Abstract
Purpose
Warfarin plays an important role in anticoagulation therapy despite the availability of the newest oral anticoagulants, and achieving optimal anticoagulation is challenging due to its narrow therapeutic range and variable dose. This study aimed to highlight polypharmacy and drug interactions in patients receiving warfarin therapy at Medani Heart Centre, Sudan.
Methods
This retrospective hospital-based study was conducted from May 2017 to October 2018. Each concurrent medication prescribed for 104 patients was collected and checked for drug-drug interactions using Medscape Reference-Drug Interaction Checker. The data were analysed by using SPSS 20, and descriptive statistics were used.
Results
The results revealed that 95.2% of patients had more than three medications in their profile, (3–5), (6–9) and more than 10 medications were prescribed for 40.4%, 44.2% and 10.6% of patients, respectively. A total of 93.3% of patients had drug-drug interactions, as follows: (1–5), (6–10), (11–15), (16–20) and more than 20 drug-drug interactions were found in 31.7%, 32.7%, 19.2%, 5.8% and 3.8% of patients, respectively. A total of 178 warfarin-drug interactions were identified in 88.5% of the patients. The INR ranged between 2 and 2.99 in 13.4% of patients, and INR values below 2 and above 5 were found in 44.2% and 21.2% of patients, respectively. Analgesics (n=54; 30.3%), cardiovascular drugs (n=51; 28.6%), and anticoagulants (n=46; 25.8%) were the most common drug classes that interact with warfarin. Significant and serious types of interactions with warfarin were found in 51% and 37.5% of patients, respectively.
Conclusion
This study highlights the complexity of managing warfarin therapy amid prevalent polypharmacy. A substantial majority of patients experienced multiple drug interactions. The identification of significant and serious interactions emphasizes the need for vigilant management strategies, including improved communication among healthcare professionals and targeted education for both providers and patients, to enhance the safety and efficacy of warfarin therapy.
Introduction
Since its introduction in the 1950s, warfarin has become a commonly used oral anticoagulant for the prevention of thromboembolism in patients with deep vein thrombosis (DVT), atrial fibrillation (AF) or prosthetic heart valve replacement.Citation1,Citation2 While warfarin boasts high efficacy, achieving the desired level of anticoagulation is challenging due to its narrow therapeutic window and considerable variability in dose response among individuals.Citation1
Although the cost-effectiveness and long-term safety of the latest generation of oral anticoagulants (dabigatran, rivaroxaban, apixaban and edoxaban) have recently become a subject of intense debate, warfarin has remained the cornerstone of anticoagulation therapy for several decades.Citation3 Direct oral anticoagulants (DOACs) are at least as effective as warfarin with less bleeding and are more convenient for administration in the management of venous thromboembolism (VTE).Citation4 Warfarin management is sophisticated; in addition to its classification as a drug with a low therapeutic index, it does not follow the dose‒response mode and has many features that make it a potential candidate for drug interactions through different pharmacokinetic approaches, such as metabolism by cytochrome P450 enzymes and high affinity for plasma protein binding.Citation5 Additionally, the wide interindividual variability and narrow therapeutic index of warfarin highlight considerable interest in identifying genetic and non-genetic variables that affect warfarin dose requirements.Citation6 In many patients receiving warfarin therapy, concomitant drugs that interact with warfarin may affect the INR values and result in thrombus formation or bleeding as adverse outcomes of combining drugs with warfarin.Citation7 Despite its proven efficacy, widespread underutilization of warfarin persists, even with refinements and standardization in its monitoring.Citation7
In the Medani Heart Centre (MHC), warfarin is extensively prescribed for mechanical valve replacement (MVR), atrial fibrillation (AF), myocardial infarction (MI), prevention and treatment of deep vein thrombosis (DVT), stroke and pulmonary embolism (PE). Many medications are prescribed concomitantly with warfarin for different indications, such as hypertension, pulmonary hypertension, diabetes mellitus and other comorbidities. This makes warfarin a possible candidate for several adverse reactions due to its narrow therapeutic index, patient compliance, and high individual variability.Citation8
Commonly interacting drugs with warfarin include quinolones and nonsteroidal anti-inflammatory drugs, including selective cyclooxygenase-2 inhibitors, selective serotonin reuptake inhibitors, lipid-lowering agents, amiodarone, azole antibiotics, macrolides, omeprazole, and fluorouracil. Therefore, coadministration of these drugs with warfarin should be approached with caution.Citation9 This study sheds light on polypharmacy and drug-drug interactions (DDIs) in patients receiving warfarin therapy at the Medani Heart Centre, Sudan.
Materials and Methods
Study Area
The study was conducted at the Medani Heart Centre (MHC), Wad Medani City, Gezira State, Sudan. This centre is situated in a key region that links Sudan’s eastern, southern, and central regions. It has been providing patient care since December 2010 and can accommodate up to 190 beds. In the MHC, approximately eight cardiac catheter procedures are performed daily, and three to five open heart surgeries are performed weekly. There are five cardiologists, fifty general practitioners, five dietitians, ninety-five nurses, and three pharmacists on staff. Every day, the MHC saw between sixty and seventy-five patients. Every week, there are two cardiac medicine referral clinics that refer between 80 and 100 patients, one cardiac surgery referral clinic that sees approximately 10 patients, and two INR referral clinics that see 90 patients each week.
Study Population
Sample Size and Technique
The total number of patients at their initial visit or routine follow-up appointments at INR referral clinics during the study period was approximately 6480. We conducted an exploratory study of 15 randomly selected patients’ medical records and demonstrated that 93.3% of patients had at least one warfarin-drug interaction in their medication profile. Accordingly, the sample size required for the study was determined using Epi-Info; considering an expected frequency of 93.3%, the prevalence of warfarin-drug interactions, and a 5% margin of error at the 95% confidence level, finally adjusting for finite population correction, a sample of 104 patients’ medical records was taken with a systematic random sampling technique.
Inclusion Criteria
Patients who were taking warfarin as an anticoagulant during their initial visit or routine follow-up appointments at the MHC between May 2017 and October 2018 and patients’ medical records with complete data were included.
Exclusion Criteria
Patients who received warfarin alone without concomitant drugs and patients whose medical records were incomplete.
Study Design
This was a retrospective hospital-based study in which all concurrent medications prescribed for each patient receiving warfarin therapy were collected and checked for drug-drug interactions (DDIs) using Medscape Reference-Drug Interaction Checker.Citation10
Medscape Reference Drug Interaction
From the medication profiles, all medications in the patient profile were entered one by one in Medscape Reference Drug Interaction Checker Software to screen drug interactions.Citation10
Data Collection Form
A pre-evaluation data collection form was constructed to obtain the primary data from medical records (Appendex.1), aiming to extract final variables that would be included in the data collection form. Patient demographics, warfarin use, comorbidities, warfarin dose, INR values for the last three months, number of interactions per patient, number of warfarin-drug interactions per patient, number of drugs per patient and type of drug-drug interactions (DDIs) were recorded (Appendex.2).
Statistical Analysis
Statistical analysis was performed using the Statistical Package for Social Science (SPSS 20). Descriptive statistics (frequencies and percentages) were used. The results are presented in tables and graphs.
Results
Throughout the study period, 104 patients were enrolled, with two-thirds (n=68; 65.4%) being females. The majority of patients (n=77; 74%) lived in rural areas. Among the age groups, the highest representation was observed in the 50–69 years age group (n=39; 37.5%), followed by the 30–49 years age group (n=29; 27.9%). Two age categories, (10–29) years and (≥ 70) years, accounted for (n=19; 18.3%) and (n=17; 16.3%) of patients, respectively. Warfarin was mainly indicated for 61 (58.6%) patients who underwent mechanical valve replacement (MVR), followed by 34 (32.7%) patients with atrial fibrillation (AF). Among the patients, 56 (53.9%) had no coexisting morbidity while on warfarin, and hypertension was the most common comorbidity in 19 (18.3%) patients, followed by 12 (11.5%) patients with both hypertension and diabetes (). Concerning the number of medications administered at the time of screening for drug interactions, 46 (44.2%) patients received 6–9 medications, 42 (40.4%) patients received 3–5 medications, and 11 (10.6%) patients received 10 or more medications. Only 5 (4.8%) patients were prescribed fewer than 3 medications (). Of the 312 patients whose INRs were assessed over the last three months (total of 3 follow-ups), 42 (13.4%) had INR values between 2.0–2.99, 138 (44.2%) had an INR < 2, and 66 (21.2%) had an INR > 5.0 ().
Table 1 Clinical Characteristics of Patients Including Indication for Warfarin, Comorbidities, and INR Values for Last Three Months
Table 2 Dose of Warfarin, Number of Drugs Administered per Patients, Drug-Drug Interactions per Patient and Warfarin-Drug Interactions per Patient
The most frequently prescribed warfarin dose was 5 mg, given to 57 (54.8%) patients, while 24 (23.1%) patients received 3 mg of warfarin. Using the Medscape Reference drug interaction checker, the overall prevalence of drug-drug interactions (DDIs) was 97 (93.3%). Specifically, 34 (32.7%) patients had 6–10 drug-drug interactions, 33 (31.7%) had 1–5 drug-drug interactions, 20 (19.3%) had 11–15 drug-drug interactions, 6 (5.8%) had 16–20 drug-drug interactions, and 4 (3.8%) had more than 20 drug-drug interactions. Only 7 (6.7%) patients had no drug-drug interactions. According to the Medscape reference drug interaction checker, 178 drug-drug interactions with warfarin were identified. Of these, 92 (88.5%) patients had at least one warfarin-drug interaction in their medication profile, with the majority having one warfarin-drug interaction 38 (36.5%), 31 (29.8%) having two, three, and four warfarin-drug interactions (15 (14.5%) and 7 (6.7%) of patients, respectively), and one patient (1%) had five warfarin-drug interactions. Only 12 (11.5%) of the studied patients had no warfarin-drug interactions ().
According to the patients’ medication profiles, the most common warfarin-drug interaction involved analgesics (n=54; 30.3%). Among the analgesics, aspirin had the most interactions with warfarin (n=36; 66.6%). Among cardiovascular drugs (n=51; 28.6%), spironolactone (n=46; 90.2%) was the most common drug that interacted with warfarin. Additionally, antibiotics (n=21; 11.8%) that interacted with warfarin, ceftriaxone (n=10; 47.6%) was the most common interactions. Followed by anticoagulants (n=46; 25.8%) and the proton pump inhibitor omeprazole (n=5; 2.8%), one patient received carbamazepine (n=1; 0.56%) ().
Table 3 Drug Classes That Interact with Warfarin and Level of Interaction
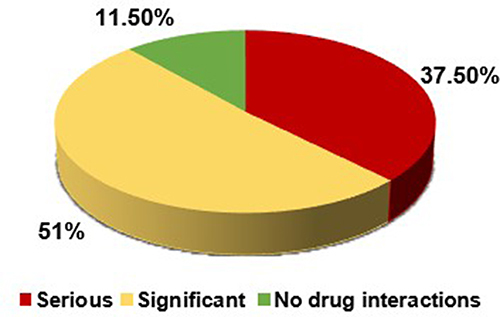
The most prevalent type of drug-drug interaction was significant, accounting for 72% of the studied patients, while 28% of the interactions were serious. According to Medscape, the most common type of interaction was the significant type, which was found in 53 (51%) patients, whereas serious drug-drug interactions occurred in 39 (37.5%) patients, with 12 (11.5%) patients having no warfarin drug-interactions ().
Discussion
To the best of our knowledge, this is the first study conducted in Sudan that focused on polypharmacy and drug interactions in patients treated with warfarin. The overall prevalence of drug-drug interactions (DDIs) was 93.3%, which was largely similar to that reported in a prospective observational cohort study conducted at Ayder Referral Hospital, Northern Ethiopia, which reported a prevalence of DDIs of 99.2% in 133 patients.Citation11 This higher prevalence of DDIs may be attributed to several factors, including patients having multiple comorbidities requiring more medication interventions, limited treatment options for which physicians had to manage a condition by the use of non-interacting medications, and potential prescribers’ knowledge, training and practice, as well as the absence of clinical pharmacists who have a significant role in identifying and managing drug interaction may also contribute to this scenario. Patients who received prescriptions from multiple prescribers or those who received their first prescription for warfarin from any other specialist were at greater risk of haemorrhage, possibly from DDIs.Citation12
Using the Medscape Reference drug interaction checker, it was found that 97 (93.3%) patients had at least one drug-drug interaction in their medication profile, a figure quite similar to that of a study reporting a drug-drug interaction prevalence of 83.42% among patients.Citation13 Notably, a surprising aspect was the presence of patients (3.8% and 5.8%) with more than 20 and between 16 and 20 drug-drug interactions, respectively. Meanwhile, 32.7% of patients had 6–10 drug-drug interactions, and 31.7% had 1–5 drug-drug interactions. Teklay et al reported an association between polypharmacy and an increased risk of major bleeding in patients receiving warfarin.Citation11 This high prevalence of drug-drug interactions (DDIs) in patients’ medication profiles could lead to an elevated risk of adverse effects, including toxicity and reduced medication efficacy.
According to the Medscape reference drug interaction checker, 178 drug-drug interactions with warfarin were identified, with 92 (88.46%) patients having at least one warfarin-drug interaction in their medication profile, this high prevalence of warfarin- drug interactions in specialized heart centre is controversial. It could be due to the complex medical regimens required for patients with advanced cardiovascular conditions, leading to polypharmacy, increasing the likelihood of interactions. Similar finding demonstrated that (97%) of patients were prescribed at least one warfarin-interacting medication during the review period.Citation14 The majority of patients had one warfarin-drug interaction (38; 36.5%), while 31 (29.8%) patients had two warfarin-drug interactions. Surprisingly, one (1%) patient had five warfarin–drug interactions, and only 12 (11.5%) of the studied patients had no warfarin-drug interactions. Verhovsek et al revealed that, with warfarin drug interactions, patients spent less time in the therapeutic range (P = 0.002),Citation14 which was supported by 86.6% of patients enrolled in this study having an INR outside of the normal therapeutic range of 2.00–3.00. One of the potential contributing factors to this high incidence of DDIs might be the absence of a crucial role by clinical pharmacist at the MHC as recognized drug experts in medication management, identifying potential interactions, and ensuring appropriate dosing and monitoring. Additionally, multiple comorbidities (46.1%) required more medications and limited treatment options.
This study demonstrated that a high polypharmacy is associated with an increased risk of bleeding.Citation15 Of these patients, forty-six (44.2%) patients received (6–9) medications at the time of screening for drug interactions, 42 (40.4%) patients were administered (3–5) medications, and 10 or more medications were administered to 11 (10.6%) patients. Unfortunately, only five (4.8%) patients were administered fewer than 3 medications. On the same approach, Piccini et al revealed that 51% of patients were on (5–9) medications, 13% were on 10 or more medications, and 36% were on (0–4) medications.Citation15
The most frequent warfarin-drug interaction involved analgesics, mainly aspirin, which closely resembled the findings of Zhang et al (27.4%) for warfarin interactions with NSAIDs/COX-2.Citation12 This is common because aspirin is widely used at the MHC for the prevention and treatment of many cardiovascular diseases. Both aspirin and warfarin increased anticoagulation, which resulted in significant drug-drug interactions. This was followed by cardiovascular drugs (n=51; 28.6%), with spironolactone (n=46; 90.2%) being the most common interacting drug with warfarin, according to Medscape, spironolactone decreased the effect of warfarin through an unknown mechanism, which resulted in a significant type of interaction. Approximately one-third of cardiovascular drugs that interact with warfarin might be due to the nature of MHC as a specialized centre for cardiovascular disorders (CVDs). Additionally, antibiotics (n=21; 11.8%) interact with warfarin, among which ceftriaxone (n=10; 47.6%) is the most common interaction, according to MHC local guidelines, ceftriaxone is the drug of choice for postoperative heart surgery, which increases the risk of bleeding when it is administered concomitantly with warfarin therapy,Citation16 followed by anticoagulants (n=46; 25.8%), which increase the effects of warfarin through pharmacodynamic synergism according to the Medscape Drug Interaction Checker. Low molecular weight heparin (enoxaparin) and heparin also increase anticoagulation.
The targeted INR for warfarin treatment depends on the indication, with the most widely accepted range being 2.0–3.0.Citation17–19 The INR at the time of screening for warfarin drug interactions was considered a measurement of coagulation status. Studies have demonstrated that drug interactions with warfarin can cause fluctuations in the INR, which can result in increased bleeding risk or thromboembolic events. In this study, most patients did not achieve a normal INR, and only 14 (13.4%) had an INR between 2.0–2.99. In contrast, 46 (44.2%) patients had an INR < 2; unfortunately, 22 (21.2%) had an INR > 5.0. This finding was more frustrating than that of Teklay et al, who reported that 30.8% of patients achieved the target INR value of 2.0–3.0, while the remaining 12.8% and 56.4% of patients achieved INR values <2.0 and > 3.0, respectively; overall, 69.7% of patients did not achieve the target INR value.Citation11 A similar study of the mean INR found that 54% of patients were within the therapeutic range, while 35% and 11% were below and above the therapeutic range, respectively. You et al demonstrated 44% of patients had an INR <2.0 and 6% had an INR > 3.0, whereas 50% had an INR within the 2–3 range.Citation20 The high prevalence of patients with sub/supratherapeutic INR values might be to large extent attributed to the high prevalence of poly pharmacy and the resultant DDIs.
Most types of drug-drug interactions were significant, accounting for 72% of the studied patients, while 28% of the interactions were serious. According to Medscape, warfarin is a drug with a narrow therapeutic index, which suggests that warfarin has no minor drug-drug interactions. Similar findings using the Micromedex online drug reference to assess drug-drug interactions revealed that the most common type of interaction was moderate, accounting for 72.4%, while the remaining 27.6% were major types of interactions.Citation11
While this research provided valuable insights, it is essential to acknowledge certain limitations. First, the single-centre design may limit the generalizability of the findings to other settings or populations. Second, the retrospective nature of the study introduces potential biases and may lack the ability to establish causation. Third, a relatively small sample size could reduce the statistical power and precision. Fourth, the study relies on the Medscape Reference-Drug Interaction Checker for assessing drug interactions. The accuracy and comprehensiveness of this tool may have limitations, and different databases or methods might yield different results. Finally, incomplete patient records, limited demographic details, and a short observation period impact the data quality and depth of insight into clinical outcomes.
Conclusion
This study sheds light on the challenges posed by polypharmacy and drug interactions in patients receiving warfarin therapy. The findings underscore the prevalence of polypharmacy, with a substantial majority of patients receiving more than three concurrent medications. Notably, a significant proportion of patients exhibited multiple drug interactions, notably with analgesics, cardiovascular drugs, and anticoagulants. Emphasizing the complex nature of managing drug regimens in individuals receiving warfarin. The identification of significant and serious interactions emphasizes the need for vigilant management strategies, including improved communication among healthcare professionals and targeted education for both health care providers and patients, to enhance the safety and efficacy of warfarin therapy.
Ethical Approval
The Health Sector Ethical Review Committee at Medani Heart Centre (MHC), Gezira State, Sudan, granted ethical clearance and approval for this study, which was carried out per the Declaration of Helsinki’s ethical standards. The study’s reference number is 55-23-15/03/2017. The need for informed consent was waived by Medani Heart Centre (MHC) ethics review committee, due to the retrospective nature for this study and the privacy and confidentiality of all patients’ medical records data were properly secured.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
I would like to thank the Faculty of Pharmacy, University of Gezira and all members of the Medani Heart Centre, especially Khandrees in the laboratory and Ahmed Elzain in the Statistic Department. Their excitement and willingness to provide support made the completion of this research an enjoyable experience.
References
- Lee MTM, Klein TE. Pharmacogenetics of warfarin: challenges and opportunities. J Hum Genet. 2013;58(6):334–338. doi:10.1038/jhg.2013.40
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. doi:10.1016/j.chest.2015.11.026
- Wang -Z-Z, Du X, Wang W, et al. Long-term persistence of newly initiated warfarin therapy in Chinese patients with nonvalvular atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2016;9(4):380–387. doi:10.1161/CIRCOUTCOMES.115.002337
- Becattini C, Agnelli G. Treatment of venous thromboembolism with new anticoagulant agents. J Am Coll Cardiol. 2016;67(16):1941–1955. doi:10.1016/j.jacc.2016.01.072
- Leite PM, Martins MAP, Das Graças Carvalho M, Castilho RO. Mechanisms and interactions in concomitant use of herbs and warfarin therapy: an updated review. Biomed Pharmacother. 2021;143:112103. doi:10.1016/j.biopha.2021.112103
- Fung E. Effect of genetic variants, especially CYP2C9 and VKORC1, on the pharmacology of warfarin. In: Seminars in Thrombosis and Hemostasis. Thieme Medical Publishers; 2012:893–904.
- Wittkowsky AK, Boccuzzi SJ, Wogen J, Wygant G, Patel P, Hauch O. Frequency of concurrent use of warfarin with potentially interacting drugs. Pharmacother J Hum Pharmacol Drug Ther. 2004;24(12):1668–1674. doi:10.1592/phco.24.17.1668.52338
- Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–19. doi:10.1136/bmj.329.7456.15
- Holbrook AM. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165(10):1095–1106. doi:10.1001/archinte.165.10.1095
- Medscape, drug interaction checker; 2018. Available from: https://reference.medscape.com/drug-interactionchecker. Accessed: March 15, 2018.
- Teklay G, Shiferaw N, Legesse B, Bekele ML. Drug‒drug interactions and risk of bleeding among inpatients on warfarin therapy: a prospective observational study. Thromb J. 2014;12(1):1–8. doi:10.1186/1477-9560-12-20
- Zhang K, Young C, Berger J. Administrative claims analysis of the relationship between warfarin use and risk of hemorrhage including drug‒drug and drug-disease interactions. J Manag Care Pharm. 2006;12(8):640–648. doi:10.18553/jmcp.2006.12.8.640
- Patel PS, Rana DA, Suthar JV, Malhotra SD, Patel VJ. A study of potential adverse drug‒drug interactions among prescribed drugs in medicine outpatient department of a tertiary care teaching hospital. J Basic Clin Pharm. 2014;5(2):44. doi:10.4103/0976-0105.134983
- Verhovsek M, Motlagh B, Crowther MA, et al. Quality of anticoagulation and use of warfarin-interacting medications in long-term care: a chart review. BMC Geriatr. 2008;8(1):1–6. doi:10.1186/1471-2318-8-13
- Piccini JP, Hellkamp AS, Washam JB, et al. Polypharmacy and the efficacy and safety of rivaroxaban versus warfarin in the prevention of stroke in patients with nonvalvular atrial fibrillation. Circulation. 2016;133(4):352–360. doi:10.1161/CIRCULATIONAHA.115.018544
- Wang M, Zeraatkar D, Obeda M, et al. Drug–drug interactions with warfarin: a systematic review and meta-analysis. Br J Clin Pharmacol. 2021;87(11):4051–4100. doi:10.1111/bcp.14833
- Hylek EM, Chang Y, Skates SJ, Hughes RA, Singer DE. Prospective study of the outcomes of ambulatory patients with excessive warfarin anticoagulation. Arch Intern Med. 2000;160(11):1612–1617. doi:10.1001/archinte.160.11.1612
- Crowther MA. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med. 2003;349(12):1133–1138. doi:10.1056/NEJMoa035241
- Kuruvilla M, Gurk-Turner C. A review of warfarin dosing and monitoring. In: Baylor University Medical Center Proceedings. Taylor & Francis; 2001:305–306.
- You J, Chan F, Wong R, Cheng G. Is INR between 2.0 and 3.0 the optimal level for Chinese patients on warfarin therapy for moderate-intensity anticoagulation? Br J Clin Pharmacol. 2005;59(5):582–587. doi:10.1111/j.1365-2125.2005.02361.x