Abstract
Globally, adverse drug reactions (ADRs), one of the leading causes of morbidity and mortality, will continue to pose a threat to public health as long as drugs are being used to treat various ailments. Prompt ADR reporting is crucial in ensuring drug safety. The aim of this narrative review was to highlight the role of pharmacists in pharmacovigilance and to identify barriers and facilitators toward ADR reporting documented in the literature. The perspective of pharmacy students on pharmacovigilance and ADR reporting has also been discussed with an aim to highlight the need to improve content related to ADR reporting and pharmacovigilance in undergraduate pharmacy curriculum. Globally, although the role of pharmacists within national pharmacovigilance systems varies, it is very well recognized. In general, pharmacists acknowledge that ADR reporting is part of their professional responsibility and have a positive attitude toward reporting ADRs. However, current research evidence suggests that there are still critical knowledge gaps with regard to ADR reporting among pharmacists, especially in countries where the role of pharmacists within the health care system is limited. These knowledge gaps can be fulfilled through continuous professional development programs and reinforcing theoretical and practical knowledge in undergraduate pharmacy curriculums. Without adequately identifying and fulfilling training needs of pharmacists and other health care professionals, the efficiency of national pharmacovigilance systems is unlikely to improve which may compromise patient’s safety.
Introduction
The World Health Organization (WHO) has defined adverse drug reactions (ADRs) as “a response to a drug which is noxious and unintended, and which occurs at doses normally used in man for the prophylaxis, diagnosis or therapy of disease, or for modifications of physiological function”.Citation1 Globally, ADRs, one of the leading causes of morbidity and mortality, will continue to pose threat to public health as long as drugs are being used to treat various ailments. A recent review estimated that ADRs were responsible for 3.5% of the hospital admissions.Citation2 Furthermore, ~1 in 10 hospitalized patients experienced an ADR in Europe.Citation2 ADRs are the fourth to sixth leading cause of death in the USA,Citation3 and it has been estimated that ADRs caused ~197,000 deaths annually in Europe.Citation4 In terms of burden on health care system, a prospective UK-based study estimated at any one time the equivalent of up to seven 800-bed hospitals may be occupied by patients admitted with ADRs.Citation5 The economic costs associated with ADRs are significant; in the USA, the cost per ADR in intensive care unit (ICU) and non-ICU wards has been estimated at USD 19,685 and USD 13,994, respectively.Citation6
Pharmacovigilance (PV) is defined by WHO as “the science and activities related to the detection, assessment, understanding and prevention of adverse drug effects or any other possible drug-related problems.”Citation7 The scope of PV to improve patients’ safety includes detection and reporting of ADR events, medication errors, counterfeit and substandard medicines, lack of efficacy of medicines, misuse and/or abuse of medicines, and drug–drug interactions. However, ADRs remain the prime focus of PV activities. Accordingly, this review focused primarily on pharmacists’ perspective pertaining to spontaneous ADR reporting.
Spontaneous reporting system (SRS) is the most widely used system globally to report adverse reactions by health care professionals, drug companies, or patients themselves to the national authorities regulating PV activities in the country.Citation8 SRS could improve the safety profile of a particular drug by detecting and reporting ADRs that may not have been detected during premarketing clinical trials or even during postmarketing surveillance.Citation9,Citation10 Therefore, it could serve as a method of detection for new, rare, or serious ADR events. One of the main advantages of SRS is that it applies to all drugs during its lifetime and not limited to a period of study.Citation11
SRS of ADR also has its limitations. Reports of low quality, known reactions, and inability to establish causal relationship are frequently associated with SRS.Citation12 In addition, SRS limits the potential of calculating the rates due to incomplete numerator data and undependable denominator.Citation13 Moreover, SRS has been associated with underreporting which could affect new drugs and serious reactions.Citation10 Despite its limitations, SRS is considered as the most cost-effective method in monitoring drug safety.Citation10
Given the voluntary nature of SRS, health care professionals, including doctors, dentists, nurses, and pharmacists, have an important role in ensuring that ADRs are well documented and reported. Health care professionals’ knowledge about and access to local ADR reporting systems, clinical skills in detecting an ADR, and attitude toward reporting ADRs are the key determinants of ADR reporting. Pharmacists being the drug experts have the central role in ensuring drug safety by detecting and reporting of ADRs. The aim of this narrative review was to highlight the role of pharmacists in SRS and to identify barriers and facilitators toward ADR reporting documented in the literature. The perspective of pharmacy students on PV and ADR reporting has also been discussed with an aim to highlight the need to improve content related to ADR reporting and PV in undergraduate pharmacy curriculum.
Role of pharmacists in ADR reporting
The role of pharmacists in ADR reporting has evolved over the past decade but still vary geographically.Citation14–Citation16 Over a decade ago, in Scandinavian countries, pharmacists were not allowed to report ADRs independently.Citation17 In the UK, pharmacists were only allowed to independently report ADRs after 10-year long debate and struggle.Citation18 Contrastingly, in Malaysia, in 2010, pharmacists contributed more than half of the total ADR reports received by the Malaysian National Pharmacovigilance Center.Citation15 An international survey of 41 member states participating in the WHO Drug Monitoring program published in 2004 also found considerable variations in the role of pharmacists in reporting ADRs.Citation14 The variation in the role of pharmacists in PV activities can be explained by the variations in pharmacists’ role within health care system across the globe from mere “dispenser” to the guardian of drug safety and patient outcomes. As the role of pharmacists within the health care systems continues to evolve, their role in ADR reporting is getting recognized. Research evidence shows that recruitment of pharmacists in public hospitals can not only detect and report ADRs but also prevent ADRs and reduce associated humanistic and financial costs.Citation18,Citation19 Furthermore, hospital pharmacists are more likely to report ADRs compared with community pharmacists.Citation14 This could be explained by the fact that pharmacists with a clinical background have greater awareness about ADR reporting system and are frequently engaged with prescribers.Citation20 Furthermore, regular contact with the patients coupled with the access to patients’ medical records allows clinical pharmacists at the hospitals to develop a better understanding of the suspected ADRs.Citation21 Nevertheless, being the most accessible health care professional, community pharmacists have a crucial role in ensuring drug safety as well by detecting and reporting ADRs, especially in areas where access to general practitioners/primary care physicians is limited.
Pharmacists’ perspective on ADR reporting
Given that both community and hospital pharmacists can play an important role in ADR reporting, a number of studies have been conducted globally to evaluate knowledge, attitudes, and practices of pharmacists toward ADR reporting with an aim to identify knowledge, attitudes, practices, and barriers to ADR reporting, so that appropriate interventions can be designed and implemented to overcome these barriers.Citation21–Citation36 A summary of studies evaluating pharmacists’ knowledge and practices toward ADR reporting published between 2011 and 2016 are presented in . In general, pharmacists expressed willingness to report ADRs and considered ADR reporting as part of their professional responsibility. However, a number of barriers to ADR reporting experienced by pharmacists have also been identified in the literature.Citation21–Citation38 Broadly, these barriers can be classified as health system-related barriers and individual pharmacist-related barriers (). Although a number of these barriers are interrelated, this broad classification is made just for ease of comprehension and to identify potential target areas for interventions aimed at improving ADR reporting rates.
Figure 1 Barriers toward ADR reporting experienced by pharmacists.
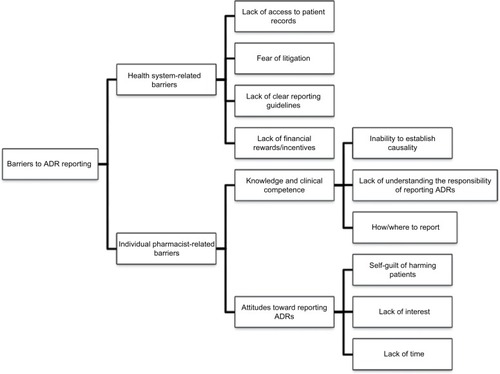
Table 1 Summary of studies evaluating knowledge, attitude, and practices of pharmacists toward ADR reporting
The most commonly reported barrier is the lack of knowledge about local reporting guidelines and policies and establishing ADR causation with suspected drug. Another well-documented misconception among pharmacists is to report only serious and/or new ADRs. In the UK, a study evaluating the knowledge and attitudes of hospital pharmacists toward ADR reporting reported that almost half of the participants were unclear about what type of ADRs should be reported.Citation37 Similarly, another study conducted in People’s Republic of China aimed to identify reasons for underreporting of ADRs by hospital pharmacists and concluded that the inability of hospital pharmacists to establish the seriousness of an ADR was one of the barriers to ADR reporting.Citation29 The uncertainty of pharmacists toward ADR reporting could have been influenced by their lack of awareness about ADR reporting. Furthermore, pharmacists may be reluctant to report minor reactions and would only report an ADR once they have established the association of the ADR with the suspected drug.Citation38 Concern of submitting inappropriate reports is also a commonly cited barrier to ADR reporting.Citation38
From the discussion above, it is evident that pharmacists’ knowledge and attitudes have influence on ADR reporting. Subsequently, in almost all the cross-sectional studies conducted across the globe, pharmacists themselves have highlighted the need for more training both in detecting and reporting ADRs. Therefore, many of the studies concluded that continuous training, providing feedback to reporters and providing incentives (either financially or in term of continuous pharmacy education points) could be the effective methods in persuading more active involvement from community pharmacists in PV activities.
Strategies to improve ADR reporting
As a broad principle, strategies for improving ADR reporting should be targeted both at the health care system level and individual pharmacist level. In addition to encouraging pharmacists to report ADRs, their knowledge and skill deficits in detecting and reporting ADRs should also be fulfilled through continuous professional development programs. Evidence suggests that provision of continuous education to health professionals is instrumental in changing their behaviors and attitudes toward ADR reporting.Citation39,Citation40 The aim of such education should not only be limited to improve the pharmacists’ knowledge about ADRs but also be directed to change their attitudes and perceptions toward ADR reporting. Studies have also reported that increased training is associated with an increased likelihood to ADR reporting.Citation37,Citation41 Nonmonetary incentives (e.g., certificate of recognition) for pharmacists who report ADRs is another method of improving spontaneous reporting of ADRs.Citation36,Citation40,Citation41
On health-system level, to engage community pharmacists more in ADR reporting, providing community pharmacists with access to patient’s medical and medication history will enable pharmacists to establish ADR causation and report ADRs as inability to establish causation deter pharmacists from reporting ADRs. Research evidence also suggests that electronic ADR tools can also improve spontaneous reporting of ADRs.Citation42,Citation43 Introduction and integration of electronic ADR reporting system with hospital information system at a 400-bed tertiary hospital in Spain led to an increase in the ADR reports submitted to the PV center.Citation43 The integrated ADR tool also allowed easier analysis of submitted ADR reports and automatic notification of suspected allergies to the allergy department.Citation43 The effectiveness of electronic reporting in improving the spontaneous reporting of ADRs has been reported at a children hospital in the UK as well.Citation42 This study was conducted to assess the impact of electronic reporting and monitoring of ADRs in children and to determine whether it could supplement the conventional Medicines and Healthcare Products Regulatory Agency Yellow card reporting system (standard ADR reporting form used in the UK). During the study period, 87 ADR reports were submitted through the electronic system compared with only 8 ADR reports, which were submitted to the Yellow card scheme suggesting the superiority of electronic reporting system compared with traditional paper-based reporting system.Citation42
Perspective of undergraduate pharmacy students
It is imperative to ensure that pharmacy students, the future pharmacists, are well trained and have sufficient knowledge pertaining to procedures and importance of ADR reporting.Citation44 They should be able to recognize, prevent, and repot an ADR. There are nine studies exploring the pharmacy students’ perspectives in PV and ADR reporting. Therefore, a number of studies have evaluated perspective of pharmacy students toward PV and ADR reporting.Citation44–Citation51 All were cross-sectional studies, using a structured validated questionnaire, involved mainly the Bachelor of Pharmacy (BPharm) students.Citation44–Citation51 Only one studyCitation44 was conducted in the Western country (i.e., the USA), whereas the rest were carried out in Asian region.Citation44–Citation51
The majority of the published studies investigated knowledge, attitudes, and perception of the pharmacy students on ADR reporting,Citation44–Citation51 except one that also looked into the practice aspect of PV and ADR reporting.Citation47 In general, most studies reported that the pharmacy students had insufficient knowledge in PV or ADR reporting.Citation34,Citation44,Citation45,Citation47,Citation48 Approximately only 24.4%–62.4% of the pharmacy students were able to define PV and ADR correctlyCitation44,Citation48,Citation49,Citation51 and less than half of them knew the exact mechanism of ADR reporting (e.g., where to obtain ADR reporting form, when to report, and whom to report).Citation45,Citation47,Citation48 In certain studies, up to 88% of the surveyed students did not know how causality assessment of an ADR is being conducted,Citation49 and 70%–80% of them did not know about the PV program in the nation,Citation48,Citation51 reflecting their poor knowledge in this area.
Of note, Doctor of Pharmacy (PharmD) students were significantly more knowledgeable in PV and ADR reporting when compared with BPharm students.Citation46,Citation47 Likewise, in the study by Saygi et al,Citation34 higher knowledge score in PV was reported among the fifth year BPharm students than those in the first year of the pharmacy study. The level of knowledge regarding PV among the pharmacy students was found to be significantly associated with their previous exposure to the relevant topics, such as PV, ADR reporting, and pharmacoepidemiology.Citation45,Citation46 Also, it was noted that pharmacy students who had any previous experience or exposure to ADR would have significantly better knowledge in PV and ADR reporting.Citation45,Citation46
Pharmacy students generally expressed a positive attitude toward PV and ADR reporting.Citation44,Citation46–Citation48,Citation50 The majority of the surveyed pharmacy students agreed that PV is crucial, and all health care professionals including pharmacists should actively report ADR in their daily practices.Citation46–Citation48,Citation50 Most believed that by reporting ADR can educate others about the risk of the drugs, improve patient safety, and it is personally rewarding.Citation44 No significant difference in attitude was observed between the PharmD and BPharm students,Citation46 except more PharmD students considered ADR reporting as their major responsibilities being a pharmacist.
The perception and barriers toward PV and ADR reporting among the pharmacy students have been explored; most BPharm students have indicated that limited training and exposure to PV and ADR reporting were the major reasons for their poor knowledge and lack of preparedness in handling ADR reporting cases.Citation44–Citation48,Citation50,Citation51 About 30%–50% of the BPharm students reported that they had taken a PV course or felt that the PV-related topics had been well covered in the curriculum.Citation44,Citation45,Citation49 However, PharmD students claimed that they were adequately trained to handle ADR reporting.Citation46 Accordingly, more emphasis in PV topics should be given in the BPharm courses to sufficiently prepare them for ADR monitoring and reporting when working as pharmacists in the future. In addition, the majority of the pharmacy students (i.e., 81.9%–86%) perceived that the paucity of information provided by the patients was one of the major barriers toward ADR reporting.Citation46 Indeed, this insufficient information had led to the difficulty in causality assessment of an ADR. The lack of encouragement and incentives by the relevant authorities in ADR reporting was another main reason of underreporting among the pharmacy students.Citation44,Citation46 The pharmacy students agreed that the use of health information technologies (e.g., online reporting system), the provision of legal protection by the relevant authorities, and the mandatory in ADR reporting would ensure a proper and effective PV process.Citation46
Conclusion
Pharmacists, being drug experts and guardian of safe and effective use of medicines, have an important role in not only detecting, reporting, and monitoring of ADRs but also preventing ADRs. Knowledge gaps with regard to ADR reporting still exist among pharmacists, especially in countries where the role of pharmacists is still in transition from being product oriented to patient oriented. These knowledge gaps can be fulfilled through continuous professional development programs and reinforcing theoretical and practical knowledge in undergraduate pharmacy curriculum. Engaging community pharmacists in ADR reporting by giving them access to patient’s medical record and introducing electronic reporting system can also improve ADR reporting rate. Without adequately identifying and fulfilling training needs of pharmacists and other health care professionals, the efficiency of national PV systems are unlikely to improve, which may compromise patient safety.
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health OrganizationInternational drug monitoring: the role of national centres. Report of a WHO MeetingGeneva1972
- BouvyJCDe BruinMLKoopmanschapMAEpidemiology of adverse drug reactions in Europe: a review of recent observational studiesDrug Saf201538543745325822400
- LazarouJPomeranzBHCoreyPNIncidence of adverse drug reactions in hospitalized patientsJAMA199827915120012059555760
- European CommissionProposal for a Regulation Amending, as Regards Pharmacovigilance of Medicinal Products for Human Use. Regulation (EC) No 726/2004. Impact Assessment2008 Available from: http://ec.europa.eu/health/files/pharmacos/pharmpack_12_2008/pharmacovigilance-ia-vol1_en.pdfAccessed September 3, 2014
- PirmohamedMJamesSMeakinSAdverse drug reactions as cause of admission to hospital: prospective analysis of 18,820 patientsBMJ20043297456151915231615
- CullenDJSweitzerBJBatesDWBurdickEEdmondsonALeapeLLPreventable adverse drug events in hospitalized patients: a comparative study of intensive care and general care unitsCrit Care Med1997258128912979267940
- The World Health OrganizationPharmacovigilance Available from http://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/Accessed February 15, 2017
- PalSNDuncombeCFalzonDOlssonSWHO strategy for collecting safety data in public health programmes: complementing spontaneous reporting systemsDrug Saf2013362758123329541
- RossiACKnappDEAnelloCDiscovery of adverse drug reactions. A comparison of selected phase IV studies with spontaneous reporting methodsJAMA198324916222622286834622
- HazellLShakirSAUnder-reporting of adverse drug reactions: a systematic reviewDrug Saf200629538539616689555
- MannRElizabethAPharmacovigilance2nd edWest Sussex, EnglandJohn Wiley & Sons Ltd2007
- FontanarosaPBRennieDDeAngelisCDPostmarketing surveillance – lack of vigilance, lack of trustJAMA2004292212647265015572723
- LexchinJIs there still a role for spontaneous reporting of adverse drug reactions?CMAJ2006174219119216415466
- van GrootheestKOlssonSCouperMde Jong-van den BergLPharmacists’ role in reporting adverse drug reactions in an international perspectivePharmacoepidemol Drug Saf2004137457464
- HadiMAMingLCImpact of pharmacist recruitment on ADR reporting: Malaysian experienceSouth Med Rev20114210210323093890
- OsemeneKPAyeniMIAfolabiMOThe role of community pharmacists in monitoring adverse drug reactions in NigeriaJ Pharm Health Serv Res201234197204
- OlssonSNational Pharmacovigilance Systems-Country profiles and Overview2nd edUppsalaUppsala Monitoring Centre1999
- RobertsPIWolfsonDJBoothTGThe role of pharmacists in adverse drug reaction reportingDrug Saf1994117117917082
- GlassmanPClinical pharmacist’s role in preventing adverse drug events: brief update reviewMaking Health Care Safer II: An Updated Critical Analysis of the Evidence for Patient Safety PracticesRockville, MDagency for Healthcare Research and Quality(US)20133Evidence Reports/Technology Assessments, No. 211 Chapter 4. Available from: https://www.ncbi.nlm.nih.gov/books/NBK133380/
- CalvertRTClinical pharmacy—a hospital perspectiveBr J Clin Pharmacol199947323123810215745
- SchliengerRGLüscherTFHaefeliWESchoenenbergerRAAcademic detailing improves identification and reporting of adverse drug eventsPharm World Sci1999213110510427579
- Abdel-LatifMMMAbdel-WahabBAKnowledge and awareness of adverse drug reactions and pharmacovigilance practices among health-care professionals in Al-Madinah Al-Munawwarah, Kingdom of Saudi ArabiaSaudi Pharm J201523215416125972735
- Al-hazmiNNNaylorILA study of community pharmacists’ awareness and contributions to adverse drug reactions (ADRs) reporting systems in the Makkah, Kingdom of Saudi ArabiaJ Clin Trials20133127
- DuarteMFerreiraPSoaresMCavacoAMartinsAPCommunity pharmacists’ attitudes towards adverse drug reaction reporting and their knowledge of the new pharmacovigilance legislation in the southern region of Portugal: a mixed methods studyDrugs Ther Perspect2015319316322
- ElkalmiRMHassaliMAIbrahimMIJamshedSQAl-LelaOQCommunity pharmacists’ attitudes, perceptions, and barriers toward adverse drug reaction reporting in Malaysia: an quantitative insightJ Patient Saf2014102818724618640
- HadiMAHelwaniRLongCMFacilitators and barriers towards adverse drug reaction reporting: perspective of Malaysian hospital pharmacistsJ Pharm Health Serv Res20134155158
- JoseJJimmyBAl-GhailaniASHAl-MajaliMAA cross sectional pilot study on assessing the knowledge, attitude and behavior of community pharmacists to adverse drug reaction related aspects in the Sultanate of OmanSaudi Pharm J201422216316924648829
- KhanTMCommunity pharmacists’ knowledge and perceptions about adverse drug reactions and barriers towards their reporting in Eastern region, Alahsa, Saudi ArabiaTher Adv Drug Saf201342455125083250
- LiuJZhouZYangSFactors that affect adverse drug reaction reporting among hospital pharmacists in Western ChinaInt J Clin Pharm201537345746425832677
- MahmoudMAAlswaidaYAlshammariTCommunity pharmacists’ knowledge, behaviors and experiences about adverse drug reaction reporting in Saudi ArabiaSaudi Pharm J201422541141825473329
- ObaraTYamaguchiTSatohMPrevalence, determinants and reasons for the non-reporting of adverse drug reactions by pharmacists in the Miyagi and Hokkaido regions of JapanAdv Pharmacoepidemiol Drug Saf20154191
- RabbaAKMohammadRPharmacovigilance Study: Exploring the Role of Community Pharmacists in Adverse Drug Reactions Reporting in Alkharj City, Saudi ArabiaLat Am J Pharm2015345901906
- QassimSMetwalyZShamsainMAl-HaririReporting adverse drug reactions: evaluation of knowledge, attitude and practice among community pharmacists in UAEIOSR J Pharm201441723
- SaygiSAlkasFBEtikanIGelisenISardasSPharmacovigilance awareness among the community pharmacists and pharmacy students in the Turkish Republic of Northern CyprusJ Pharmacovigil20164204
- SuyaghMFarahDAbu-FarhaRPharmacist’s knowledge, practice and attitudes toward pharmacovigilance and adverse drug reactions reporting processSaudi Pharm J201523214715325972734
- YuYMLeeEKooBSPredictive factors of spontaneous reporting of adverse drug reactions among community pharmacistsPLoS One2016115e015551727192159
- GreenCFMottramDRRowePHPirmohamedMAttitudes and knowledge of hospital pharmacists to adverse drug reaction reportingBr J Clin Pharmacol2001511818611167664
- GreenCFMottramDRRowePBrownAMAttitudes of hospital pharmacists to adverse drug reaction reporting: a qualitative surveyInt J Pharmacy Prac199974247255
- PagottoCVaralloFRMastroianniPCImpact of educational interventions on adverse drug events reportingInt J Technol Assess Health Care201329441041724290334
- Gonzalez-GonzalezCLopez-GonzalezEHerdeiroMTFigueirasAStrategies to improve adverse drug reaction reporting: a critical and systematic reviewDrug Saf201336531732823640659
- SweisDWongICA survey on factors that could affect adverse drug reaction reporting according to hospital pharmacists in Great BritainDrug Saf200023216517210945377
- LynnRMRidingKMcIntoshNThe use of electronic reporting to aid surveillance of ADRs in children: a proof of concept studyArch Dis Child201095426226520335236
- OrtegaAAguinagaldeALacasaCAquerretaIFernández-BenítezMFernándezLMEfficacy of an adverse drug reaction electronic reporting system integrated into a hospital information systemAnn Pharmacother200842101491149618780808
- GavazaPBuiBCandidatePPharmacy students’ attitudes toward reporting serious adverse drug eventsAm J Pharm Educ201276101622412200
- ElkalmiRMHassaliMAIbrahimMIWidodoRTEfanQMHadiMAPharmacy students’ knowledge and perceptions about pharmacovigilance in Malaysian public universitiesAm J Pharm Educ20117559621829270
- AhmadAKhanMUMoorthyJKumarBDKumarGSPatelIComparison of knowledge, attitudes and perceived barriers towards adverse drug reactions reporting between bachelor of pharmacy and doctor of pharmacy students in southern IndiaJ Pharma Health Serv Res201676369
- FarhaRAAlsousMElayehEHattabDA cross-sectional study on knowledge and perceptions of pharmacovigilance among pharmacy students of selected tertiary institutions in JordanTropical J Pharm Res20151418991905
- IsfahaniMEMousaviSRakhshanAAssarianMKutiLEslamiKAdverse drug reactions: knowledge, attitude and practice of pharmacy studentsJ Pharm Care20131145148
- RajiahKMaharajanMKNairSPharmacy students’ knowledge and perceptions about adverse drug reactions reporting and pharmacovigilanceSaudi Pharm J201624560060427752233
- ShakeelSIffatWAnjumFBushraRIbrahimSShafiqSEmerging need of pharmacovigilance: perspectives of future pharmacist in PakistanInt J Pharm Teach & Pract20145966971
- KothariNMirzaNAgrawalNChoudaryMPichholiyaMMatuleSMGoyalBKAn evaluation of knowledge and perception of pharmacy students toward pharmacovigilance and adverse drug event reportingAsian J Pharmacol20159262265
