Abstract
Multiple sclerosis is a chronic inflammatory demyelinating disease of the central nervous system. Both genetic and environmental factors are believed to contribute to the pathogenesis of the disease. Histopathological findings suggest that multiple sclerosis is an immune-mediated disease, involving both the cellular and humoral immune systems. Within the last 20 years, several disease-modifying therapies for the treatment of multiple sclerosis were established. Moreover, promising new substances are currently being tested in clinical trials and will likely broaden the therapeutic opportunities available within the upcoming years.
Immunopathogenesis of multiple sclerosis
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS), affecting approximately 1.3 million worldwide.Citation1 The majority of MS patients will develop significant disability over time. Although the pathogenesis has been the focus of MS research for several decades, the mechanisms leading to evolution and progression of the disease are still uncertain. Several lines of evidence point to an autoimmune pathogenesis, with both genetic and environmental factors contributing to disease susceptibility.Citation2–Citation4 Large-scale genetic studies in MS have revealed more than 50 gene loci associated with MS, with the HLADRB1*1501 allele being the most important.Citation5 Interestingly, there is a great overlap with loci observed in other autoimmune diseases, such as diabetes and rheumatoid arthritis. Among the possible environmental factors, infection with Epstein Barr virus and low vitamin D levels seem to be the most important contributors to susceptibility.Citation3,Citation4,Citation6 Other factors, such as the gut microbiome, have been discussed as possible susceptibility factors based on findings in experimental animal models.Citation7,Citation8
A large body of evidence suggests that MS is an autoimmune disease.Citation9 CNS antigens seem to be the likely targets of the autoimmune response. It is conceivable that in genetically susceptible individuals, an infection or release of CNS proteins into the periphery may trigger loss of self-tolerance towards CNS proteins, probably by activation of myelin-reactive T cells.Citation10 Viral infections can probably cause bystander activation of T cells in an immunostimulatory context.Citation11 Moreover, release of autoantigens due to cellular damage by a viral agent can lead to activation of autoreactive T cells due to cross-reactivity between viral antigens and CNS antigens, a mechanism known as molecular mimicry.Citation10,Citation12,Citation13 After migration into the CNS, autoreactive T cells may become reactivated by antigen-presenting cells presenting CNS autoantigens on major histocompatibility complex molecules to the invading T cells ().
Figure 1 Immunopathogenesis of multiple sclerosis.
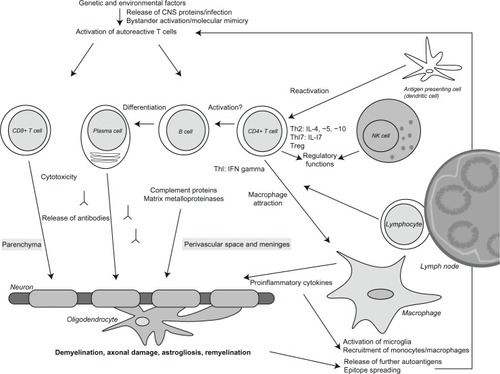
Histopathologically, MS lesions are characterized by inflammatory infiltrates consisting of activated T cells, B cells, plasma cells, and macrophages. Whereas CD4+ T cells are mainly found in the perivascular spaces and meninges, CD8+ T cells are located in the parenchyma of MS lesions.Citation14 In MS lesions, profound demyelination, axonal damage, astrogliosis, and remyelination is observed.Citation15–Citation19 Besides, deposits of complement proteins and immunoglobulins are seen. Several proinflammatory cytokines and matrix metalloproteinases are active in MS lesions.Citation13,Citation14,Citation16,Citation20
In the pathogenesis of MS, CD4+ T cells are believed to release cytokines and immune mediators, which lead to attraction of macrophages and further release of proinflammatory cytokines. CD4+ T cells require for their activation an interaction with major histocompatibility complex class II expressing cells, such as dendritic cells, macrophages, or B cells. Animal experiments suggest that T-helper (Th) 1 cells, which release interferon-gamma and Th17 cells, which secrete Th17, play a key role in inflammation within the CNS. In contrast, Th2 cells, characterized by secretion of interleukins 4, 5, and 10, and regulatory T cells expressing Foxp3, counter-regulate encephalitogenic Th1 and Th17 responses.Citation21 Moreover, some T cells may not only cause harm to CNS tissue, but also prime regeneration of MS lesions.Citation22
CD8+ T cells also seem to be involved in the pathogenesis. In contrast with CD4+ T cells, CD8+ T cells can directly interact with and damage major histocompatibility complex I/antigen-expressing cells, such as neurons and oligodendrocytes.Citation23
As a consequence of the release of proinflammatory cytokines and cellular damage, microglia are activated and monocytes and macrophages are recruited into the lesion. Further CNS antigens are released and presented to potentially autoreactive T cells. Epitope spreading may lead to a broadened autoimmune response involving further autoantigens.Citation24
Alongside T cells, B cells are believed to play an important role in the pathogenesis of MS. B cells are important antigen-presenting cells in the peripheral immune system and possibly also in the CNS. They can capture soluble proteins by their specific B cell receptor, process and present peptide antigens bound to major histocompatibility complex class II molecules to autoreactive T cells. Plasmablasts and plasma cells can release immunoglobulins which probably bind to autoantigens on glial cells.Citation25–Citation27 Possible mechanisms of antibody-mediated pathogenicity include complement activation or antibody-dependent cellular cytotoxicity.Citation26,Citation28 Indeed, the complement protein C9neo, which is part of the terminal lytic membrane attack complex, and immunoglobulin G deposits have been detected at the border of MS plaques.Citation20,Citation26 Moreover, the presence of intrathecal immunoglobulin G synthesis and detection of clonally expanded B cells in the cerebrospinal fluid and brain lesions of MS patients argue for a substantial role of B cells in MS.Citation29 The clinical relevance of these findings is supported by a clinical trial demonstrating that patients with lesions characterized by complement and immunoglobulin G deposition respond exceptionally well to therapeutic plasma exchange.Citation30 More recently, the role of cortical lesions has come more into the focus of MS research. In contrast with white matter lesions, cortical lesions seem to be mainly driven by meningeal inflammatory infiltrates and soluble factors from the cerebrospinal fluid (CSF).Citation31,Citation32 Therefore, histopathological findings suggest a role of both the cellular and humoral immune systems in the pathogenesis of MS.
Disease-modifying therapies in multiple sclerosis
During the past 20 years, several disease-modifying substances have been developed for treatment of MS. and summarize established therapies and drugs currently evaluated in clinical trials.
Figure 2 Established and emerging multiple sclerosis therapies.
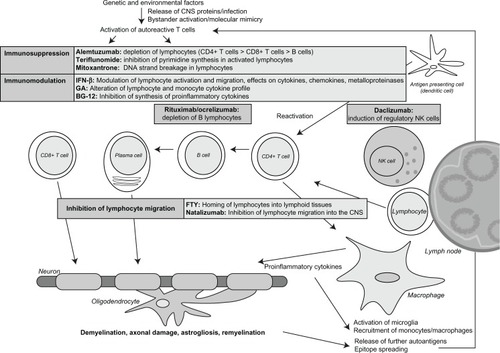
Table 1 Summary of application, assumed mode of action, and current status of drug development
Immunomodulatory and immunosuppressive therapies
This group of drugs aims to suppress or alter immune responses in the periphery. The mechanisms are rather nonselective and affect a broad range of immune cells.
Interferon beta
The first immunomodulatory drug approved for the treatment of relapsing-remitting forms of MS was interferon-beta 1b in 1993. The pivotal trial showed a significant reduction in relapse rate and the number of active lesions on magnetic resonance imaging.Citation33 Within the following years, several formulations of interferon-beta 1a and 1b came onto the market. In secondary progressive MS, interferon-beta 1b delayed disease progression in the European trial but failed in the US trial.Citation34,Citation35 Detailed analysis of the trials suggested that interferon-beta 1b is active in secondary progressive MS, as long as relapse activity is present.Citation36 Accordingly, the drug was approved for treatment of secondary progressive MS with ongoing relapse activity in Europe. Moreover, in patients with clinically isolated syndrome, conversion to clinically definite MS was significantly delayed by early treatment with interferon-beta.Citation37,Citation38 The mechanism by which interferon-beta decreases inflammatory disease activity in MS is still unknown. Different mechanisms are likely to be important, such as its effects on cytokines, chemokines, and metalloproteinases, and also the modulation of lymphocyte activation and migration and proliferation of regulatory T cells.Citation39,Citation40
Glatiramer acetate
Glatiramer acetate is an amino acid copolymer consisting of L-alanine, L-lysine, L-glutamic acid, and L-tyrosine, and was discovered in a model of experimental allergic encephalomyelitis in 1974.Citation41 It is believed to inhibit the T cell response towards myelin antigens.Citation42–Citation45 Pivotal studies showed a reduction of relapse rate in patients with relapsing-remitting MS by 29% within two yearsCitation46 and a significant decrease in the total number of enhancing lesions.Citation47 Glatiramer acetate was approved for treatment of clinically isolated syndrome in 2009, having been shown to delay conversion to clinically definite MS in the PreCISe Study.Citation48 The mechanism by which glatiramer acetate works in MS also remains unclear. It potentially includes alteration of the cytokine profile of T cells and monocytes, induction of regulatory T cells, and induction of neurotrophic factors in immune cells.Citation49–Citation51
Mitoxantrone
Mitoxantrone is an anthracenedione which induces DNA strand breakage by intercalation, and also inhibits the DNA repair enzyme, topoisomerase II. In a placebo-controlled Phase III study (MIMS), mitoxantrone reduced disability progression and relapse rates (reduction of relapse rate by 63% during the first year and 68% during the second year of treatment compared with placebo) in highly active relapsing-remitting and secondary progressive MS.Citation52 Mitoxantrone was approved by the US Food and Drug Administration and several European countries in 2000 for worsening relapsing-remitting MS, progressive relapsing MS, and secondary progressive MS. Suppression of lymphocytes seems to be its main mode of action. Mitoxantrone may cause cardiotoxicity and acute myeloid leukemia at a rate that was not anticipated when the drug was approved. Accordingly, mitoxantrone is primarily used in highly progressive secondary progressive MS and as a third-line therapy in patients with highly active relapsing-remitting MS.
Therapies affecting immune cell migration
These drugs primarily impact on migration of immune cells within the peripheral immune system or across the blood–brain-barrier.
Natalizumab
Natalizumab is a humanized monoclonal antibody against α4β1-integrin (very late activating antigen-4), an adhesion molecule expressed on the surface of activated T cells and other mononuclear leukocytes, which is essential for entry of leukocytes into the CNS.Citation53 Accordingly, migration of lymphocytes is severely impaired in patients treated with natalizumab. In the AFFIRM trial,Citation54 natalizumab significantly reduced the relapse rate by almost 70% and disease progression by more than 50% in relapsing-remitting MS compared with placebo. Similar effects were observed in the SENTINEL trial,Citation55 when natalizumab in combination with interferon-beta 1a was compared with interferon-beta 1a alone.Citation54,Citation55 Natalizumab was approved by the Food and Drug Administration in 2006 for relapsing-remitting MS.
However, during the SENTINEL study, two patients died of progressive multifocal leukencephalopathy, an opportunistic brain infection caused by the John Cunningham (JC) virus. Until September 2012, more than 250 natalizumab-treated patients developed progressive multifocal leukencephalopathy. Therefore, natalizumab is only approved as monotherapy for treatment of highly active or breakthrough relapsing-remitting MS. The risk of developing progressive multifocal leukencephalopathy correlates with both treatment duration and JC virus antibody status. In particular, after 24 months of treatment, the risk of progressive multifocal leukencephalopathy increases to approximately 4.6 cases per 1000 per year in JC virus antibody-positive patients without prior immunosuppressive therapy. Moreover, a history of immunosuppressive therapy before initiation of treatment with natalizumab increases the risk of developing progressive multifocal leukencephalopathy.Citation56 After discontinuation of therapy, disease activity seems to return to the initial level.Citation57
Fingolimod
In 2010, a new oral drug was approved for the treatment of relapsing-remitting MS, ie, fingolimod (FTY720), a sphingosine 1-phosphate receptor agonist. After phosphorylation in vivo, FTY720-P binds to the sphingosine 1-phosphate receptor, promoting internalization of the receptor and homing of lymphocytes into lymphoid tissues.Citation58 This results in a profound decrease of circulating CD4+ T cells and B cells in the blood, and to a lesser extent, in the CSF.Citation59 Two large Phase III studies have shown significant effects on relapse rate (relative reduction of annualized relapse rate by 54% at a dose of 0.5 mg daily compared with placebo) and CNS inflammation as measured by magnetic resonance imaging compared with interferon-beta 1a (TRANSFORMS study)Citation60 and with placebo (FREEDOMS study)Citation61 in relapsing-remitting MS. Relevant side effects include viral infections (herpes viruses), macula edema, skin tumors, elevated liver enzymes, and upper and lower respiratory tract infections. Two patients died in the Phase III trials as a result of viral infections. However, both patients were treated with a high dose of fingolimod, which is no longer used to treat MS. In the US, fingolimod is approved for baseline therapy of relapsing-remitting MS, whereas in Europe it is restricted to patients with highly active disease, comparable with the labeling for natalizumab. More recently, the possible cardiac side effects of the drug have received attention. In December 2011, a patient died suddenly within 24 hours of taking fingolimod for the first time. Therefore, treatment with fingolimod is not recommended without cardiology advice in patients suffering from cardiovascular diseases or taking antiarrhythmic or heart rate-lowering drugs. Cardiac monitoring after the first exposure is mandatory.Citation62,Citation63
Emerging therapies
The drugs described in this section target specific molecules on immune cells and lead to depletion of those cells that express the target molecules.
Alemtuzumab
Alemtuzumab is a humanized monoclonal antibody against CD52, which is expressed on lymphocytes, monocytes, eosinophils, and thymocytes.Citation64 Therefore, alemtuzumab leads to depletion of these cells in peripheral blood. In a Phase II study (CAMMS223), alemtuzumab reduced the relapse rate in relapsing-remitting MS by 74% compared with interferon-beta.Citation65
Two Phase III studies (CARE-MS I/II) evaluated the safety and efficacy of alemtuzumab compared with interferon-beta in patients with relapsing-remitting MS.Citation66,Citation67 In both studies, a significant reduction in relapse rate compared with interferon-beta 1a was observed. In one of the trials, a significant reduction in disease progression compared with interferon-beta 1a was also seen. The main side effects of alemtuzumab are autoimmune diseases of the thyroid in up to 19% of patients, idiopathic thrombocytopenic purpura (1%), and, rarely, Goodpasture syndrome.
Rituximab/ocrelizumab/ofatumumab
Rituximab is a chimeric antibody directed against CD20, which is expressed on B cells from the pre-B cell to memory B cell stage. The antibody depletes all B cells from the peripheral blood and to a certain extent from lymph nodes, although it has no impact on plasma cells in the bone marrow and inflamed tissue. A Phase II trial in relapsing-remitting MS showed a significant reduction of gadolinium-enhancing brain lesions in patients treated with rituximab compared with placebo.Citation68 In another Phase II study of patients with primary progressive MS, a reduction in disease progression was observed in a subgroup of young patients with lesion activity on magnetic resonance imaging.Citation69
Ocrelizumab is a humanized antibody directed against CD20. In a Phase II study, ocrelizumab significantly reduced the annualized relapse rate (reduction by 80% in the 600 mg group and 73% in the 2000 mg group) and the number of gadolinium-enhancing brain lesions in patients with relapsing-remitting MS.Citation70 One patient died from systemic inflammatory response syndrome after the first treatment cycle in week 14 of the trial, and a brain autopsy showed signs of hypoxia and brain edema with transcranial herniation. The relationship between treatment and death has remained uncertain. Two Phase III trials are currently recruiting patients with primary progressive MS and relapsing-remitting MS, respectively.Citation71,Citation72 A small pilot trial with ofatumumab, another CD20-specific antibody, showed significant reduction of magnetic resonance imaging activity in a treatment compared with a placebo group.Citation73
New immunosuppressants and immunomodulators
These drugs impact on immune cells but seem to have fewer side effects than traditional immunosuppressive drugs.
Laquinimod
The exact mode of action of laquinimod is not fully understood, although a possible mechanism seems to be modulation of the Th1/Th2 balance towards a Th2 response.Citation74 In a mouse model of experimental autoimmune encephalomyelitis, laquinimod inhibited the development of disease.Citation75 In a Phase II study, laquinimod reduced the number of gadolinium-enhancing lesions in relapsing-remitting MS compared with placebo.Citation76 The Phase III ALLEGRO study showed a reduction in the annualized relapse rate by 23% and of disability progression by 36%.Citation77 In the Phase III BRAVO trial, the drug was not superior to interferon-beta 1a.Citation78 Few side effects were observed in the trial and no deaths occurred related to the drug.
BG00012 (dimethyl fumarate)
According to in vitro data, BG00012 may exert anti-inflammatory effects by inhibiting synthesis of proinflammatory cytokines.Citation79 In a Phase II study, BG00012 reduced the number of new gadolinium-enhancing and enlarging lesions, as well as the annualized relapse rate in patients with relapsing-remitting MS compared with placebo.Citation80 Recently, the results of two Phase III trials were published. In both trials, BG00012 significantly reduced relapse rates, compared with placebo, by around 50%. Whereas in the Phase III CONFIRM study,Citation81 reduction of disability progression by BG00012 was not significant compared with placebo, the DEFINE studyCitation82 showed a significant reduction of disability progression by more than 30%. Further, all secondary endpoints were positive. Treatment with BG00012 was associated with gastrointestinal side effects and flushing. No major side effects or deaths related to treatment were observed.Citation81,Citation82 Based on the results from the Phase III trials, BG00012 has recently been approved for the treatment of relapsing remitting MS in the US.
Teriflunomide
Teriflunomide is the active metabolite of leflunomide and acts as an inhibitor of dihydroorotate dehydrogenase, which is essential for pyrimidine synthesis in activated lymphocytes.Citation83 In the Phase III TEMSO study, teriflunomide significantly reduced the annualized relapse rate in relapsing-remitting MS by 31% compared with placebo.Citation84 The TOWER trial in relapsing-remitting MS (teriflunomide versus placebo) showed similar results, with a significant decrease in relapse rates and confirmed progression in patients receiving teriflunomide at 14 mg daily compared with placebo.Citation85 The ongoing Phase III TENERE trial is comparing the efficacy of teriflunomide with that of interferon-beta 1a.Citation86,Citation87 Moreover, the Phase III TOPIC study is currently recruiting patients with clinically isolated syndrome.Citation88 No major side effects were observed in the clinical trials. Based on two positive Phase III trials, teriflunomide has been approved for the treatment of MS in the US and awaits approval in Europe.
Daclizumab
Daclizumab is a monoclonal immunoglobulin G antibody directed against the α-subunit of the interleukin-2 receptor (CD25), which is expressed on several immune cells. In the Phase II CHOICE study, add-on daclizumab together with interferon-beta reduced the number of new or enlarged gadolinium-enhancing lesions compared with interferon-beta alone.Citation89
The Phase IIb SELECT study showed a significant reduction in the annualized relapse rate by 50% in the 300 mg dose arm and 54% in the 150 mg dose arm, and of disease activity on magnetic resonance imaging, as measured by number of new gadolinium-enhancing and new or newly enlarging hyperintense T2 lesions in patients with relapsing-remitting MS treated with daclizumab as compared with placebo.Citation90 The Phase III DECIDE study comparing the efficacy and safety of daclizumab with interferon-beta 1a in patients with relapsing-remitting MS is currently ongoing.Citation91
Stem cell therapy
In the past few years, case reports and results of small, open-label, nonrandomized Phase I and II studies on hematopoietic and mesenchymal stem cell transplantation mainly in relapsing forms of MS were reported.Citation92–Citation97 Until now, there have not been any Phase III studies on stem cell therapy in MS, although the first randomized, open-label Phase III study on hematopoietic stem cell transplantation is currently recruiting patients.Citation98 Large randomized controlled trials are needed to evaluate whether stem cell transplantation is more efficacious than the commonly used MS drugs and outweighs the risk of transplantation-related mortality.
Outlook
During the last two decades, a number of drugs have been approved for the treatment of MS. All drugs significantly reduce relapse rates, and some have profound effects on disease progression, even during the first years of treatment. However, the increasing efficacy of MS therapies has been accompanied by new and sometimes life-threatening side effects. During the next decade, a number of new drugs will become available for the treatment of relapsing-remitting MS and clinically isolated syndrome. With the increasing number of drugs, safety will become a key issue, especially for baseline therapies. Unfortunately, the safest drugs are also those with the lowest efficacy. We expect that an increase in specificity by selectively targeting particular immune cells that are deeply involved in the disease pathogenesis will eventually pave the way for safe and efficacious drugs for use in clinically isolated syndrome and relapsing-remitting MS. Moreover, treatments that impact on the course of primary and secondary progressive MS are desperately needed. Accordingly, a number of trials have been initiated that will hopefully result in better treatment of these entities. In the long run, a better understanding of the pathogenesis involved is essential to develop highly specific and effective MS therapies that do not have severe side effects.
Disclosure
BH and his Department of Neurology have received invitational financial support for research activities from Bayer Healthcare Pharmaceuticals, Biogen Idec, Merck Serono, Novartis, Metanomics, Protagen, and Roche, and fees or honoraria for consulting from Bayer Healthcare Pharmaceuticals, Biogen Idec, Genzyme, Merck Serono, Novartis, Teva Pharmaceuticals, GlaxoWellcome, Chugai Pharmaceuticals, and Sanofi-aventis. RCS has no conflicts of interest to report in this work.
References
- Multiple Sclerosis International FederationAtlas of MS Database Available from: http://www.atlasofms.orgAccessed September 19, 2012
- SadovnickADEbersGCDymentDARischNJEvidence for genetic basis of multiple sclerosis. The Canadian Collaborative Study GroupLancet1996347172817308656905
- AscherioAMungerKLEnvironmental risk factors for multiple sclerosis. Part I: the role of infectionAnn Neurol20076128829917444504
- AscherioAMungerKLEnvironmental risk factors for multiple sclerosis. Part II: noninfectious factorsAnn Neurol20076150451317492755
- SawcerSHellenthalGPirinenMGenetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosisNature201147621421921833088
- AscherioAMungerKLSimonKCVitamin D and multiple sclerosisLancet Neurol2010959961220494325
- HughesLESmithPABonellSCross-reactivity between related sequences found in Acinetobacter sp, Pseudomonas aeruginosa, myelin basic protein and myelin oligodendrocyte glycoprotein in multiple sclerosisJ Neuroimmunol200314410511514597104
- SwanborgRHWhittum-HudsonJAHudsonAPInfectious agents and multiple sclerosis – are Chlamydia pneumoniae and human herpes virus 6 involved?J Neuroimmunol20031361812620637
- HemmerBArchelosJJHartungHPNew concepts in the immunopathogenesis of multiple sclerosisNat Rev Neurosci2002329130111967559
- FujinamiRSOldstoneMBAmino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunityScience1985230104310452414848
- AichelePBachmannMFHengartnerHZinkernagelRMImmunopathology or organ-specific autoimmunity as a consequence of virus infectionImmunol Rev199615221458930666
- WucherpfennigKWStromingerJLMolecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic proteinCell1995806957057534214
- O’ConnorKCBar-OrAHaflerDAThe neuroimmunology of multiple sclerosis: possible roles of T and B lymphocytes in immunopathogenesisJ Clin Immunol200121819211332657
- GayFWDryeTJDickGWEsiriMMThe application of multifactorial cluster analysis in the staging of plaques in early multiple sclerosis. Identification and characterization of the primary demyelinating lesionBrain1997120Pt 8146114839278635
- HauserSLBhanAKGillesFKempMKerrCWeinerHLImmunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesionsAnn Neurol1986195785873524414
- LucchinettiCBruckWParisiJScheithauerBRodriguezMLassmannHHeterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelinationAnn Neurol20004770771710852536
- FergusonBMatyszakMKEsiriMMPerryVHAxonal damage in acute multiple sclerosis lesionsBrain1997120Pt 33933999126051
- BruckWKuhlmannTStadelmannCRemyelination in multiple sclerosisJ Neurol Sci200320618118512559508
- LassmannHBruckWLucchinettiCRodriguezMRemyelination in multiple sclerosisMult Scler199731331369291167
- BarnettMHPrineasJWRelapsing and remitting multiple sclerosis: pathology of the newly forming lesionAnn Neurol20045545846815048884
- SakaguchiSNaturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-selfNat Immunol2005634535215785760
- Hvilsted NielsenHToft-HansenHLambertsenKLOwensTFinsenBStimulation of adult oligodendrogenesis by myelin-specific T cellsAm J Pathol20111792028204121872562
- NeumannHMedanaIMBauerJLassmannHCytotoxic T lymphocytes in autoimmune and degenerative CNS diseasesTrends Neurosci20022531331912086750
- TuohyVKYuMWeinstock-GuttmanBKinkelRPDiversity and plasticity of self recognition during the development of multiple sclerosisJ Clin Invest199799168216909120012
- UccelliAAloisiFPistoiaVUnveiling the enigma of the CNS as a B-cell fostering environmentTrends Immunol20052625425915866238
- StorchMKPiddlesdenSHaltiaMIivanainenMMorganPLassmannHMultiple sclerosis: in situ evidence for antibody- and complement-mediated demyelinationAnn Neurol1998434654719546327
- SrivastavaRAslamMKalluriSRPotassium channel KIR4.1 as an immune target in multiple sclerosisN Engl J Med201236711512322784115
- MorandiBBramantiPBonaccorsiIRole of natural killer cells in the pathogenesis and progression of multiple sclerosisPharmacol Res2008571518182304
- ColomboMDonoMGazzolaPAccumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patientsJ Immunol20001642782278910679121
- KeeganMKonigFMcClellandRRelation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchangeLancet200536657958216099294
- HowellOWReevesCANicholasRMeningeal inflammation is widespread and linked to cortical pathology in multiple sclerosisBrain20111342755277121840891
- LucchinettiCFPopescuBFBunyanRFInflammatory cortical demyelination in early multiple sclerosisN Engl J Med20113652188219722150037
- No authors listedInterferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study GroupNeurology1993436556618469318
- No authors listedPlacebo-controlled multicentre randomised trial of interferon-beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon-beta-1b in secondary progressive MSLancet1998352149114979820296
- PanitchHMillerAPatyDWeinshenkerBNorth American Study Group on Interferon beta-1b in Secondary ProgressiveMSInterferon beta-1b in secondary progressive MS: results from a 3-year controlled studyNeurology2004631788179515557491
- KapposLWeinshenkerBPozzilliCInterferon beta-1b in secondary progressive MS: a combined analysis of the two trialsNeurology2004631779178715557490
- JacobsLDBeckRWSimonJHIntramuscular interferon-beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study GroupN Engl J Med200034389890411006365
- KapposLPolmanCHFreedmanMSTreatment with interferon-beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromesNeurology2006671242124916914693
- Ann MarrieRRudickRADrug insight: interferon treatment in multiple sclerosisNat Clin Pract Neurol20062344416932519
- ChenMChenGDengSLiuXHuttonGJHongJIFN-beta induces the proliferation of CD4+CD25+Foxp3+ regulatory T cells through upregulation of GITRL on dendritic cells in the treatment of multiple sclerosisJ Neuroimmunol2012242394622112394
- TeitelbaumDWebbCBreeMMeshorerAArnonRSelaMSuppression of experimental allergic encephalomyelitis in Rhesus monkeys by a synthetic basic copolymerClin Immunol Immunopathol197432562624141659
- TeitelbaumDAharoniRArnonRSelaMSpecific inhibition of the T-cell response to myelin basic protein by the synthetic copolymer Cop 1Proc Natl Acad Sci U S A198885972497282462252
- GranBTranquillLRChenMMechanisms of immunomodulation by glatiramer acetateNeurology2000551704171411113226
- TeitelbaumDFridkis-HareliMArnonRSelaMCopolymer 1 inhibits chronic relapsing experimental allergic encephalomyelitis induced by proteolipid protein (PLP) peptides in mice and interferes with PLP-specific T cell responsesJ Neuroimmunol1996642092178632064
- Ben-NunAMendelIBakimerRThe autoimmune reactivity to myelin oligodendrocyte glycoprotein (MOG) in multiple sclerosis is potentially pathogenic: effect of copolymer 1 on MOG-induced diseaseJ Neurol1996243S14S228965116
- JohnsonKPBrooksBRCohenJACopolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a Phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study GroupNeurology199545126812767617181
- ComiGFilippiMWolinskyJSEuropean/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging – measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study GroupAnn Neurol20014929029711261502
- ComiGMartinelliVRodegherMEffect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trialLancet20093741503151119815268
- LalivePHNeuhausOBenkhouchaMGlatiramer acetate in the treatment of multiple sclerosis: emerging concepts regarding its mechanism of actionCNS Drugs20112540141421476611
- HaasJKorporalMBalintBFritzschingBSchwarzAWildemannBGlatiramer acetate improves regulatory T-cell function by expansion of naive CD4(+)CD25(+)FOXP3(+)CD31(+) T-cells in patients with multiple sclerosisJ Neuroimmunol200921611311719646767
- HongJLiNZhangXZhengBZhangJZInduction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3Proc Natl Acad Sci U S A20051026449645415851684
- HartungHPGonsetteRKonigNMitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trialLancet20023602018202512504397
- BielekovaBBeckerBLMonoclonal antibodies in MS: mechanisms of actionNeurology201074Suppl 1S31S4020038761
- PolmanCHO’ConnorPWHavrdovaEA randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosisN Engl J Med200635489991016510744
- RudickRAStuartWHCalabresiPANatalizumab plus interferon-beta-1a for relapsing multiple sclerosisN Engl J Med200635491192316510745
- BloomgrenGRichmanSHotermansCRisk of natalizumab-associated progressive multifocal leukoencephalopathyN Engl J Med20123661870188022591293
- O’ConnorPWGoodmanAKapposLDisease activity return during natalizumab treatment interruption in patients with multiple sclerosisNeurology2011761858186521543733
- BrinkmannVBillichABaumrukerTFingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosisNat Rev Drug Discov2010988389721031003
- KowarikMCPellkoferHLCepokSDifferential effects of fingolimod (FTY720) on immune cells in the CSF and blood of patients with MSNeurology2011761214122121464424
- CohenJABarkhofFComiGOral fingolimod or intramuscular interferon for relapsing multiple sclerosisN Engl J Med201036240241520089954
- KapposLRadueEWO’ConnorPA placebo-controlled trial of oral fingolimod in relapsing multiple sclerosisN Engl J Med201036238740120089952
- European Medicines AgencyEuropean Medicines Agency gives new advice to better manage risk of adverse effects on the heart with Gilenya Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/public_health_alerts/2012/04/human_pha_detail_000059.jsp&mid=WC0b01ac058001d126Accessed September 15, 2012
- US Food Drug AdministrationFDA Drug Safety Communication: revised recommendations for cardiovascular monitoring and use of multiple sclerosis drug Gilenya (fingolimod) Available from: http://www.fda.gov/Drugs/DrugSafety/ucm303192.htmAccessed September 15, 2012
- JonesJLColesAJCampath-1H treatment of multiple sclerosisNeurodegener Dis20085273118075272
- ColesAJCompstonDASelmajKWAlemtuzumab versus interferon-beta-1a in early multiple sclerosisN Engl J Med20083591786180118946064
- CohenJAColesAJArnoldDLCARE-MS I investigatorsAlemtuzumab versus interferon-beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled Phase 3 trialLancet20123801819182823122652
- ColesAJTwymanCLArnoldDLCARE-MS II investigatorsAlemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled Phase 3 trialLancet20123801829183923122650
- HauserSLWaubantEArnoldDLB-cell depletion with rituximab in relapsing-remitting multiple sclerosisN Engl J Med200835867668818272891
- HawkerKO’ConnorPFreedmanMSRituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trialAnn Neurol20096646047119847908
- KapposLLiDCalabresiPAOcrelizumab in relapsing-remitting multiple sclerosis: a Phase 2, randomised, placebo-controlled, multicentre trialLancet20113781779178722047971
- ClinicalTrials.govA study of ocrelizumab in comparison with interferon beta-1a (Rebif) in patients with relapsing multiple sclerosis Available from: http://www.clinicaltrials.gov/show/NCT01247324 and http://www.clinicaltrials.gov/show/NCT01412333Accessed September 23, 2012
- ClinicalTrials.govA study of ocrelizumab in patients with primary progressive multiple sclerosis Available from: http://www.clinicaltrials.gov/show/NCT01194570Accessed September 23, 2012
- Soelberg SorensenPDrulovicJHavrdovaELisbySGraffOShackelfordSMagnetic resonance imaging (MRI) efficacy of ofatumumab in relapsing-remitting multiple sclerosis (RRMS) – 24-week results of a Phase II study [abstract]Mult Scler201016Suppl 10S37S38
- ZouLPAbbasNVolkmannISuppression of experimental autoimmune neuritis by ABR-215062 is associated with altered Th1/Th2 balance and inhibited migration of inflammatory cells into the peripheral nerve tissueNeuropharmacology20024273173911985832
- BrunmarkCRunstromAOhlssonLThe new orally active immunoregulator laquinimod (ABR-215062) effectively inhibits development and relapses of experimental autoimmune encephalomyelitisJ Neuroimmunol200213016317212225898
- ComiGPulizziARovarisMEffect of laquinimod on MRI-monitored disease activity in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled Phase IIb studyLancet20083712085209218572078
- ComiGJefferyDKapposLPlacebo-controlled trial of oral laquinimod for multiple sclerosisN Engl J Med20123661000100922417253
- ClinicalTrials.govBRAVO study: laquinimod double blind placebo controlled study in RRMS patients with a rater blinded reference arm of interferon β-1a (Avonex®) Available from: http://clinicaltrials.gov/show/NCT00605215Accessed September 17, 2012
- WilmsHSieversJRickertURostami-YazdiMMrowietzULuciusRDimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammationJ Neuroinflammation201073020482831
- KapposLGoldRMillerDHEfficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled Phase IIb studyLancet20083721463147218970976
- FoxRJMillerDHPhillipsJTPlacebo-controlled Phase 3 study of oral BG-12 or glatiramer in multiple sclerosisN Engl J Med20123671087109722992072
- GoldRKapposLArnoldDLPlacebo-controlled Phase 3 study of oral BG-12 for relapsing multiple sclerosisN Engl J Med20123671098110722992073
- SchorlemmerHUMilbertUZeitterDHaunGWunschelMBartlettRRCell cycle regulation and inhibition of de novo pyrimidine biosynthesis by leflunomideInflamm Res199948Suppl 2S115S11610667841
- O’ConnorPWolinskyJSConfavreuxCRandomized trial of oral teriflunomide for relapsing multiple sclerosisN Engl J Med20113651293130321991951
- KapposLComiGConfavreuxCThe efficacy and safety of teriflunomide in patients with relapsing MS: results from TOWER, a Phase III, placebo-controlled studyAbstract presented at the 28th Congress of the European Committee for Treatment and Research in Multiple SclerosisOctober 10–13, 2012Lyon, France
- ClinicalTrials.govAn Efficacy Study of Teriflunomide in Patients with Relapsing Multiple Sclerosis (TOWER) Available from: http://clinicaltrials.gov/show/NCT00751881Accessed September 17, 2012
- ClinicalTrials.govA study comparing the effectiveness and safety of Teriflunomide and Interferon Beta-1a in Patients with Relapsing Multiple Sclerosis (TENERE) Available from: http://clinicaltrials.gov/show/NCT00883337Accessed September 17, 2012
- ClinicalTrials.govPhase III study with Teriflunomide Versus Placebo in Patients with First Clinical Symptom of Multiple Sclerosis (TOPIC) Available from: http://clinicaltrials.gov/show/NCT00622700Accessed September 17, 2012
- WynnDKaufmanMMontalbanXDaclizumab in active relapsing multiple sclerosis (CHOICE study): a Phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon-betaLancet Neurol2010938139020163990
- GiovannoniGGoldRSelmajKA randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of daclizumab HYP monotherapy in relapsing-remitting multiple sclerosis: primary results of the SELECT trialAbstract presented at the 5th Joint triennial congress of the European and Americas Committees for Treatment and Research in Multiple SclerosisOctober 19–22, 2011Amsterdam, The Netherlands
- ClinicalTrials.govEfficacy and Safety of Daclizumab High Yield Process Versus Interferon β 1a in Patients with Relapsing-Remitting Multiple Sclerosis (DECIDE) Available from: http://clinicaltrials.gov/show/NCT01064401Accessed September 17, 2012
- ConnickPKolappanMCrawleyCAutologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label Phase 2a proof-of-concept studyLancet Neurol20121115015622236384
- NashRAHuttonGJRackeMKTreatment of severe relapsing-remitting multiple sclerosis with high-dose immunosuppressive therapy and autologous hematopoietic cell trans plantation: early results of the HALT MS clinical trial (Immune Tolerance Network: ITN033AI)ASH Annual Meeting Abstracts20111183075
- BurtRKLohYCohenBAutologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a Phase I/II studyLancet Neurol2009824425319186105
- MancardiGLMurialdoARossiPAutologous stem cell transplantation as rescue therapy in malignant forms of multiple sclerosisMult Scler20051136737115957523
- KimiskidisVSakellariITsimourtouVAutologous stem-cell transplantation in malignant multiple sclerosis: a case with a favorable long-term outcomeMult Scler20081427828317942513
- FagiusJLundgrenJObergGEarly highly aggressive MS successfully treated by hematopoietic stem cell transplantationMult Scler20091522923718805841
- ClinicalTrials.govHematopoietic stem cell therapy for patients with inflammatory multiple sclerosis failing interferon: a randomized study Available from: http://clinicaltrials.gov/show/NCT00273364Accessed January 14, 2013