Abstract
Recent US Food and Drug Administration approvals of Provenge® (sipuleucel-T) as the first cell-based cancer therapeutic factor and ipilimumab (Yervoy®/anticytotoxic T-lymphocyte antigen-4) as the first “checkpoint blocker” highlight recent advances in cancer immunotherapy. Positive results of the clinical trials evaluating additional checkpoint blocking agents (blockade of programmed death [PD]-1, and its ligands, PD-1 ligand 1 and 2) and of several types of cancer vaccines suggest that cancer immunotherapy may soon enter the center stage of comprehensive cancer care, supplementing surgery, radiation, and chemotherapy. This review discusses the current status of the clinical evaluation of different classes of therapeutic cancer vaccines and possible avenues for future development, focusing on enhancing the magnitude and quality of cancer-specific immunity by either the functional reprogramming of patients’ endogenous dendritic cells or the use of ex vivo-manipulated dendritic cells as autologous cellular transplants. This review further discusses the available strategies aimed at promoting the entry of vaccination-induced T-cells into tumor tissues and prolonging their local antitumor activity. Finally, the recent improvements to the above three modalities for cancer immunotherapy (inducing tumor-specific T-cells, prolonging their persistence and functionality, and enhancing tumor homing of effector T-cells) and rationale for their combined application in order to achieve clinically effective anticancer responses are addressed.
Keywords:
Introduction
Current comprehensive cancer care is centered on reducing the bulk of disease through surgery, chemotherapy, and radiation. Despite the increasing effectiveness of these cornerstones of treatment and high cure rates of multiple cancer forms, cancer remains a leading cause of death.Citation1 Recent breakthroughs in cancer immunotherapy have added several promising new therapies to the traditional armamentarium of oncology treatment regimens.
The strategy of utilizing the immune system in the treatment of cancer dates back to the 1890s and the work of William Coley.Citation2 Coley observed that some tumors regress in the setting of acute bacterial infection. He attempted to recapitulate this phenomenon by studying the injection of heat-inactivated Streptococcus erysipelas and Serratia marcescens (Coley’s toxins) in cancer patients. The field of cancer immunology and immunotherapy has greatly advanced since Coley’s initial studies, a time when little was known about the mechanisms underlying the antitumor effects of bacterial toxins. There is now a growing understanding of how the immune system identifies tumor cells and targets them for elimination. Just as important is the growing understanding of how tumors can undermine the immune system’s ability to recognize and eliminate cancer cells.
Briefly, an adaptive immune response against tumor cells is classically believed to be initiated when tissue-resident antigen-presenting cells, such as dendritic cells, take up and process tumor-specific or tumor-associated antigens, and present these antigens in the context of major histocompatibility complex (MHC) complexes to naïve T-cells in secondary lymphoid organs. Naïve T-cells can differentiate and expand into different classes of antigen-specific T-cells, including cluster of differentiation (CD)4+ T helper cells and CD8+ effector cytotoxic T lymphocytes (CTLs). At each step of this process, various signals shape whether an antitumor T-cell response will be produced, or conversely, an immunosuppressive and/or tolerogenic response will be made by such mediators as regulatory T-cells and myeloid-derived suppressor cells (reviewed by Palucka and Banchereau,Citation3 Chen and Mellman,Citation4 and Blattman and GreenbergCitation5). Immunotherapies for cancer can target each or many of these steps to skew toward an antitumor response and away from an immunosuppressive response.
Cancer immunotherapies can be categorized as non-antigen-specific or antigen-specific therapies. Non-antigen-specific immunotherapies aim to either enhance the immune response in a general fashion or to decrease the immunosuppression present in the tumor environment. Non-antigen-specific therapies include cytokines and immune growth factors (eg, interferon (IFN]-α, interleukin [IL]-2, or granulocyte macrophage colony-stimulating factor), immunologic adjuvants (eg, Bacille Calmette-Guérin); Toll-like receptor (TLR)-3 agonists, such as poly-I:C (Rintatolimod, Ampligen®; Hemispherx Biopharma, Inc., Philadelphia, PA, USA) and poly-ICLC (Hiltonol®; Oncovir, Washington, DC, USA); TLR-4 agonists, such as monophosphoryl lipid A; the TLR-7 agonist, imiquimod; immune checkpoint blockers, eg, anticytotoxic T-lymphocyte antigen-4 (CTLA-4) antibody;Citation6,Citation7 and the programmed death-1 (PD-1) pathway agents, nivolumab and lambrolizumab.Citation8–Citation11
Compared with non-specific immunotherapies, antigen-specific therapies, such as therapeutic vaccines against cancer, aim to induce immune cells to target cancer cells that express a particular set of antigens. Different classes of cancer vaccines include peptide-based or protein-based vaccines, cancer cell-based vaccines, viral vector vaccines, DNA vaccines, messenger RNA vaccines, and carbohydrate vaccines.Citation12–Citation19 In all cases, these vaccines involve two components, an antigen and an adjuvant, aimed at promoting local inflammation and the resulting immunization. Additionally, all of the above types of cancer vaccines rely on the patients’ endogenous dendritic cells (DCs) for their uptake and effective antigen presentation to tumor-specific CD8+ and CD4+ T-cells.
Another category of cell-based cancer vaccines is use of patients’ ex vivo-generated and tumor antigen-loaded DCs (or more precisely, autologous cellular therapeutics). This strategy limits the dependence of the immune system on patients’ resident DCs, which have been shown to be defective in the advanced stages of cancerCitation3,Citation20,Citation21 or even redirected to differentiate toward myeloid-derived suppressor cells.Citation22,Citation23 Regardless of whether endogenous or ex vivo-generated DCs are utilized for immunization, therapeutic cancer vaccines need to overcome several common challenges to induce immunity in the presence of established tumors and can benefit from recent developments in the area of DC biology.
Challenges in therapeutic cancer vaccination
For a therapeutic cancer vaccine to be effective, it must be capable of inducing a high number of antigen-specific T-cells against an established tumor, which can migrate to the tumor and perform their effector functions at the tumor site (). However, challenges are present for each of these three goals. The first challenge is achieving high numbers of antitumor T-cells when the vaccine is being administered in the presence of an ongoing, although dysfunctional, immune response. Due to the ongoing antitumor immune response, the vaccine-carrying antigen-presenting cells (using either endogenous DCs that have taken up vaccine-introduced antigens or ex vivo-generated tumor antigen-loaded DCs), may be recognized by the CD8+ T-cells as “tumor”.Citation24,Citation25 Since this encounter occurs in the periphery, away from the immunosuppressive tumor microenvironment, the CD8+ T-cells may be capable of eliminating the vaccine, and thus limiting the vaccine’s effectiveness before it can induce an immune response.Citation3
Figure 1 Elements of effective antitumor immunity.
Abbreviations: CD, cluster of differentiation; DC, dendritic cell; IFN, interferon; IL, interleukin; PD, programmed death-1; PDL, programmed death ligand; TLR, toll-like receptor.
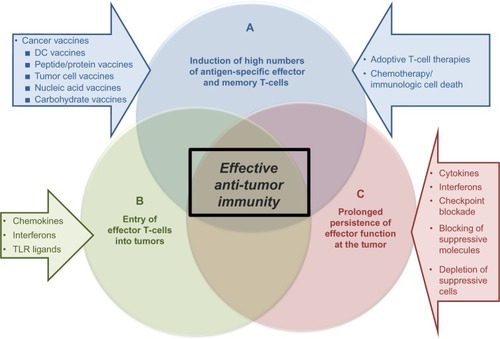
Additionally, there is a lack of the proinflammatory signals required to promote effective immune responses. These signals are replaced by tumor-induced immunosuppressive/anti-inflammatory signals predominating in cancer patients. Therefore, to achieve the goal of inducing high numbers of tumor-specific T-cells, the vaccine-carrying antigen-presenting cells must not only survive long enough to present antigen, but must also provide the inflammatory signals to drive effector cell functions.Citation3,Citation26–Citation28
Unfortunately, the presence of high numbers of antigen-specific T-cells does not ensure an effective antitumor response if these T-cells are unable to home to the tumor. In a normal infection scenario, where the immune response is targeting invading pathogens, the microorganisms and local tissue damage induce chemokines that recruit effector cells such as CTLs, type 1 helper CD4+ T-cells, or natural killer cells to the site of pathogen entry.Citation27,Citation29 However, one of the immune evasion mechanisms evoked by tumors to support tumor growth and metastatic spread is downregulation of the chemokines that attract immune effector cellsCitation28,Citation29 and upregulation of chemokines that attract suppressor cells, such as regulatory T-cells,Citation30–Citation32 suppressive plasmacytoid DCs,Citation33 and myeloid-derived suppressor cells.Citation34,Citation35 Thus, a therapeutic vaccine needs to either induce T-cells that can respond to the spontaneously expressed tumor-associated chemokines or be administered as part of a combinatorial therapy with additional factors to alter the chemokine profile in the tumor microenvironment.Citation32,Citation34
Once high numbers of vaccine-induced tumor-specific T-cells have been generated and arrive at the tumor site, the T-cells must be capable of killing the tumor cells in order for the vaccine to be effective. Most types of cancer (including melanoma, ovarian, breast, renal, prostate, lung, and head and neck cancer) produce many factors, including IL-10, transforming growth factor beta, vascular endothelial growth factor, IL-6, and cyclooxygenase-2 products like prostaglandin E2, that contribute to immune dysfunction by suppressing the functions of endogenous or adoptively transferred DCs and T-cells.Citation21,Citation36–Citation38 These factors not only act to directly suppress DC and T-cell functions, but they can also promote cell-mediated immune suppression by enhancing the recruitment, expansion, and activation of regulatory T-cells and myeloid-derived suppressor cells.Citation30,Citation31,Citation39,Citation40 While in some patients the high numbers of tumor-specific T-cells induced by the vaccine may be able to overcome the immunosuppressive tumor microenvironment, most therapeutic cancer vaccine strategies would greatly benefit from a combinatorial approach that alters the tumor to reduce immunosuppressive factors.
Promise and challenges in therapeutic cancer vaccines: clinical trials
The development of clinically effective therapeutic cancer vaccines has been challenging. Currently, the only therapeutic cancer vaccine approved by the US Food and Drug Administration is sipuleucel-T, a treatment for metastatic androgen-independent prostate cancer that was approved in 2010.Citation41,Citation42
Sipuleucel-T consists of antigen-presenting cells that are activated ex vivo from autologous peripheral blood mononuclear cells by a fusion protein, PA2024, which is comprised of granulocyte macrophage colony-stimulating factor and prostatic acid phosphatase, a prostate adenocarcinoma-associated antigen.Citation41,Citation43 In two randomized, double-blind, placebo-controlled multicenter Phase III trials, sipuleucel-T increased median survival by 4 months when compared with placebo.Citation43,Citation44 Sipuleucel-T was administered in three doses at weeks 0, 2, and 4, each at 2 days following leukapheresis. In the D9901/D9902A trials of 225 patients with asymptomatic metastatic hormone-refractory prostate cancer randomized in a 2:1 ratio to treatment with sipuleucel-T or a control infusion, the primary objective was time to disease progression. While there was no statistically significant difference in time to progression (median 11.1 weeks with sipuleucel-T versus 9.7 weeks with control), there was a 33% reduction in risk of death with sipuleucel-T compared with control and a statistically significant difference in survival (median 23.2 months for sipuleucel-T versus 18.9 months for control, P=0.011).Citation44 In the IMPACT (Immunotherapy for Prostate Adenocarcinoma Treatment) study of 127 metastatic castration-resistant prostate cancer patients with the primary endpoint of overall survival, there was a 22% adjusted relative reduction in risk of death and a statistically significant increase in median survival of 4.1 months (median 25.8 months for sipuleucel-T versus 21.7 months for placebo, P=0.03), although there was no difference in disease progression.Citation43 Patients in the treatment group who had antibody titers of more than 400 against PA2024 had an increased survival compared with those who had titers of less than 400 (P<0.001).Citation43 Cumulative antigen-presenting cell activation measured by CD54 upregulation, antigen-presenting cell number, total nucleated cell number, and antigen-specific immune responses to PA2024 and/or prostatic acid phosphatase in the treatment group correlated with overall survival (P<0.05).Citation41
The ClinicalTrials.gov registry gives an insight into upcoming cancer vaccines in development that show promise in improving outcomes.Citation45 A query of this website in November 2013 with a targeted search of Phase III and IV clinical trials with known statuses and “cancer” listed as the condition, “vaccine” as the intervention, and “survival” as the outcome measure, resulted in 42 studies. A summary of selected cancer-specific vaccines from this query is listed in with additional information from publications and abstracts.Citation43,Citation46–Citation55
Table 1 Selected Phase III trials of cancer-specific vaccines
In addition to the sipuleucel-T trials, a Phase III trial of a glycoprotein 100 peptide vaccine also posted positive results. In a randomized, multicenter trial, patients with advanced melanoma received IL-2 and glycoprotein 100:209–217 (210 M) peptide vaccination or IL-2 alone.Citation47 For the primary endpoint of clinical response, the IL-2 with vaccination group had a significantly higher response rate of 20% (complete response 11%, partial response 9%) versus a response rate of 10% in the IL-2 only group (complete response 2%, partial response 8%; P=0.05). Median progression-free survival was also significantly longer in the IL-2 with vaccination group (2.2 months) than in the IL-2 alone group (1.6 months; P=0.008). There was a trend of increased overall survival with the addition of vaccination to IL-2 (17.8 months) compared with IL-2 alone (11.1 months; P=0.06). It is important to note that this study was not powered to detect a difference in overall survival.
Several Phase III trials of therapeutic cancer vaccines are currently in progress. Another vaccine in Phase III trials is TG4010, a poxvirus vector vaccine encoding for the tumor-associated antigen Mucin-1 (MUC1) and IL-2, which is being investigated in non-small cell lung cancer (NSCLC).Citation49 IMA901, a multiple peptide vaccine for renal cell carcinoma, has also completed accrual for its Phase III study and its results are pending. The ten peptides for IMA901 were uniquely chosen using an antigen discovery platform that analyzed renal cell carcinoma tissue.Citation56 The HyperAcute® vaccines (NewLink Genetics, Ames, IA, USA) for pancreatic and NSCLC consist of allogeneic cancer cells that have been genetically modified to express murine α(1,3)galactosyl.Citation50,Citation51
ProstAtak™ (Advantagene Inc., Auburndale, MA, USA) and Prostvac®-V/F-TRICOM™ (Bavarian Nordic; Washington, DC, USA) are viral-based vaccines for prostate cancer. ProstAtak involves intratumoral injection of an adenovirus containing a Herpes virus thymidine kinase gene followed by valaciclovir. Prostvac-V/F-TRICOM is composed of recombinant vaccinia and fowlpox viral vectors that encode for prostate-specific antigen and TRICOM, a combination of three costimulatory molecules, LFA-3, B7.1, and intercellular adhesion molecule-1.Citation52
A few Phase III trials have failed to meet their primary endpoints, and highlight the difficulties of cancer vaccine development. One of the largest studies in NSCLC, the MAGRIT (MAGE-A3 as Adjuvant NSCLC Immunotherapy) trial, which utilized a melanoma-associated antigen 3 (MAGE-A3) protein vaccine, was stopped in early 2014 after failing to increase the primary endpoint of disease-free survival in MAGE-A3-positive patients overall or MAGE-A3-positive patients without chemotherapy treatment, compared with control.Citation57–Citation59 This was following a double-blind, randomized, placebo-controlled Phase II study that showed clinical activity, with all treated patients developing anti-MAGE-A3 antibodies and with a pretreatment 84-gene expression signature being associated with increased disease-free response.Citation60,Citation61 However, the subsequent Phase III trial was not able to determine a subpopulation of gene signature-positive patients who would benefit from treatment since there was an insufficient treatment effect.Citation59
Belagenpumatucel-L, an allogeneic genetically modified NSCLC tumor cell vaccine, showed a trend toward increased median survival but this did not reach statistical significance.Citation53 However, the subgroup of patients who received vaccination within 12 weeks of chemotherapy had a statistically significant improvement, and the study is continuing in this subgroup of patients. Similarly, tecemotide, a MUC1 peptide vaccine for NSCLC, failed to demonstrate a statistically significant difference in overall survival compared with placebo, but a significant increase in median overall survival in the subgroup of patients who had concurrent chemoradiation has led to plans for a randomized trial of tecemotide with concurrent chemoradiation in stage III NSCLC patients.Citation54
One of the largest studies in metastatic melanoma was MMAIT-IV (Malignant Melanoma Active Immunotherapy Trial for Stage IV Disease), an international, multicenter, randomized, double-blind Phase III trial in 1,656 stage III and IV patients of an allogeneic whole melanoma cell vaccine, Canvaxin™ (CancerVax Corporation, Carlsbad, CA, USA) with a Bacille Calmette-Guérin adjuvant, compared with placebo plus Bacille Calmette-Guérin, that was closed early after interim analysis showed a low probability of demonstrating a significant increase in survival in the Canvaxin with Bacille Calmette-Guérin arm.Citation62 Although the trial had negative results, an ancillary study of pretreatment and post-treatment circulating tumor cell biomarkers for melanoma antigen recognized by T-cells 1 (MART1), MAGE-A3, and paired box 3 (PAX3) from patients in the MMAIT-IV trial was able to demonstrate that pretreatment and serial circulating tumor cell levels were significantly associated with decreased disease-free survival and overall survival.Citation63
Another large melanoma vaccine study, the randomized Phase III trial of adjuvant ganglioside (GM2) conjugated to Keyhole Limpet hemocyanin (KLH) admixed with adjuvant QS-21 (GM2-KLH/QS-21) vaccine versus observation in 1,314 stage II melanoma patients, was terminated after the second interim analysis due to failure to increase recurrence-free survival and a trend toward increased overall survival in the observation arm, which was also confirmed on final analysis after a median follow-up of 4 years.Citation64
A challenge in evaluating therapeutic cancer vaccines is appropriate patient selection. While clinical trials of new oncologic therapies are traditionally first tested in patients with advanced cancers who have failed multiple treatment regimens, vaccines may be more effective when the disease burden is low.Citation65 Another challenge in trial design and evaluation is that the kinetics of tumor growth rates for vaccine therapy differ from those of traditional chemotherapy and radiotherapy.Citation66 Compared with these directly cytotoxic therapies in which the treatment response occurs immediately following their administration and the tumor growth rate often returns to pretreatment levels following termination of treatment, positive responses to vaccine therapy may begin months after treatment, with a potentially prolonged treatment effect persisting long after administration.Citation67 Therefore, the intermediate endpoint of progression-free survival based on the commonly used Response Evaluation Criteria in Solid Tumors or World Health Organization criteria has very limited value in vaccine therapies, and more relevant immunologic endpoints are needed.Citation66–Citation68 A common phenomenon with immunotherapy trials is that overall survival may improve without a change in progression-free survival.Citation43,Citation46,Citation69 In fact, there may even be a treatment response after initial progression or tumor growth.Citation67 In result, the recently formulated immune-related response criteriaCitation67 are better predictors of prolonged overall survival of patients treated with immunotherapy than the classical response criteria used to evaluate the effectiveness of chemotherapeutic agents (Response Evaluation Criteria In Solid Tumors [RECIST] and World Health Organization).Citation67
Finally, another important trial design consideration is immunologic selection and response monitoring of patients. Pretreatment markers would help to determine which patients would benefit the most from vaccine treatment but this work is still in its infancy.Citation70 The discovery of markers to monitor immune responses that correlate with clinical outcomes is still in development. Current biomarkers to evaluate the immune response focus on CTL antigen recognition and the humoral response. Markers shown to correlate with clinical outcome include antigen-specific T-cell response based on IFN-γ enzyme-linked ImmunoSpot (ELISPOT) assays, cytokine expression levels, and reduction in regulatory T-cells.Citation41,Citation71–Citation73
Furthermore, two clinical trials involving DC vaccines indicated a role of DC-produced IL-12p70 as a predictive marker of the clinical benefit of vaccination.Citation74,Citation75
Avenues for improved immunization: exploiting the biology of dendritic cells
The primary aim of cancer vaccines is to generate a CTL response against cancer cells.Citation76 An important advantage of therapeutic immunizations, compared with traditional cancer treatments, is that the treatment effect is typically durable due to the induction of long-lived effector memory and central memory T-cells, which can persist for prolonged periods after administration of the vaccine. The second advantage is the very high selectivity of the immune response in targeting tumor cells, while not damaging healthy tissue. As mentioned before, several strategies, such as protein or DNA vaccines, utilize a patient’s endogenous DCs at the injection site for uptake and presentation of tumor antigens, but the observed dysfunction of DCs in cancer patients due to tumor-related suppressive factors may limit the effectiveness of these vaccines, which rely on endogenous DCs for antigen uptake.Citation37,Citation77–Citation79 Therefore, the use of ex vivo-generated DC vaccines is an attractive option for circumventing this issue, enabling DCs to mature in the absence of tumor-related immunosuppression and allowing more control of the DC maturation process to direct the nature of the immune response.
Effective induction of an antigen-specific T-cell response requires delivery of at least four types of signals () by DCs, each of which can be optimized to improve the cancer vaccine.Citation80 The first signal (signal 1) is the presentation of processed antigen in the context of MHC molecules by DCs to naïve T-cells via the T-cell receptor.Citation81 One of the key characteristics of DCs that makes these cells a unique tool for cancer vaccination is their ability to take up different forms of antigens, process them, and then cross-present these antigens to naïve CD4+ and CD8+ T-cells. This broadens the source of antigens that can be used in vaccines to include not only peptides (which are MHC-restricted and limited to known, well characterized tumor antigens and thus only applicable to patients who express the appropriate MHC haplotype and have tumors that express the specific antigen), but also recombinant proteins, tumor lysates, or whole tumor cells from either autologous or allogeneic sources. The use of proteins or whole cell sources of antigen increases the ability to prime immune responses to undefined patient-specific tumor antigens. Various methods of processing tumor cells for loading have been studied, such as freeze-thaw lysates, irradiation, and oxidation of tumor cells, to enhance the uptake and cross-presentation of whole tumor cells by DCs.Citation79,Citation82–Citation84 Of note, loading DCs with apoptotic cells was shown to be more effective in stimulating CTLs compared with loading with necrotic cells.Citation85
Figure 2 Four types of DC-mediated signals regulating the magnitude and quality of tumor-specific T-cell responses.
Abbreviations: COX2, cyclooxygenase-2; CTL, cytotoxic T lymphocyte; DC, dendritic cell; IFN, interferon; IL, interleukin; MDSCs, myeloid derived suppressor cells; TLR, toll-like receptor; Th1, type 1 helper; Th2, type 2 helper; Th17, type 17 helper; Tregs, regulatory T-cells.
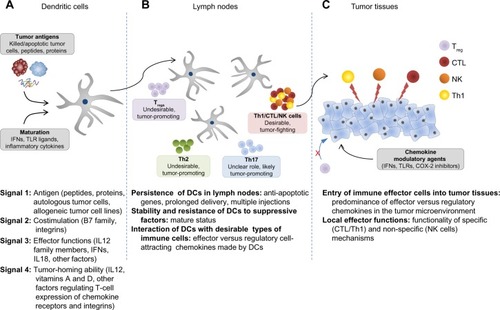
Signal 2 involves costimulatory signals that amplify the T-cell receptor signal and prolong the MHC:T-cell receptor interaction to ensure T-cell activation. This amplification signal is provided by B7 family molecules, such as CD80 and CD86, that bind to CD28 on the T-cell.Citation86,Citation87 The MHC:T-cell receptor and CD28:CD80/CD86 interactions are stabilized by integrins, notably leukocyte function-associated antigen-1 (LFA-1): intercellular adhesion molecule-1 interactions, so that the cell-cell interactions are not prematurely terminated, resulting in incomplete activation.Citation88 The absence of costimulation during antigen presentation by DCs can induce CD8+ T-cell tolerance to the antigen.Citation89 The molecules involved in costimulation are upregulated upon DC maturation, when the DC also gains the ability to respond to the lymph node-homing chemokines CCL (chemokine [CC motif ligand]-19 and CCL22) by upregulating CC chemokine receptor type 7 (CCR7).Citation90,Citation91 The first generation of DC vaccines utilized immature or partially matured DCs capable of cross-presentation of antigens but deficient in costimulatory and lymph node-homing abilities.Citation86 This led to protocols for DC maturation producing the “second generation” of DC vaccines that are able to provide both signals 1 and 2. In these protocols, the DCs are matured using either monocyte-conditioned mediumCitation92 or a cytokine cocktail consisting of IL-6, IL1β, tumor necrosis factor alpha, and prostaglandin E2.Citation93 While these maturation strategies induce upregulation of costimulatory molecules and CCR7, and have enhanced immunogenicity in vitro and in vivo in healthy volunteers, their initial promise diminished in a randomized multicenter Phase III trial for advanced melanoma when less than 5% of patients receiving the vaccine demonstrated a clinical response and there was no impact on overall survival.Citation94
Signal 3 is the DC-produced cytokine profile that skews the type of immune response generated (ie, type 1 cell- mediated versus type 2 humoral responses), and provides survival and differentiation signals to naïve T-cells. A prototypical example of a signal 3 cytokine that promotes cell-mediated immunity is IL-12p70,Citation95 which is produced by DCs when they are matured in the presence of IFN-γ, a cytokine produced by activated natural killer cells at the site of infection, and in the absence of the chronic inflammatory cytokine, prostaglandin E2. One possible factor in the negative results of the clinical trials using “second generation” DCs is the use of prostaglandin E2-containing maturation cocktails, since prostaglandin E2 has subsequently been shown to have a deleterious effect on IL-12p70.Citation95–Citation97
In order to generate mature DCs with high costimulatory molecules and lymph node-homing ability, as well as high IL-12-producing capacity to promote the desirable cell-mediated immunity, a “third generation” of DCs was generated.Citation73,Citation74 The “third generation” DCs are generally matured in conditions mimicking viral infection, which predominantly drives cell-mediated immunity. Some of the strategies to mature DCs are: to coculture immature DCs with other immune cells, such as IL-18 activated natural killer cellsCitation98 or memory CD8+ T-cells;Citation25,Citation99 to mature with conditioned medium from activated CTLs;Citation100,Citation101 or to use cytokine cocktails that include viral-mimicking TLR ligands.Citation102–Citation107 Each of these strategies generate DCs that are “type 1 polarized” (DC1), possessing not only high antigen cross-presentation and costimulatory abilities, but also a superior ability to secrete IL12 for up to 48 hours after interaction with CD40L-expressing CD4+ T-cells.Citation102,Citation103,Citation108–Citation110 Additional inclusion of IFN-α to a “type 1 polarizing” cytokine cocktail consisting of IFN-γ, IL1β, tumor necrosis factor alpha, and poly-I:C enhanced the expression of the lymph node homing chemokine receptor CCR7.Citation111–Citation113 These αDC1s also preferentially produce chemokines that promote migration of naïve, memory, and effector T-cells, but show reduced expression of chemokines that promote immunosuppressive cell recruitment, further enhancing the ability of αDC1s to interact and prime strong antitumor immune responses.Citation111–Citation113 A recent clinical trial utilizing αDC1 vaccines and an alternative type of “type 1 polarized DCs” induced by the combination of CD40L and IFN-γ demonstrated that the ability of DC1 vaccines to produce high IL-12p70 levels was the strongest predictor of prolonged progression-free survival in vaccinated patients.Citation74,Citation75
The last type of signal (signal 4) delivered to T-cells during priming interactions with DCs results in programming of specific chemokine receptor expression on activated T-cells that directs them to specific tissues.Citation86 In vitro and ex vivo studies have demonstrated that different DC subsets isolated from various tissues can modulate the chemokine expression profile on activated T-cells, thereby directing T-cells back to the tissues of DC origin.Citation114,Citation115 This differential chemokine expression programming is not limited to DCs developed in various tissues in vivo, but also extends to ex vivo-generated, cytokine-matured DCs. A comparison of CD8+ T-cells from melanoma patients sensitized ex vivo by either prostaglandin E2-matured DCs (second generation) or type 1 polarized DC1s (third generation) demonstrated different chemokine expression on the activated CD8+ T-cells.Citation97 Specifically, T-cells sensitized by DC1s had higher expression of CCR5 and CXC chemokine receptor 3 (CXCR3), two chemokine receptors involved in peripheral homing to the skin and entry into melanoma and other tumors, compared with T-cells sensitized by prostaglandin E2-matured DCs.Citation111,Citation112,Citation114,Citation116
Helping vaccination-induced T-cells to work: conditioning tumor microenvironments for effective CTL entry and function
Future developments in cancer immunotherapy research will likely focus on the challenges that vaccine-induced CTLs encounter in reaching the tumor microenvironment and performing their antitumor cytotoxic functions. Areas of current investigation in changing the tumor milieu include promoting CTL entry via chemokine modulation, inhibiting immune checkpoints that block CTL effector function, and decreasing immunosuppressive cells, such as regulatory T-cells and myeloid-derived suppressor cells.
Chemokine modulation aims to shift the balance of the tumor environment toward expression of effector T-cell attracting chemokines, and away from regulatory T-cell attracting chemokines.Citation32 Tumor infiltration of certain immune cells such as CTLs, type 1 helper CD4+ T-cells, DCs, and M1 macrophages has positive prognostic value, while infiltration by regulatory T-cells, type 2 helper CD4+ T-cells, myeloid-derived suppressor cells, and M2 macrophages is associated with poor outcomes.Citation117–Citation120 There are currently several monoclonal antibodies and small molecule inhibitors targeting various chemokine receptors in clinical trials.Citation121 Our group has also shown that ex vivo treatment of tumor tissue with type 1 IFNs, a TLR-3 ligand, and a cyclo-oxygenase-2 inhibitor increased the production of the effector T-cell attracting chemokines, CCL5 and CXCL10, while decreasing the production of regulatory T-cells attracting chemokine CCL22.Citation32
Combining vaccines with agents that reduce the levels of immunosuppressive cells (such as myeloid-derived suppressor cells and regulatory T-cells) has also been an attractive strategy. Low-dose cyclophosphamide has been used extensively for its ability to suppress regulatory T-cells since it is inexpensive and easily obtained.Citation122 A randomized Phase II study of the renal cell cancer peptide vaccine, IMA901, demonstrated that a single cyclophosphamide dose was effective in reducing the number of regulatory T-cells, and that among patients who were immune responders, those treated with cyclophosphamide had increased survival.Citation56 Another Phase I/II trial of a multipeptide-loaded DC vaccine in advanced ovarian cancer showed a trend toward increased survival with the addition of cyclophosphamide treatment.Citation123 Other combination strategies to reduce regulatory T-cells in vaccine trials have included anti-CD25 monoclonal antibodies and a CD25 targeting immunotoxin.Citation124,Citation125 Preliminary data from an ongoing randomized DC vaccine trial targeting myeloid-derived suppressor cells using all-trans-retinoic acid show that the treatment arm receiving all-trans-retinoic acid and vaccination had an improved immune response compared with vaccination alone.Citation126 Other inhibitors of immunosuppressive targets shown to correlate with decreased survival, such as prostaglandin E2, indoleamine 2,3-dioxygenase, and nitric oxide synthase, are also potential targets for combinatorial therapy with cancer vaccines.Citation127–Citation130
In contrast with combinatorial therapies that reverse immunosuppressive cells, cancer vaccines may be combined with cytokine treatments that promote effector T-cell activity and prolong T-cell memory (see ). IL-7, IL-15, IL-21, and IL-27 are similar to IL-2 as part of the common gamma chain cytokine receptor family.Citation131 IL-7 has a role in development, homeostasis, and survival of T-cells and B-cells.Citation132,Citation133 Administration of recombinant IL-7 to cancer patients has been shown to be safe and to rapidly expand circulating CD4 and CD8 cells that express CD127, but not regulatory T-cells.Citation134 IL-15 has a role in T-cell and natural killer cell activation and proliferation and maintenance of memory T-cell responses.Citation135,Citation136 Early phase clinical trials utilizing IL-15 for cancer treatment are ongoing or recently completed, but without published results as yet.Citation136 IL-21 is produced by activated CD4+ T-cells and natural killer T-cells, and contributes to antitumor immunity by its induction and activation of CD8+ T-cells, natural killer cells, and natural killer T-cells.Citation131,Citation137,Citation138 Early Phase I and II studies have shown encouraging results in metastatic melanoma and metastatic renal cell carcinoma.Citation139–Citation142 IL-27 is produced by antigen-presenting cells and can enhance CD8+ T-cell and natural killer cell activation, but development of IL-27 as a therapeutic is still in preclinical stages.Citation143,Citation144
The approval by the US Food and Drug Administration of ipilimumab for metastatic melanoma in 2010 signaled a change in the landscape of cancer therapies. Ipilimumab (MDX-010, Yervoy®; Bristol-Myers Squibb, New York, NY, USA) is a fully human monoclonal antibody against CTLA-4, and a homologue of CD28 with greater affinity to B7 molecules which outcompetes CD28 binding, effectually preventing the costimulatory signal 2.Citation11 Anti-CTLA-4 antibodies block this inhibitory interaction or immune checkpoint and restore signal 2 for T-cell activation. In a randomized, double-blind, three-arm Phase III trial comparing ipilimumab with and without a glycoprotein 100 vaccine (MDX-1379) with vaccination alone in patients with metastatic melanoma, subjects in the ipilimumab treatment groups were found to have a significantly higher median survival compared with those receiving vaccination alone (10 months versus 6.4 months).Citation46 The failure of the vaccination arms in the Phase III study to improve overall survival was unexpected, but it is possible that this resulted from the application of a single-epitope glycoprotein 100 peptide vaccine. A similar glycoprotein 100 vaccine did not show an improvement in survival, although that study was only powered to detect a difference in progression-free survival and not overall survival.Citation47 Furthermore, the original Phase III study had ipilimumab and vaccination administration occurring concurrently, whereas there is more recent evidence from a murine model that sequential therapy of vaccination followed by anti-CTLA-4 antibody was superior to the anti-CTLA-4 antibody when administered first.Citation145 Some of the early preclinical studies of ipilimumab indeed focused on using it in combination with cell-based cancer vaccines, and other anti-CTLA-4/vaccine combinations are in clinical trials.Citation11,Citation146–Citation148 A recent Phase II study comparing ipilimumab alone or in combination with GM-CSF-secreting whole cell vaccine showed a higher survival rate when ipilimumab was combined with vaccine.Citation146
Another actively studied immune checkpoint receptor is PD-1 (CD279).Citation149 PD-1 and its ligands, PD-1 ligand 1 and 2 (PDL1 and PDL2), are expressed on more cell types than CTLA-4. PD-1 expression can be induced not only on activated T-cells, but also on B-cells and natural killer cells, while PDL1 and PDL2 can be upregulated on tumor cells, antigen-presenting cells, and other cells in inflammatory conditions. Several clinical trials of anti-PD-1 and anti-PDL1 antibodies have shown durable response rates.Citation6,Citation150–Citation152 While studies using combinatorial PD pathway agents and vaccine therapy are not as advanced as those with anti-CTLA-4 agents, there is promising preclinical and early clinical trial data suggesting that the dual combination or even the triple combination with anti-CTLA-4/PD pathway blockade/vaccination therapy will have increased clinical benefit by further enhancing the antigen-specific T-cell response from vaccination and decreasing regulatory T-cells.Citation153–Citation157
An effective combinatorial vaccine therapy will likely need to address three goals: building a robust antigen-specific CTL response; altering the tumor microenvironment to allow CTL infiltration and reduce migration of regulatory T-cells and myeloid-derived suppressor cells; and counteracting CTL inhibitory mechanisms such as immune checkpoints that lead to immunosuppression (). An encouraging study using a combination of a peptide vaccine, anti-PD-1 antibody, and low-dose cyclophosphamide in a murine tumor model demonstrated that this combination of drugs synergized in increasing survival and reducing tumor burden.Citation158 One of the concerns about optimal application of complex immunotherapies is determination of the optimal sequence and duration of application of each of the components. It also needs to be determined how to optimally incorporate immunotherapy, different forms of which can either suppress or enhance both the induction of immune responses and the susceptibility of cancer tissues to immune attack.
Conclusion
Several of the new cancer vaccines have recently shown promise in prolonging patient survival. The next era of vaccine development is likely to involve both continued improvement of the vaccines themselves as well as combinatorial application of vaccines with agents that target the tumor microenvironment to promote entry of vaccination-induced cells, while eliminating local predominance of suppressive cells, and amplifying and prolonging the duration of the effector phase of antitumor immunity at tumor sites. The development of optimized immunotherapies for advanced cancer will also benefit from identification of the most relevant laboratory correlates of clinical effectiveness and integration of immunotherapy with other elements of comprehensive cancer care.
Acknowledgments
This work was supported by grants from the National Institutes of Health (CA113263, CA132714, CA159981).
Disclosure
Alpha-type 1 polarized DCs, one of the types of DC discussed in this paper, are a topic of a recently issued US patent. None of the authors receives any form of support or remuneration related to that intellectual property. The authors report no other conflicts of interest in this work.
References
- SiegelRNaishadhamDJemalACancer statistics, 2013CA Cancer Clin20136311130
- NautsHCSwiftWEColeyBLThe treatment of malignant tumors by bacterial toxins as developed by the late Coley WB, reviewed in the light of modern researchCancer Res19466420521621018724
- PaluckaKBanchereauJCancer immunotherapy via dendritic cellsNat Rev Cancer201212426527722437871
- ChenDSMellmanIOncology meets immunology: the cancer-immunity cycleImmunity201339111023890059
- BlattmanJNGreenbergPDCancer immunotherapy: a treatment for the massesScience2004305568120020515247469
- HamidORobertCDaudASafety and tumor responses with lambrolizumab (anti-PD-1) in melanomaN Engl J Med2013369213414423724846
- WolchokJDKlugerHCallahanMKNivolumab plus ipilimumab in advanced melanomaN Engl J Med2013369212213323724867
- VacchelliEGalluzziLEggermontATrial watch: FDA-approved Toll-like receptor agonists for cancer therapyOncoimmunology20121689490723162757
- EggermontAMSuciuSTestoriALong-term results of the randomized phase III Trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanomaJ Clin Oncol201230313810381823008300
- ClementJMMcDermottDFThe high-dose aldesleukin (IL-2) “SELECT” trial: a trial designed to prospectively validate predictive models of response to high-dose IL-2 treatment in patients with metastatic renal cell carcinomaClin Genitourin Cancer200972E7E919692326
- LipsonEJDrakeCGIpilimumab: an anti-CTLA-4 antibody for metastatic melanomaClin Cancer Res201117226958696221900389
- GoldmanBDeFrancescoLThe cancer vaccine roller coasterNat Biotechnol200927212913919204689
- RiceJOttensmeierCHStevensonFKDNA vaccines: precision tools for activating effective immunity against cancerNat Rev Cancer20088210812018219306
- LaroccaCSchlomJViral vector-based therapeutic cancer vaccinesCancer J201117535937121952287
- BartlettDLLiuZSathaiahMOncolytic viruses as therapeutic cancer vaccinesMol Cancer201312110324020520
- MockeyMBourseauEChandrashekharVmRNA-based cancer vaccine: prevention of B16 melanoma progression and metastasis by systemic injection of MART1 mRNA histidylated lipopolyplexesCancer Gene Ther200714980281417589432
- DikenMKreiterSSelmiATureciOSahinUAntitumor vaccination with synthetic mRNA: strategies for in vitro and in vivo preclinical studiesMethods Mol Biol201396923524623296938
- Fotin-MleczekMDuchardtKMLorenzCMessenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activityJ Immunother201134111521150709
- Heimburg-MolinaroJLumMVijayGJainMAlmogrenARittenhouse-OlsonKCancer vaccines and carbohydrate epitopesVaccine201129488802882621964054
- AlmandBResserJRLindmanBClinical significance of defective dendritic cell differentiation in cancerClin Cancer J20006517551766
- Della BellaSGennaroMVaccariMAltered maturation of peripheral blood dendritic cells in patients with breast cancerBr J Cancer20038981463147214562018
- ObermajerNMuthuswamyRLesnockJEdwardsRPKalinskiPPositive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cellsBlood2011118205498550521972293
- ObermajerNKalinskiPKey role of the positive feedback between PGE(2) and COX2 in the biology of myeloid-derived suppressor cellsOncoimmunology20121576276422934275
- NakamuraYWatchmakerPUrbanJHelper function of memory CD8+ T cells: heterologous CD8+ T cells support the induction of therapeutic cancer immunityCancer Res20076720100121001817942935
- WatchmakerPBUrbanJABerkEMemory CD8+ T cells protect dendritic cells from CTL killingJ Immunol200818063857386518322193
- CooperAMKhaderSAThe role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosisImmunol Rev200822619120419161425
- HaringJSBadovinacVPHartyJTInflaming the CD8+ T cell responseImmunity2006251192916860754
- KalinskiPOkadaHPolarized dendritic cells as cancer vaccines: directing effector-type T cells to tumorsSemin Immunol201022317318220409732
- HartlDKrauss-EtschmannSKollerBInfiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseasesJ Immunol2008181118053806719017998
- ZouWRegulatory T cells, tumour immunity and immunotherapyNat Rev Immunol20066429530716557261
- CurielTJCoukosGZouLSpecific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survivalNat Med200410994294915322536
- MuthuswamyRBerkEJuneckoBFNF-kappaB hyperactivation in tumor tissues allows tumor-selective reprogramming of the chemokine microenvironment to enhance the recruitment of cytolytic T effector cellsCancer Res201272153735374322593190
- ZouWMachelonVCoulomb-L’HerminAStromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cellsNat Med20017121339134611726975
- ObermajerNMuthuswamyROdunsiKEdwardsRPKalinskiPPGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environmentCancer Res201171247463747022025564
- KryczekIWeiSKellerELiuRZouWStroma-derived factor (SDF-1/CXCL12) and human tumor pathogenesisAm J Physiol Cell Physiol20072923C987C99516943240
- MeliefCJCancer immunotherapy by dendritic cellsImmunity200829337238318799145
- RabinovichGAGabrilovichDSotomayorEMImmunosuppressive strategies that are mediated by tumor cellsAnnu Rev Immunol20072526729617134371
- UchidaKSchneiderSYochimJMIntratumoral COX-2 gene expression is a predictive factor for colorectal cancer response to fluoropyrimidine-based chemotherapyClin Cancer Res20051193363336815867236
- BanerjeeDKDhodapkarMVMatayevaESteinmanRMDhodapkarKMExpansion of FOXP3 high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patientsBlood200610882655266116763205
- ViewegJSuZDahmPKusmartsevSReversal of tumor-mediated immunosuppressionClin Cancer Res2007132 Pt 2727s732s17255301
- SheikhNAPetrylakDKantoffPWSipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancerCancer Immunol Immunother201362113714722865266
- CheeverMAHiganoCSProvenge (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccineClin Cancer Res201117113520352621471425
- KantoffPWHiganoCSShoreNDSipuleucel-T immunotherapy for castration-resistant prostate cancerN Engl J Med2010363541142220818862
- HiganoCSSchellhammerPFSmallEJIntegrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancerCancer2009115163670367919536890
- ClinicalTrialsgovNational Institutes of HealthBethesda, MD, USA Available from: http://www.ClinicalTrials.govAccessed November 28, 2013
- HodiFSO’DaySJMcDermottDFImproved survival with ipilimumab in patients with metastatic melanomaN Engl J Med2010363871172320525992
- SchwartzentruberDJLawsonDHRichardsJMgp100 peptide vaccine and interleukin-2 in patients with advanced melanomaN Engl J Med2011364222119212721631324
- MiddletonGWValleJWWadsleyJA phase III randomized trial of chemoimmunotherapy comprising gemcitabine and capecitabine with or without telomerase vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancerJ Clin Oncol201331Suppl 15 Abstr LBA4004
- QuoixERamlauRWesteelVTherapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trialLancet Oncol201112121125113322019520
- HardacreJMMulcahyMSmallWAddition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 studyJ Gastrointest Surg20131719410023229886
- MorrisJCRossiGRHaroldNPotential chemo-sensitization effect of tergenpumatucel-L immunotherapy in treated patients with advanced non-small cell lung cancer (NSCLC)J Clin Oncol201331Suppl 15 Abstr 8094
- BandmanODelcayreALausRPROSPECT: A randomized, double-blind, phase III efficacy trial of PROSTVACJ Clin Oncol201230Suppl 15 Abstr TPS4699
- GiacconeGBazhenovaLNemunaitisJA phase III study of belagenpumatucel-L therapeutic tumor cell vaccine for non-small cell lung cancer (NSCLC) Available from: http://eccamsterdam2013.ecco-org.eu/Scientific-Programme/Abstract-search.aspx?abstractid=8961Accessed November 28, 2013
- ButtsCSocinskiMAMitchellPLTecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trialLancet Oncol2014151596824331154
- LawsonDHLeeSJTarhiniAAMargolinKAErnstoffMSKirkwoodJME4697: Phase III cooperative group study of yeast-derived granulocyte macrophage colony-stimulating factor (GM-CSF) versus placebo as adjuvant treatment of patients with completely resected stage III–IV melanomaJ Clin Oncol201028Suppl 15 Abstr 8504
- WalterSWeinschenkTStenzlAMultipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survivalNat Med20121881254126122842478
- TherassePVansteenkisteJFZielinskiMMAGRIT phase III trial: MAGE-A3 antigen-specific cancer immunotherapy (ASCI) as adjuvant therapy in patients with completely resected stage IB-IIIA NSCLCJ Clin Oncol201129Suppl 15 Abstr TPS210
- GlaxoSmithKlineThe investigational MAGE-A3 antigen-specific cancer immunotherapeutic does not meet first co-primary endpoint in Phase III melanoma clinical trial2013 Available from: http://www.gsk.com/media/press-releases/2013/the-investigational-mage-a3-antigen-specific-cancer-immunotherap.htmlAccessed January 14, 2014
- GlaxoSmithKlineUpdate on phase III clinical trial of investigational MAGE-A3 antigen-specific cancer immunotherapeutic in non-small cell lung cancer2014 Available from: http://www.gsk.com/media/press-releases/2014/update-on-phase-III-clinical-trial-of-investigational-MAGE-A3-antigen-specific-cancer-immunotherapeutic-in-non-small-cell-lung-cancer.htmlAccessed June 19, 2014
- Ulloa-MontoyaFLouahedJDizierBPredictive gene signature in MAGE-A3 antigen-specific cancer immunotherapyJ Clin Oncol201331192388239523715562
- VansteenkisteJZielinskiMLinderAAdjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study resultsJ Clin Oncol201331192396240323715567
- MortonDLMozzilloNThompsonJFAn international, randomized, phase III trial of bacillus Calmette-Guerin (BCG) plus allogeneic melanoma vaccine (MCV) or placebo after complete resection of melanoma metastatic to regional or distant sitesJ Clin Oncol200725Suppl 18 Abstr 8508
- HoshimotoSFariesMBMortonDLAssessment of prognostic circulating tumor cells in a phase III trial of adjuvant immunotherapy after complete resection of stage IV melanomaAnn Surg2012255235736222202581
- EggermontAMSuciuSRutkowskiPAdjuvant ganglioside GM2-KLH/QS-21 vaccination versus observation after resection of primary tumor .1.5 mm in patients with stage II melanoma: results of the EORTC 18961 randomized phase III trialJ Clin Oncol201331303831383724019551
- GulleyJLMadanRASchlomJImpact of tumour volume on the potential efficacy of therapeutic vaccinesCurr Oncol2011183e150e15721655153
- SchlomJTherapeutic cancer vaccines: current status and moving forwardJ Natl Cancer Inst2012104859961322395641
- WolchokJDHoosAO’DaySGuidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteriaClin Cancer Res200915237412742019934295
- SchlomJArlenPMGulleyJLCancer vaccines: moving beyond current paradigmsClin Cancer Res200713133776378217606707
- BilusicMGulleyJLEndpoints, patient selection, and biomarkers in the design of clinical trials for cancer vaccinesCancer Immunol Immunother201261110911722120693
- HarropRTreasurePde BelinJAnalysis of pre-treatment markers predictive of treatment benefit for the therapeutic cancer vaccine MVA-5T4 (TroVax)Cancer Immunol Immunother201261122283229422692758
- SkachkovaOVKhranovskaNMGorbachOISvergunNMSydorRINikulinaVVImmunological markers of anti-tumor dendritic cells vaccine efficiency in patients with non-small cell lung cancerExp Oncol201335210911323828386
- CornforthANLeeGJFowlerAWCarbonellDJDillmanROIncreases in serum TARC/CCL17 levels are associated with progression-free survival in advanced melanoma patients in response to dendritic cell-based immunotherapyJ Clin Immunol200929565766419421847
- LopezMNPeredaCSegalGProlonged survival of dendritic cell-vaccinated melanoma patients correlates with tumor-specific delayed type IV hypersensitivity response and reduction of tumor growth factor beta-expressing T cellsJ Clin Oncol200927694595219139436
- OkadaHKalinskiPUedaRInduction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant gliomaJ Clin Oncol201129333033621149657
- CarrenoBMBecker-HapakMHuangAIL-12p70-producing patient DC vaccine elicits Tc1-polarized immunityJ Clin Invest201312383383339423867552
- PaluckaKUenoHBanchereauJRecent developments in cancer vaccinesJ Immunol201118631325133121248270
- HerberDLCaoWNefedovaYLipid accumulation and dendritic cell dysfunction in cancerNat Med201016888088620622859
- Pinzon-CharryAMaxwellTLopezJADendritic cell dysfunction in cancer: a mechanism for immunosuppressionImmunol Cell Biol200583545146116174093
- DillmanROCornforthANDepriestCTumor stem cell antigens as consolidative active specific immunotherapy: a randomized phase II trial of dendritic cells versus tumor cells in patients with metastatic melanomaJ Immunother201235864164922996370
- KalinskiPDendritic cells in immunotherapy of established cancer: roles of signals 1, 2, 3 and 4Curr Opin Investig Drugs2009106526535
- BanchereauJSteinmanRMDendritic cells and the control of immunityNature199839266732452529521319
- ChiangCLKandalaftLETanyiJA dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedsideClin Cancer Res201319174801481523838316
- ReyesDSalazarLEspinozaETumour cell lysate-loaded dendritic cell vaccine induces biochemical and memory immune response in castration-resistant prostate cancer patientsBr J Cancer201310961488149723989944
- StromeSEVossSWilcoxRStrategies for antigen loading of dendritic cells to enhance the antitumor immune responseCancer Res20026261884188911912169
- AlbertMLSauterBBhardwajNDendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLsNature1998392667186899510252
- KalinskiPMuthuswamyRUrbanJDendritic cells in cancer immunotherapy: vaccines and combination immunotherapiesExp Rev Vaccines2013123285295
- AndersenBMOhlfestJRIncreasing the efficacy of tumor cell vaccines by enhancing cross primingCancer Lett2012325215516422809568
- GrakouiABromleySKSumenCThe immunological synapse: a molecular machine controlling T cell activationScience1999285542522122710398592
- HawigerDInabaKDorsettYDendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivoJ Exp Med2001194676977911560993
- de VriesIJLesterhuisWJScharenborgNMMaturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patientsClin Cancer Res20039145091510014613986
- AdemaGJde VriesIJPuntCJFigdorCGMigration of dendritic cell based cancer vaccines: in vivo veritas?Curr Opin Immunol200517217017415766677
- ReddyASappMFeldmanMSubkleweMBhardwajNA monocyte conditioned medium is more effective than defined cytokines in mediating the terminal maturation of human dendritic cellsBlood1997909364036469345048
- JonuleitHKuhnUMullerGPro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditionsEur J Immunol19972712313531429464798
- SchadendorfDUgurelSSchuler-ThurnerBDacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOGAnn Oncol200617456357016418308
- KalinskiPHilkensCMWierengaEAKapsenbergMLT-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signalImmunol Today1999201256156710562707
- KalinskiPRegulation of immune responses by prostaglandin E2J Immunol20121881212822187483
- WatchmakerPBBerkEMuthuswamyRIndependent regulation of chemokine responsiveness and cytolytic function versus CD8+ T cell expansion by dendritic cellsJ Immunol2010184259159720018619
- MailliardRBSonYIRedlingerRDendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper functionJ Immunol200317152366237312928383
- MailliardRBEgawaSCaiQComplementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responsesJ Exp Med2002195447348311854360
- BerkEKalinskiPLymphocyte-polarized DC1s: Effective inducers of tumor-specific CTLsOncoimmunology2012181443144423243623
- BerkEMuthuswamyRKalinskiPLymphocyte-polarized dendritic cells are highly effective in inducing tumor-specific CTLsVaccine201230436216622422561311
- MailliardRBWankowicz-KalinskaACaiQalpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activityCancer Res200464175934593715342370
- VieiraPLde JongECWierengaEAKapsenbergMLKalinskiPDevelopment of Th1-inducing capacity in myeloid dendritic cells requires environmental instructionJ Immunol200016494507451210779751
- WesaAKalinskiPKirkwoodJMTatsumiTStorkusWJPolarized type-1 dendritic cells (DC1) producing high levels of IL-12 family members rescue patient TH1-type antimelanoma CD4+ T cell responses in vitroJ Immunother2007301758217198085
- RosesREXuSXuMKoldovskyUKoskiGCzernieckiBJDifferential production of IL-23 and IL-12 by myeloid-derived dendritic cells in response to TLR agonistsJ Immunol200818175120512718802116
- PaustianCCaspellRJohnsonTEffect of multiple activation stimuli on the generation of Th1-polarizing dendritic cellsHum Immunol2011721243120951755
- Ten BrinkeAKarstenMLDiekerMCZwagingaJJvan HamSMThe clinical grade maturation cocktail monophosphoryl lipid A plus IFNgamma generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarizationVaccine200725417145715217719152
- Lopez-AlbaiteroAMailliardRHackmanTMaturation pathways of dendritic cells determine TAP1 and TAP2 levels and cross-presenting functionJ Immunother200932546547319609238
- WieckowskiEChattaGSMailliardRMType-1 polarized dendritic cells loaded with apoptotic prostate cancer cells are potent inducers of CD8(+) T cells against prostate cancer cells and defined prostate cancer-specific epitopesProstate201171212513320717900
- LeeJJFoonKAMailliardRBMuthuswamyRKalinskiPType 1- polarized dendritic cells loaded with autologous tumor are a potent immunogen against chronic lymphocytic leukemiaJ Leukoc Biol200884131932518426971
- MuthuswamyRMueller-BerghausJHaberkornUReinhartTASchadendorfDKalinskiPPGE(2) transiently enhances DC expression of CCR7 but inhibits the ability of DCs to produce CCL19 and attract naive T cellsBlood201011691454145920498301
- MuthuswamyRUrbanJLeeJJReinhartTABartlettDKalinskiPAbility of mature dendritic cells to interact with regulatory T cells is imprinted during maturationCancer Res200868145972597818632653
- GustafssonKIngelstenMBergqvistLNystromJAnderssonBKarlsson-ParraARecruitment and activation of natural killer cells in vitro by a human dendritic cell vaccineCancer Res200868145965597118632652
- KunzMToksoyAGoebelerMEngelhardtEBrockerEGillitzerRStrong expression of the lymphoattractant C-X-C chemokine Mig is associated with heavy infiltration of T cells in human malignant melanomaJ Pathol1999189455255810629557
- CalzasciaTMassonFDi Berardino-BessonWHoming phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCsImmunity200522217518415723806
- WenzelJBekischBUerlichMHallerOBieberTTutingTType I interferon-associated recruitment of cytotoxic lymphocytes: a common mechanism in regressive melanocytic lesionsAm J Clin Pathol20051241374815923172
- SalamaPPhillipsMGrieuFTumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancerJ Clin Oncol200927218619219064967
- SenovillaLVacchelliEGalonJTrial watch: prognostic and predictive value of the immune infiltrate in cancerOncoimmunology2012181323134323243596
- FridmanWHPagesFSautes-FridmanCGalonJThe immune contexture in human tumours: impact on clinical outcomeNat Rev Cancer201212429830622419253
- GalonJCostesASanchez-CaboFType, density, and location of immune cells within human colorectal tumors predict clinical outcomeScience200631357951960196417008531
- LazennecGRichmondAChemokines and chemokine receptors: new insights into cancer-related inflammationTrends Mol Med201016313314420163989
- LeDTJaffeeEMRegulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspectiveCancer Res201272143439344422761338
- ChuCSBoyerJSchulleryDSPhase I/II randomized trial of dendritic cell vaccination with or without cyclophosphamide for consolidation therapy of advanced ovarian cancer in first or second remissionCancer Immunol Immunother201261562964122021066
- MorseMAHobeikaACOsadaTDepletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccinesBlood2008112361061818519811
- JacobsJFPuntCJLesterhuisWJDendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patientsClin Cancer Res201016205067507820736326
- IclozanCAntoniaSChiapporiAChenDTGabrilovichDTherapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancerCancer Immunol Immunther2013625909918
- OuXCaiSLiuPEnhancement of dendritic cell-tumor fusion vaccine potency by indoleamine-pyrrole 2,3-dioxygenase inhibitor, 1-MTJ Cancer Res Clin Oncol2008134552553317909857
- BasuGDTinderTLBradleyJMCyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDOJ Immunol200617742391240216888001
- ZhangHTianMXiuCWangYTangGEnhancement of antitumor activity by combination of tumor lysate-pulsed dendritic cells and celecoxib in a rat glioma modelOncol Res2013201044745524308155
- TakaokaKHidakaSHashitaniSEffect of a nitric oxide synthase inhibitor and a CXC chemokine receptor-4 antagonist on tumor growth and metastasis in a xenotransplanted mouse model of adenoid cystic carcinoma of the oral floorInt J Oncol201343373774523835861
- SantegoetsSJTurksmaAWPowellDJJrHooijbergEde GruijlTDIL-21 in cancer immunotherapy: at the right place at the right timeOncoimmunology201326e2452223894713
- PernaSKPagliaraDMahendravadaAInterleukin-7 mediates selective expansion of tumor-redirected cytotoxic T lymphocytes (CTLs) without enhancement of regulatory T-cell inhibitionClin Cancer Res201420113113924097874
- MackallCLFryTJGressREHarnessing the biology of IL-7 for therapeutic applicationNat Rev2011115330342
- RosenbergSASportesCAhmadzadehMIL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cellsJ Immunother200629331331916699374
- WaldmannTAThe biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine designNat Rev Immunol20066859560116868550
- SteelJCWaldmannTAMorrisJCInterleukin-15 biology and its therapeutic implications in cancerTrends Pharm Sci2012331354122032984
- SkakKKraghMHausmanDSmythMJSivakumarPVInterleukin 21: combination strategies for cancer therapyNat Rev Drug Discov20087323124018259184
- FrederiksenKSLundsgaardDFreemanJAIL-21 induces in vivo immune activation of NK cells and CD8(+) T cells in patients with metastatic melanoma and renal cell carcinomaCancer Immunol Immunother200857101439144918286285
- PetrellaTMTozerRBelangerKInterleukin-21 has activity in patients with metastatic melanoma: a phase II studyJ Clin Oncol201230273396340122915661
- ThompsonJACurtiBDRedmanBGPhase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinomaJ Clin Oncol200826122034203918347008
- SchmidtHBrownJMouritzenUSafety and clinical effect of subcutaneous human interleukin-21 in patients with metastatic melanoma or renal cell carcinoma: a phase I trialClin Cancer Res201016215312531920959407
- HashmiMHVan VeldhuizenPJInterleukin-21: updated review of Phase I and II clinical trials in metastatic renal cell carcinoma, metastatic melanoma and relapsed/refractory indolent non-Hodgkin’s lymphomaExpert Opin Biol Ther201010580781720384523
- MurugaiyanGSahaBIL-27 in tumor immunity and immunotherapyTrends Mol Med201319210811623306374
- SwarbrickAJunankarSRBattenMCould the properties of IL-27 make it an ideal adjuvant for anticancer immunotherapy?Oncoimmunology201328e2540924083081
- WadaSJacksonCMYoshimuraKSequencing CTLA-4 blockade with cell-based immunotherapy for prostate cancerJ Transl Med2013118923557194
- LeDTLutzEUramJNEvaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancerJ Immunother201336738238923924790
- MadanRAMohebtashMArlenPMIpilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trialLancet Oncol201213550150822326924
- RibasAComin-AnduixBChmielowskiBDendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanomaClin Cancer Res200915196267627619789309
- PardollDMThe blockade of immune checkpoints in cancer immunotherapyNat Rev Cancer201212425226422437870
- BrahmerJRDrakeCGWollnerIPhase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlatesJ Clin Oncol201028193167317520516446
- BrahmerJRTykodiSSChowLQSafety and activity of anti-PD-L1 antibody in patients with advanced cancerN Engl J Med2012366262455246522658128
- LipsonEJSharfmanWHDrakeCGDurable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibodyClin Cancer Res201319246246823169436
- SierroSRDondaAPerretRCombination of lentivector immunization and low-dose chemotherapy or PD-1/PD-L1 blocking primes self-reactive T cells and induces anti-tumor immunityEur J Immunol20114182217222821538347
- RosenblattJGlotzbeckerBMillsHPD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccineJ Immunother201134540941821577144
- LiBVanRoeyMWangCChenTHKormanAJoossKAnti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor- secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumorsClin Cancer Res20091551623163419208793
- DuraiswamyJKaluzaKMFreemanGJCoukosGDual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumorsCancer Res201373123591360323633484
- WeberJSKudchadkarRRGibneyGTPhase I/II trial of PD-1 antibody nivolumab with peptide vaccine in patients naive to or that failed ipilimumabJ Clin Oncol201331Suppl 15 Abstr 9011
- MkrtichyanMNajjarYGRaulfsECAnti-PD-1 synergizes with cyclophosphamide to induce potent anti-tumor vaccine effects through novel mechanismsEur J Immunol201141102977298621710477
