Purpose
To evaluate how the growth of neovascularization elsewhere (NVE) was delineated in an eye with hemicentral retinal vein occlusion (CRVO) using optical coherence tomography (OCT) angiography.
Patients and methods
We examined a 64-year-old man diagnosed with hemi-CRVO. The area around the occluded vein was scanned using a spectral-domain OCT device (RTVue XR Avanti). Blood flow was detected using the split-spectrum amplitude-decorrelation angiography (SSADA) algorithm. Color fundus photography, fluorescein angiography (FA), and OCT angiography examinations were performed at the first visit and at 3 and 6 months postpresentation.
Results
At the first visit, FA revealed delayed retinal venous filling and extensive areas of capillary nonperfusion. The patient underwent a trial of intravitreal ranibizumab injection (0.5 mg/0.05 mL) for the treatment of macular edema. At 3 months postpresentation, there was no NVE around the occluded vein in the en-face SSADA image, but at 6 months, NVE appeared on the occluded veins. The en-face SSADA image showed the NVE structure in the fibrovascular membrane on the occluded vein more clearly than FA images.
Conclusion
OCT angiography clearly visualized the sprouting of NVE in an eye with hemi-CRVO. New findings of the vascular structure of NVE in hemi-CRVO were revealed using the en-face SSADA algorithm.
Keywords:
Introduction
Central retinal vein occlusion (CRVO) is a cause of visual loss in developed countries.Citation1,Citation2 CRVO is divided into two types: nonischemic and ischemic CRVO.Citation1,Citation3 The visual prognosis for patients with ischemic CRVO is worse than that for patients with nonischemic CRVO.Citation2,Citation3 Patients with ischemic CRVO and branch retinal vein occlusion have a greater risk of developing neovascularization elsewhere (NVE),Citation3 which can lead to vitreous hemorrhage and neovascular glaucoma, and result in loss of vision.Citation3,Citation4
Fluorescein angiography (FA) is a very important tool for evaluating nonperfusion areas and detecting neovascularization (NV) in ischemic CRVO.Citation5 Although FA is vital for CRVO examination, intravenous fluorescein injection should be carefully performed because dye injection can occasionally cause nausea and, rarely, anaphylaxis. Furthermore, it is difficult to evaluate the detailed vasculature in NV by FA because of leakage of the dye toward the vitreous cavity.
Recent developments in theoretically-based optical coherence tomography (OCT) angiography allow for noninvasive evaluation of capillary vasculature.Citation6–Citation10 In particular, use of the split-spectrum amplitude-decorrelation angiography (SSADA) algorithm improves the signal-to-noise ratio of flow detection.Citation8 The en-face SSADA images provide a clearer image of the vasculature than FA. Furthermore, the en-face SSADA images can help in the clear visualization of the microvasculature in different retinal layers.Citation11 Some studies have evaluated choroidal NV using OCT angiography by applying the SSADA algorithm,Citation7,Citation12 and we previously reported that the NV structures could be clearly visualized at the disc in en-face SSADA images obtained from patients with diabetic retinopathy.Citation11 No studies to date have evaluated retinal NV in CRVO using en-face SSADA imaging.
In this study, we used OCT angiography with application of the SSADA algorithm to evaluate the microvasculature of NVE in ischemic hemi-CRVO.
Patient and methods
A 64-year-old man diagnosed with hemi-CRVO in his right eye was recruited. This patient was examined in the Department of Ophthalmology of Asahikawa Medical University. Best-corrected visual acuity (BCVA), slit-lamp examination, fundus biomicroscopy, color fundus photograph, intraocular pressure (IOP), FA, OCT, and OCT angiography (vide infra) were evaluated at the first visit and at 3 and 6 months postpresentation. Central macular thickness was defined as the region of tissue between the inner limiting membrane and the outer retinal pigment epithelium boundary at the fovea for OCT images. The FA was performed with Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany). This study was performed in adherence to the tenets of the Declaration of Helsinki and was approved by our institutional review board at the Asahikawa Medical University. Informed consent was obtained from the patient.
The instrument used for the OCT angiographic images was based on the RTVue XR Avanti (Optovue Inc., Fremont, CA, USA) and was used to obtain en-face SSADA images, as previously described.Citation11 The scanning area was captured in 3×3 mm2 sections and focused on the occluded vein near the optic disc head. The OCT angiograms were segmented as follows; the en-face image was segmented with an inner boundary set at 3 µm beneath the internal limiting membrane and an outer boundary set at 15 µm beneath the inner plexiform layer (IPL) to obtain images of the superficial vascular layers (defined as the superficial plexus), the en-face image was segmented with an inner boundary at 15 µm beneath the IPL and the outer boundary was set at 70 µm beneath the IPL to obtain images of the deep vascular layers (defined as the deep plexus).
Results
At the first visit, BCVA in the right eye was 20/100 and IOP was 14 mmHg. Fundus examination revealed numerous retinal hemorrhages in half of the inferior part, dilated and tortuous retinal veins, macular edema, and cotton wool spots (). The left eye had a completely normal fundus. The central macular thickness in his right eye was 721 µm, indicating severe macular edema. FA revealed delayed retinal venous filling and extensive areas of capillary nonperfusion (). The patient was diagnosed with ischemic hemi-CRVO and underwent intravitreal injection of ranibizumab (0.5 mg/0.05 mL) for the treatment of macular edema.
Figure 1 Color fundus photography and FA at the first visit (A and B) and at 6 months postpresentation (C and D).
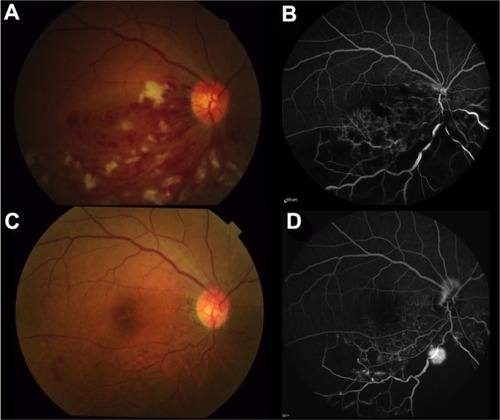
One month after the first visit, his BCVA had improved to 20/25 and central macular thickness decreased significantly to 296 µm. At 3 months postpresentation, his BCVA had further improved to 20/20. Fundus examination revealed a reduction in the retinal hemorrhage, cotton wool spots, and macular edema (256 µm). At 6 months after the first visit, there was no macular edema. His BCVA was 20/20, and his IOP was 15 mmHg. Fundus examination revealed almost no change from the appearance at 3 months (). FA, however, revealed an extensive nonperfused area and emergence of NVE at the occluded inferior temporal vein ().
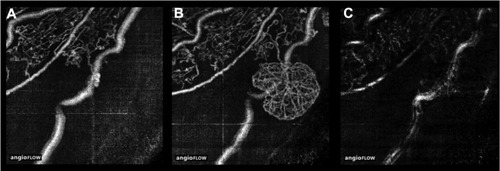
In the en-face SSADA images of the superficial plexus, there was no NVE around the occluded vessel () at 3 months postpresentation, but at 6 months, remarkable NVE growth was observed on the occluded vein (). NVE was not visualized in the en-face SSADA image of the deep plexus (). The en-face SSADA image more clearly revealed the NVE structure in the fibrovascular membrane () than the FA image ().
Figure 2 En-face SSADA image of the superficial plexus at 3 months (A) and 6 months (B) postpresentation. NVE appeared around the occluded veins. (C) The deep plexus image at 6 months postpresentation.
Abbreviations: SSADA, split-spectrum amplitude-decorrelation angiography; NVE, neovascularization elsewhere.
Discussion
This is the first study to show the growth of NVE in a hemi-CRVO eye using en-face SSADA imaging. Although NVE can also be evaluated by FA examination, the vascular structure of NVE cannot be clearly visualized by FA because of excessive leakage from NVE, even in the early phase of FA. The current study demonstrated that en-face SSADA imaging, which is not affected by leakage, clearly visualizes the growth and detailed microvasculature of NVE in hemi-CRVO.
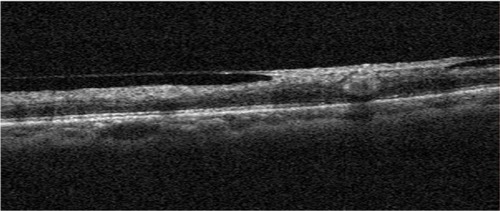
Some studies have described NV at the disc in diabetic retinopathy using spectral-domain OCT.Citation13,Citation14 Few studies, however, have examined NVE.Citation9,Citation13 Cho et alCitation13 reported that a patient with diabetic retinopathy and NVE had a thickened posterior hyaloid, and NVE was observed as hyperreflective material within the inner retinal layer and as projecting in spectral-OCT images. Moreover, in a recent Doppler OCT study, Miura et alCitation9 evaluated NVE in diabetic retinopathy. In this study, we observed that the posterior hyaloid rigidly adhered at the point of the NVE in the hemi-CRVO eye in OCT B-scan images using OCT angiography (). This finding is quite similar to NVE in diabetic retinopathy.
Figure 3 OCT B-scan image of NVE.
Abbreviations: OCT, optical coherence tomography; NVE, neovascularization elsewhere.
The findings of this study also elucidated that NVE of hemi-CRVO was located only in the superficial retinal layer because there was no vascular structure of NVE in the en-face SSADA image of the deep plexus (). The origin of NVE vessels in hemi-CRVO may be the radial peripapillary capillaries because 3 months before the growth of NVE we observed the growth of radial peripapillary capillaries into the occluded vein at the same point of the growth of NVE. Furthermore, the NVE of hemi-CRVO was round in shape, and the outer border of the NVE terminus of the vessels created loops. SpaideCitation12 reported that in some lesions of choroidal NV, the outer terminus was composed of vascular loops. This feature of NV is similar to what we observed. Taken together, the NVE in hemi-CRVO, comprising looping radial peripapillary capillaries, sprouted on the occluded vein in the superficial retinal layer.
This study has some limitations. First, this study was a case study. A large number of patients are needed to evaluate the significant features of NVE of hemi-CRVO. Second, we have not evaluated the growth processes of NVE in hemi-CRVO. In this patient, we performed photocoagulation in the area of nonperfusion. It would be interesting to determine whether the NVE might eventually disappear using en-face SSADA images.
Conclusion
This study demonstrates that NVE sprouting can be observed in hemi-CRVO by en-face SSADA imaging.
Disclosure
The authors report no conflicts of interest in this work.
References
- BhagatNGoldbergMFGasconPBellWHabermanJZarbinMACentral retinal vein occlusion: review of managementEur J Ophthalmol19999316518010544972
- HayrehSSManagement of central retinal vein occlusionOphthalmologica2003217316718812660480
- RehakMWiedemannPRetinal vein thrombosis: pathogenesis and managementJ Thromb Haemost2010891886189420492457
- ChanCKIpMSVanveldhuisenPCSCORE Study report #11: incidences of neovascular events in eyes with retinal vein occlusionOphthalmology201111871364137221440942
- HayrehSSRetinal vein occlusionIndian J Ophthalmol19944231091327829175
- HuangYZhangQThorellMRSwept-source OCT angiography of the retinal vasculature using intensity differentiation-based optical microangiography algorithmsOphthalmic Surg Lasers Imaging Retina201445538238925230403
- JiaYBaileySTWilsonDJQuantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degenerationOphthalmology201412171435144424679442
- JiaYTanOTokayerJSplit-spectrum amplitude-decorrelation angiography with optical coherence tomographyOpt Express20122044710472522418228
- MiuraMHongYJYasunoYMuramatsuDIwasakiTGotoHThree-dimensional vascular imaging of proliferative diabetic retinopathy by Doppler optical coherence tomographyAm J Ophthalmol20151593528538.e52325498353
- SpaideRFKlancnikJMJrCooneyMJRetinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiographyJAMA Ophthalmol20151331455025317632
- IshibazawaANagaokaTTakahashiAOptical coherence tomography angiography in diabetic retinopathy: a prospective pilot studyAm J Ophthalmol20151601354425896459
- SpaideRFOptical coherence tomography angiography signs of vascular abnormalization with antiangiogenic therapy for choroidal neovascularizationAm J Ophthalmol2015160161625887628
- ChoHAlwassiaAARegiatieriCVRetinal neovascularization secondary to proliferative diabetic retinopathy characterized by spectral domain optical coherence tomographyRetina201333354254723400083
- MuqitMMStangaPEFourier-domain optical coherence tomography evaluation of retinal and optic nerve head neovascularisation in proliferative diabetic retinopathyBr J Ophthalmol2014981657224158844
