Abstract
High-mobility group box 1 (HMGB1) protein is a member of the highly conserved non-histone DNA binding protein family. First identified in 1973, as one of a group of chromatin-associated proteins with high acidic and basic amino acid content, it was so named for its characteristic rapid mobility in polyacrylamide gel electrophoresis. HMGB1 was later discovered to have another function. It is released from a variety of cells into the extracellular milieu to act on specific cell-surface receptors. In this latter role, HMGB1 is a proinflammatory cytokine that may contribute to many inflammatory diseases, including sepsis. Therefore, HMGB1 regulates intracellular cascades influencing immune cell functions, including chemotaxis and immune modulation. The bioactivity of the HMGB1 is determined by specific posttranslational modifications that regulate its role in inflammation and immunity. During tumor development, HMGB1 has been reported to play paradoxical roles in promoting both cell survival and death by regulating multiple signaling pathways. In this review, we focus on the role of HMGB1 in physiological and pathological responses, as well as the mechanisms by which it contributes to immunity, inflammation, and cancer progression.
Introduction
The high-mobility group box 1 (HMGB1), a member of the high-mobility group (HMG) protein family, was identified in calf thymus in 1973 by Goodwin et al.Citation1 The name “HMG” is derived from its high electrophoretic mobility in polyacrylamide gels.
HMG proteins are subdivided into three families named HMGB, HMGA, and HMGN. All HMGB proteins, including HMGB1–4, contain the functional HMG-box motif, have architectural functions, and are capable of organizing dynamic active chromatin structures. In adults, HMGB1 is expressed everywhere except in neurons, whereas HMGB2 and HMGB4 are expressed in the testes, and HMGB3 primarily in lymphoid organs.Citation2 Prior to its naming, HMGB1 was also known as HMG1, amphoterin, and SBP-1.Citation3
HMGB1 senses and coordinates the cellular stress response and plays a critical role not only inside of the cell as a DNA chaperone, chromosome guardian, autophagy sustainer, and protector from apoptotic cell death, but also outside the cell as the prototypic damage-associated molecular pattern molecule (DAMP).Citation4
Therefore, to elucidate the pleiotropic roles of HMGB1, in this review, we will emphasize the characteristics that make HMGB1 a critical molecular target in diseases including infectious diseases, ischemia, immune disorders, neurodegenerative diseases, metabolic disorders, and cancer.
HMGB1: structural characteristics
HMGB1 is expressed in all vertebrate cells, in yeast, plants, and bacteria, localized in cytoplasm and nuclei. The protein abundance is high in thymus (106 molecules/cell) and in undifferentiated tissues; it is highly conserved through evolution, and has 99% identity among all mammals. It probably originated more than 500 million years ago, before the split between the animal and plant kingdoms.Citation5
HMGB1 structure is composed of two tandem DNA-binding HMG-box domains (N-terminal A and central B) and an acidic C-terminal tail, mediating protein–protein interactions ().
Figure 1 Structure of HMGB1 protein.
Abbreviations: Cys, cysteine; HMGB1, high-mobility group box 1.
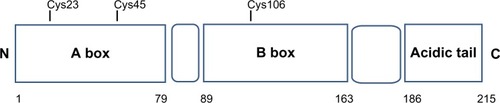
HMGB1 is a 215 amino acid protein of 30 kDa, composed of three domains: two positively charged domains (A box and B box) and a negatively charged carboxyl terminus (acidic tail). This structure confers onto HMGB1 the peculiar feature to recognize and specifically bind DNA structures, containing sharp bends or kinks, such as four-way junctions.Citation6,Citation7
Structurally, HMGB1 consists of 215 residues organized in three main functional domains: the A and B boxes (positively charged) and the acidic tail (negatively charged). The A and B boxes, residues 1–79 and 89–163, respectively, are functionally DNA-binding domains.Citation8,Citation9
This region is composed of α-helical structures; in the nucleus, HMGB1 binds to the minor groove of linear DNA and bends it into a helical structure.Citation10 The C-terminal acidic tail, residues 186–215, plays an important role in nuclear functions.Citation11,Citation12
At the N-terminus of HMGB1 (residues 6–12) is a heparin-binding sequence that likely contributes to the heparin and heparin sulfate binding capacity of HMGB1.Citation12
A C-terminal region of HMGB1 at amino acids 150–183 mediates the receptor for advanced glycation end-product (RAGE) and Toll-like receptor (TLR) binding.Citation13
The RAGE is a cell-bound receptor of the immunoglobulin superfamily that is activated by a variety of proinflammatory ligands, including HMGB1, advanced glycation end-products, members of the S100 family of proteins, and amyloid β-peptide.Citation14
Three cysteines (Cys) residues, crucial for the biological activities of extracellular HMGB1, are encoded within the HMGB1: two vicinal Cys in box A (C23 and C45) and a single one in box B (C106).Citation15,Citation16
Extracellular HMGB1 can bind and activate different signaling transduction cell receptors, such as the receptor RAGE; the TLRs 2, 4, and 9; macrophage antigen-1; syndecan-3; CD24-Siglec-10; CXCR4; and certain integrin.Citation17
RAGE is expressed in endothelial cells, vascular smooth muscle cells, monocytes, macrophages, dendritic cells, neurons, and glial cells.Citation18 The interaction of RAGE–HMGB1 leads to the activation of different signal transduction pathways and culminates in cell responses ranging from cell motility to cell proliferation, as well as the production and release of cytokines/chemokines. RAGE blockade suppresses the HMGB1 effects, but only partially, strongly suggesting the existence of additional receptors for HMGB1. TLR2 and 4 are involved in HMGB1-induced proinflammatory activation of macrophages and neutrophils. TLRs blockade does not completely abolish HMGB1 effect.
Recently, new receptor-mediating HMGB1 effects were demonstrated;Citation19 these authors evaluated that at concentrations of agonist that were per se ineffective, HMGB1 potentiates the activation of the ionotropic glutamate N-methyl-d-aspartate receptor, interacting with the ion channel through the sequence corresponding to the peptide located in the box B at the amino acids 130–139.Citation9
Known roles in DNA replication, DNA repair, and transcription
HMGB1 is a member of the HMG family whose nuclear localization and affinity for DNA is regulated through phosphorylation and acetylation. HMGB1 has a dynamic relationship with chromatin and plays an important role in DNA architecture and transcriptional regulation.Citation20
HMGB1 is stored in the nucleus as a result of the presence of two lysine-rich nuclear localization sequences (NLSs) located in the box A (residues 28–44) and in the box B (residues 179–185). Hyperacetylation of NLSs promotes HMGB1 translocation from the nucleus to the cytosol, and the subsequent release of HMGB1.Citation21
The subcellular localization of HMGB1 depends on the type and activation state of the cell. The HMGB1 is usually localized in the cell nucleus and diffusely distributed in cytoplasm.Citation22 HMGB1 can be released from the nucleus to the extracellular space in response to different stimuli in two ways: following cellular injury it is passively released during cellular apoptosis or necrosis; or it is released actively following inflammatory signals from activated immune cells or neuronal cells. Hyperacetylated HMGB1 molecules accumulate in the cytosol where they are packaged into secretory lysosomes and then released into the extracellular environment.Citation23
HMGB1 binds the minor groove of DNA facilitating the assembly of site-specific DNA-binding proteins, including nuclear hormone/nuclear hormone receptor complexes and p53 or p73 transcriptional complexes.Citation24
HMGB1 is involved in the facilitation of protein–protein interaction and recognition of DNA damage in the process of mismatch repair.Citation25
HMGB1 is the structural protein of chromatin and regulates nuclear homeostasis and genome stability in several ways: HMGB1 binds to nucleosomes at the dyad axis, promotes nucleosome sliding, relaxes nucleosome structure, and makes chromatin more accessible by its ability to bend DNA.Citation26
HMGB1 has been found to increase the binding affinity of many sequence-specific transcription factors to their cognate DNA, such as p53, p73, the retinoblastoma (Rb) protein, nuclear factor-κB (NF-κB), and the estrogen receptor.
HMGB1 directly binds to a variety of bulky DNA lesions which allows it to participate in DNA repair pathways including nucleotide excision repair, base excision repair, mismatch repair, and double strand break repair via nonhomologous end-joining.
Loss of HMGB1 reduces telomerase activity, decreases telomere length, and increases chromosomal instability.Citation27
HMGB1 as an alarmin: roles in inflammation, response to infection, and wound repair
For HMGB1 to function as a cytokine, it must be released into the extracellular milieu. HMGB1 is released passively during cellular necrosis by almost all cells that have a nucleus and signals neighboring cells of ongoing damage.
In 1999, Wang et al first reported that HMGB1 was liberated from cells stimulated with cytokines and that HMGB1 played an important role in mediating experimental sepsis.Citation28 Then, Scaffidi et alCitation29 showed that HMGB1 is released from cells undergoing necrosis, but not from apoptotic cells, because HMGB1 is tightly bound to chromatin.
Recent reports demonstrate that HMGB1 can also be released by apoptotic cells,Citation30 but in this case, the released HMGB1 appears to have a tolerogenic rather than a proinflammatory effect.Citation31
Active secretion of HMGB1 is generally by nontraditional, leaderless pathways which are not routed through the endoplasmic reticulum or Golgi apparatus. During normal cellular homeostasis the dynamic relationship of HMGB1 with the nucleus and cytoplasm heavily favors the nucleus. However, HMGB1 concentrates in secretory lysosomes when hyperacetylated on lysine residues and then is released upon appropriate signaling stimuli.Citation28
When HMGB1 is not acetylated, it remains localized to the nucleus and is not secreted or released, such as during apoptosis.Citation31
Because HMGB1 does not contain a leader sequence, it is released via a nonclassical secretory pathway that may involve specialized vesicles of the endolysosomal compartment. Stimuli for secretion of HMGB1 are diverse and include pathogen-associated molecular patterns, cytokines, and certain states of cellular stress.
HMGB1 can promote inflammatory responses by numerous mechanisms. Comparisons of the necrotic cell debris from HMGB1-deficient and wild-type cells demonstrate that HMGB1-deficient cells have a profoundly reduced capacity to induce cytokines.Citation29
Recombinant HMGB1 also induces cytokine production from human monocytes but not from lymphocytes.Citation32 However, several groups have more recently shown that highly purified HMGB1 does not induce significant amounts of proinflammatory cytokines.Citation6
However, it should also be noted that recombinant HMGB1 may be different from the native protein, with changes in the oxidative status having a profound impact on its biological activity.Citation31
HMGB1 is not released only from cells of the immune system; for other type of cells, it has been demonstrated an active secretion pathway. The first nonimmune cell to be studied for active HMGB1 secretion was the pituicyte, which was found to release HMGB1 in response to IL-1 or TNF stimulation.Citation28
Sepsis is a lethal syndrome after infection or injury, associated with a high mortality rate,Citation33 and proinflammatory cytokines released from monocyte/macrophages and other cells have been implicated as important mediators of the lethal effect of endotoxin.Citation28 Proinflammatory cytokines can lead to the development of tissue damage, metabolic acidosis, hypotension, multiple organ failure, and even death.Citation33
Endogenous danger signals released from necrotic or stressed cells that trigger the inflammatory response after trauma have been termed alarmins or danger-associated molecular patterns (DAMPs).
HMGB1 was recognized as a late mediator of sepsis in animal models of systemic endotoxemia.Citation28 Therapy with anti-HMGB1 antibodies before or after endotoxin exposure confers significant protection against lethality, and administration of HMGB1 itself was lethal, suggesting that HMGB1 is an endogenous mediator of endotoxin lethality.Citation28
Some research reported that cytokines may stimulate release of HMGB1 from pituicytes and astrocytes.Citation34 These findings suggest a role for HMGB1 in neurological disorders. It also demonstrates that HMGB1 is highly expressed in mouse brain neurons and astrocytes, is released during brain ischemia, and contributes to neuroinflammation and postischemic brain damage.Citation35
Clinical studies have demonstrated that HMGB1 is a late mediator of sepsis and some investigations have reported elevated serum/plasma HMGB1 concentrations in patients with sepsis.Citation28
Until now, it was not known to what extent local HMGB1 release contributes to serum HMGB1 levels, and to what extent it contributes to damage at the site of infection and to distant organs in patients with sepsis.Citation33 It has been reported that genetic variation in the HMGB1 gene could affect the outcome of patients with systemic inflammatory response syndrome and sepsis, suggesting a possible role for HMGB1 genetics.Citation36
More recently, HMGB1 has been associated, as a putative danger signal, to pathogenesis of a variety of noninfectious inflammatory conditions, ie, autoimmunity, trauma, hemorrhagic shock, and ischemia-reperfusion injury.Citation37 Furthermore, a role for HMGB1 has been suggested in some normal and pathological conditions for a huge number of organs and systems including liver, heart, pancreas, brain, bone, and kidney.Citation38 In fact, HMGB1 levels are elevated in patients with mechanical trauma, such as strokes, acute myocardial infarction, acute respiratory distress, and liver transplantation.Citation39,Citation40 This pleiotropic inflammatory role for HMGB1 could be due to the existence of multiple receptors that mediate HMGB1 responses. The different responses to HMGB1 stimulation may rely on the levels of HMGB1, on the expression of the receptors in the target organ, and on the ability of HMGB1 to interact with other molecules. Potentially, there is significant cross talk between receptors, and the deficient or defective receptor may influence the function of other receptors.Citation41
There is growing interest in the role of alarmins, such as HMGB1, in contrast to their role in promoting inflammation, to promote tissue repair and regeneration.Citation42 HMGB1 may act as a trigger of tissue repair, recruiting stem cells and promoting their proliferation.Citation43
The physiological meaning of the role of HMGB1 in wound healing remains to be elucidated. For cutaneous wound healing, some studiesCitation42,Citation44 allowed us to postulate that HMGB1 release may occur from skin cells as the result of cell activation, enhanced membrane permeability, or membrane ruptures. After its release in the wounded area, HMGB1 could operate the recruitment of different cell types, influencing keratinocyte and fibroblast proliferation and migration.Citation42
Taken together, the regenerative properties of HMGB1 represent a potentially important way by which the functions of such alarmin can be manipulated to promote healing as opposed to injury.
Emerging roles in innate and adaptive immunity and autoimmunity
It has also been found that HMGB1 behaves as an endogenous immune adjuvant.Citation45 HMGB1 elicits a striking increase in the titers of high-affinity hypermutated IgG when coinjected with the soluble nominal antigen ovalbumin. Moreover, it was sufficient to reconstitute the immunogenicity of apoptotic “self” cells in vivo.Citation46
HMGB1 triggers inflammation, attracting other cells, inducing tissue repair, recruiting stem cells, and promoting their proliferation. HMGB1 also activates dendritic cells and promotes their functional maturation and their response to lymph node chemokines. Therefore, HMGB1 acts in an autocrine/paracrine fashion and sustains long-term repair and defense programs. HMGB1 secretion is critical for the immunity system because dendritic cells, when reaching the lymph nodes, secrete HMGB1, sustaining the proliferation of antigen-specific T-cells, to prevent their activation-dependent apoptosis, and to promote their polarization toward a T-helper 1 phenotype.Citation46
Considerable evidence suggests the implication of extracellular HMGB1 in the pathogenesis of a variety of autoimmune diseases.
Anti-HMGB1 antibodies are present in the serum of patients with rheumatoid arthritis and drug-induced systemic lupus erythematosus.Citation47 HMGB1 is overexpressed in synovial biopsy specimens of rheumatoid patients and in experimental arthritis.Citation48
HMGB1 induces arthritis in mice, while treatment with HMGB1 antagonists ameliorates collagen-induced arthritis in both rats and mice.Citation49
Increased extracellular HMGB1 is also detectable in the dermis and epidermis of skin lesions in patients with cutaneous lupus erythematosus and in biopsy specimens of the minor salivary glands of patients with Sjogren’s syndrome.Citation50
In multiple sclerosis, HMGB1 expression was found to be upregulated in brain active lesions from patients.Citation51 In the animal model of multiple sclerosis, experimental autoimmune encephalomyelitis, neutralization of HMGB1 ameliorated clinical severity, reduced central nervous system pathology, and blocked proinflammatory cytokine production.Citation52
New recent data support a novel concept of brain–immune interaction after acute brain lesions in which soluble danger signals from the brain mediate a complex immune-modulatory reaction in the peripheral immune system.Citation53,Citation54 The HMGB1-RAGE-mediated pathway could be a key mechanism explaining the complex postischemic brain–immune interactions. This mechanism indicates that the cytokine-inducing, fully reduced isoform of HMGB1 was released from the ischemic brain in the hyperacute phase of stroke in mice and patients. Cytokines secreted in the periphery in response to brain injury induced sickness behavior, which could be abrogated by inhibition of the HMGB1-RAGE pathway or direct cytokine neutralization.Citation54 Therefore, HMGB1 in the central nervous system could a useful biomarker and may contribute to the chronicity of neuroinflammation through the proposed positive-feedback loop involving infiltrating macrophages and resident microglia.Citation51
Posttranslational modifications and molecular interactions in HMGB1 function
HMGB1 contains three Cys residues, which are critical for the biological activity of the protein (). Cys are modified by different redox signals with oxidation of thiol group side-chains (-SH) to several reversible redox states, such as disulfide (R-S-S-R), sulfenic acid (R-SOH), and sulfonate (R-SO3H) moieties.Citation37
Figure 2 Redox modulation of HMGB1 activity.
Abbreviation: HMGB1, high-mobility group box 1.
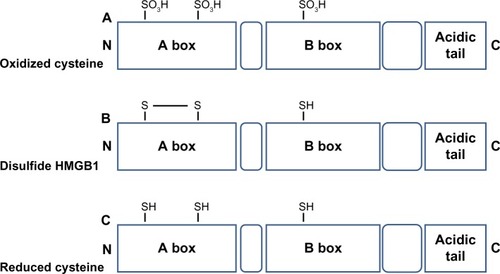
HMGB1 can form a Cys23–Cys45 intermolecular disulfide bond, while the Cys106 remains in the reduced form.Citation15
Replacement of Cys23 and/or Cys45 with serines did not affect the nuclear distribution of the mutant proteins, whereas C106 and triple Cys mutations impaired nuclear localization of HMGB1.Citation55
The redox status of HMGB1 recognized its cytokine-inducing and chemokine activity.Citation16 C106 is necessary for the binding of HMGB1 to TLR4 to stimulate cytokine release and inflammation.Citation56
Recent observations suggested that the disulfide bond between C23 and C45 is also required for HMGB1 cytokine activity.Citation16 Indeed, mutations of C45 or C23 abolish HMGB1 cytokine-inducing activity.
Reactive oxygen species oxidizes HMGB1 at C106 released from apoptotic cells, thereby neutralizing its cytokine potential.Citation31 All-cysteine-oxidized HMGB1 conformation prevents HMGB1’s cytokine or chemokine activity.Citation16 Therefore, the redox modifications of HMGB1 play a crucial role for the protein functionality during infection and inflammation.Citation56
The complex and fine-tuned process of autophagy is also regulated by HMGB1 activities. During stress, starvation, oxidative stress, and chemotherapy, HMGB1 translocate from nucleus to cytoplasm for binding beclin-1, which initiates the autophagosome process. The intramolecular disulfide bond (C23–C45) of HMGB1 is required for binding beclin-1. Once released, this reduced-HMGB1 triggers autophagy in a RAGE-dependent manner in cancer cells.Citation56
The functional in vivo relevance of redox-modified HMGB1 has been confirmed in experimental animal models.Citation20
HMGB1 may undergo other extensive posttranslational modifications, including reversible acetylation, methylation, ADP ribosylation, glycation, and phosphorylation.Citation57
Acetylation of key lysine residues within the two NLS sites of HMGB1 is a regulatory mechanism for intracellular shuttling, allowing the release of protein from activated monocytes and macrophages.Citation58 Hyperacetylation of these residues unbalances the HMGB1 from its nuclear position toward cytoplasmic accumulation and its continuous shuttling between nucleus and cytoplasm.Citation59 The acetylated HMGB1 in serum of patients is a specific biomarker of acetaminophen-induced hepatotoxicity that is useful for clinical outcome.Citation60 Other findings suggest that the hyperacetylated HMGB1 is a newly identified biomarker for pyroptosis. Pyroptosis is a proinflammatory cell-death mode associated with osmotic lysis and the release of the intracellular content into the extracellular milieu.Citation61 Redox status of three Cys of HMGB1 suggest that HMGB1 released during pyroptosis may contain active (disulfide-bonded) form, whereas HMGB1 during necrosis may contain all-thiol forms. Moreover, other observations revealed that HMGB1 released during pyroptosis is acetylated. So, HMGB1 released during pyroptosis can subsequently be detected by TLR4 and RAGE receptors to stimulate cytokine release and cell migration.Citation61,Citation62
Emerging roles in the pathogenesis of cancer: HMGB1 as a therapeutic target?
There is growing evidence that HMGB1 may be associated with hallmarks of cancer such as: unlimited replicative potential ability to develop blood vessels (angiogenesis); evasion of programmed cell death (apoptosis); self-sufficiency in growth signals; insensitivity to inhibitors of growth; inflammation; tissue invasion and metastasis; and enhancement of inflammation.Citation63
HMGB1 is one of the most cancer-specific genes. Overexpression of HMGB1 in tumor tissue and increased HMGB1 serum level are near-universal in solid tumors, including colon, gastric, lung, breast, ovarian, pancreatic, and prostate cancer.Citation27
Serum HMGB1 levels were significantly associated with depth of invasion, lymph node metastasis, tumor size, and poor prognosis. The selective upregulation of HMGB1 in tumors contrasts with the other DAMPs, which are expressed under normal conditions and display only modest increased expression in malignancy.Citation64
Interestingly, the mechanisms by which HMGB1 expression is normally suppressed are not fully elucidated. We know that HMGB1 can associate with other molecules, including DNA and cytokines, and activate cells through the differential surface receptors such as TLR4, RAGE, CD24, and TIM-3, influencing the cancer cell’s fate through the cross talk of many different pathways.Citation65
HMGB1 functions as a tumor suppressor in breast cancer, binding to well-known tumor suppressor protein Rb,Citation66 causing Rb-dependent G1 arrest and apoptosis induction. The overexpression of HMGB1 inhibits Rb-positive breast cancer cells growth in vitro, also preventing growth in in vivo tumor models.Citation67
Genomic instability is a hallmark of most cancers.Citation68 Some studies also demonstrate that mammalian HMGB1 and its yeast homologue are critical for maintaining genome stability.Citation68,Citation69 High levels of aneuploidy and spontaneous chromosome aberrations were observed in HMGB1-deficient mouse embryonic fibroblasts (MEFs).Citation69
HMGB1 is involved in major DNA repair pathways, but it could interact with telomerase catalytic component as well as DNA repair enzymes and cofactors to regulate telomere length and DNA repair efficiency.Citation70 The complex roles of HMGB1 in coordinating DNA repair and genomic stability should be further elucidated.
The tumor growth is normally accompanied by reduced microvessel density, therefore resulting in chronic hypoxia. These hypoxic and necrotic regions exhibit increased expression of angiogenic growth factors, recruiting macrophages which produce some angiogenetic cytokines and growth factors.Citation71
Activation of HMGB1 and its receptor RAGE results in the activation of NF-κB pathway, which in turn upregulates leukocyte adhesion molecules and the production of proinflammatory cytokines and angiogenic factors, thereby promoting inflammation and angiogenesis.Citation72 Antibody-targeting HMGB1 inhibits angiogenesis in vitro and in vivo.Citation72
Several HMGB1-targeting agents have been already used in cancer research. These agents include soluble RAGE, HMGB1-neutralizing antibody, platinating agent, ethyl pyruvate, quercetin, and glycyrrhizin.Citation27 HMGB1-neutralizing antibody can block activity of extracellular HMGB1 in tumor therapy.Citation73 Blockade of RAGE signaling pathways could also result in attenuation of tumor development and growth. Several strategies that block HMGB1-RAGE signaling have been described.Citation74 Soluble RAGE acts as a decoy to prevent RAGE signaling and has been used successfully in animal models.Citation75 Platinating agents such as cisplatin and oxaliplatin have the ability to retain HMGB1 within the nucleus.Citation76 HMG domain motifs bind specifically to the major platinum DNA adducts, creating a shield against the human excision nucleases accomplished through the HMGB1 acidic domain.Citation77 Ethyl pyruvate, the first HMGB1 inhibitor used in animal models, inhibits the release of TNF and HMGB1 from endotoxin-stimulated murine macrophages, and also attenuates activation of both the p38 mitogen-activated protein kinase and NF-κB signaling pathways.Citation78 Ethyl pyruvate, interacting with NF-κB, inhibits liver tumor growth.Citation79 In addition, glycyrrhizin and quercetin, potential HMGB1 inhibitors by direct binding to HMGB1 or inhibition of PI3K, improve the effectiveness of anticancer agents in several different tumor models.Citation63 So, targeting the HMGB1 or its receptor represents an important potential application in cancer therapy.Citation80
Conclusion
In 1973, the discovery of HMGB1 as a non-histone chromosomal protein was reported. HMGB1 in the nucleus regulates a number of DNA-related mechanisms.Citation1 In 1999, with the discovery of HMGB1 as a late mediator of sepsis, a new research field was born.Citation28 During the past years, HMGB1 receptors, which balance its activity in multiple cellular processes, have been isolated. In addition to its receptors, HMGB1 posttranslational modifications play an important role in mediating HMGB1 activity.Citation23
Intracellular and extracellular HMGB1 play significantly different roles in inflammation, injury, and cancer. A new function for HMGB1 is as an autophagy regulator, linking the HMGB1 to sterile inflammation, infection, neurodegenerative disease, and cancer.Citation4
Few molecules have as many structures and functions as HMGB1. The understanding of molecular plasticity and multiplicity of HMGB1 is an exciting focus of research, in particular as a prototype for development of anti-inflammatory and immunosuppressive therapies. Future work investigating the details of HMGB1 location, structure, modification, and partners will uncover the secrets of HMGB1’s multiple functions.
Disclosure
The authors report no conflicts of interest in this work.
References
- GoodwinGHSandersCJohnsEWA new group of chromatin-associated proteins with a high content of acidic and basic amino acidsEur J Biochem197338114194774120
- CatenaREscoffierECaronCKhochbinSMartianovIDavidsonIHMGB4, a novel member of the HMGB family, is preferentially expressed in the mouse testis and localizes to the basal pole of elongating spermatidsBiol Reprod200980235836618987332
- BustinMRevised nomenclature for high mobility group (HMG) chromosomal proteinsTrends Biochem Sci200126315215311246012
- KangRChenRZhangQHMGB1 in health and diseaseMol Aspects Med201440111625010388
- PaonessaGFrankRCorteseRNucleotide sequence of rat liver HMG1 cDNANucleic Acids Res1987152190773684582
- StrosMHMGB proteins: interactions with DNA and chromatinBiochim Biophys Acta201017991–210111320123072
- BianchiMEBeltrameMPaonessaGSpecific recognition of cruciform DNA by nuclear protein HMG1Science19892434894 Pt 1105610592922595
- ReadCMCaryPDCrane-RobinsonCDriscollPCNormanDGSolution structure of a DNA-binding domain from HMG1Nucleic Acids Res19932115342734368346022
- WeirHMKraulisPJHillCSRaineARLaueEDThomasJOStructure of the HMG box motif in the B-domain of HMG1EMBO J1993124131113198467791
- HockRFurusawaTUedaTBustinMHMG chromosomal proteins in development and diseaseTrends Cell Biol2007172727917169561
- AizawaSNishinoHSaitoKKimuraKShirakawaHYoshidaMStimulation of transcription in cultured cells by high mobility group protein 1: essential role of the acidic carboxyl-terminal regionBiochemistry1994334914690146957993897
- BustinMRegulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteinsMol Cell Biol19991985237524610409715
- ParkJSSvetkauskaiteDHeQInvolvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 proteinJ Biol Chem200427997370737714660645
- BierhausAHumpertPMMorcosMUnderstanding RAGE, the receptor for advanced glycation end productsJ Mol Med (Berl)2005831187688616133426
- YangHLundbäckPOttossonLRedox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1)Mol Med20121825025922105604
- VenereauECasalgrandiMSchiraldiMMutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine releaseJ Exp Med201220991519152822869893
- BianchiMEHMGB1 loves companyJ Leukoc Biol200986357357619414536
- SchmidtAMYanSDYanSFSternDMThe biology of the receptor for advanced glycation end products and its ligandsBiochim Biophys Acta200014982–39911111108954
- PedrazziMAvernaMSparatoreBPotentiation of NMDA receptor-dependent cell responses by extracellular high mobility group box 1 proteinPLoS One201278e4451822952988
- LiJKokkolaRTabibzadehSStructural basis for the proinflammatory cytokine activity of high mobility group box 1Mol Med200391–2374512765338
- LuBAntoineDJKwanKJAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocationProc Natl Acad Sci U S A201411183068307324469805
- BustinMNeihartNKAntibodies against chromosomal HMG proteins stain the cytoplasm of mammalian cellsCell1979161181189369705
- YangHHreggvidsdottirHSPalmbladKA critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine releaseProc Natl Acad Sci U S A201010726119421194720547845
- BrezniceanuMLVölpKBösserSHMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinomaFASEB J200317101295129712759333
- YuanFGuLGuoSWangCLiGMEvidence for involvement of HMGB1 protein in human DNA mismatch repairJ Biol Chem200427920209352094015014079
- TraversAAPriming the nucleosome: a role for HMGB proteins?EMBO Rep20034213113612612600
- KangRZhangQZehHJ3rdLotzeMTTangDHMGB1 in cancer: good, bad, or both?Clin Cancer Res201319154046405723723299
- WangHBloomOZhangMHMG-1 as a late mediator of endotoxin lethality in miceScience1999285542524825110398600
- ScaffidiPMisteliTBianchiMERelease of chromatin protein HMGB1 by necrotic cells triggers inflammationNature2002418689419119512110890
- BellCWJiangWReichCF3rdPisetskyDSThe extracellular release of HMGB1 during apoptotic cell deathAm J Physiol Cell Physiol20062916C1318C132516855214
- KazamaHRicciJEHerndonJMHoppeGGreenDRFergusonTAInduction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 proteinImmunity2008291213218631454
- AnderssonUGTraceyKJHMGB1, a pro-inflammatory cytokine of clinical interest: introductionJ Intern Med2004255331831914871455
- HuangWTangYLiLHMGB1, a potent proinflammatory cytokine in sepsisCytokine201051211912620347329
- PassalacquaMPatroneMPicottiGBStimulated astrocytes release high-mobility group 1 protein, an inducer of LAN-5 neuroblastoma cell differentiationNeuroscience1998824102110289466426
- FaracoGFossatiSBianchiMEHigh mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivoJ Neurochem2007103259060317666052
- KornblitBMunthe-FogLMadsenHOStrømJVindeløvLGarredPAssociation of HMGB1 polymorphisms with outcome in patients with systemic inflammatory response syndromeCrit Care2008123R8318577209
- MagnaMPisetskyDSThe role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseasesMol Med20142013814624531836
- AsavarutPZhaoHGuJMaDThe role of HMGB1 in inflammation-mediated organ injuryActa Anaesthesiol Taiwan2013511283323711603
- KohnoTAnzaiTNaitoKRole of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodellingCardiovasc Res200981356557318984601
- GoldsteinRSGallowitsch-PuertaMYangLElevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemiaShock200625657157416721263
- SimsGPRoweDCRietdijkSTHerbstRCoyleAJHMGB1 and RAGE in inflammation and cancerAnnu Rev Immunol20102836738820192808
- RanzatoEMartinottiSPedrazziMPatroneMHigh mobility group box protein-1 in wound repairCells20121469971024710526
- RanzatoEPatroneMPedrazziMBurlandoBHmgb1 promotes wound healing of 3T3 mouse fibroblasts via RAGE-dependent ERK1/2 activationCell Biochem Biophys201057191720361273
- RanzatoEPatroneMPedrazziMBurlandoBHMGb1 promotes scratch wound closure of HaCaT keratinocytes via ERK1/2 activationMol Cell Biochem20093321–219920519588230
- Rovere-QueriniPCapobiancoAScaffidiPHMGB1 is an endogenous immune adjuvant released by necrotic cellsEMBO Rep20045882583015272298
- BianchiMEManfrediAAHigh-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunityImmunol Rev2007220354617979838
- WittemannBNeuerGMichelsHTruckenbrodtHBautzFAAutoantibodies to nonhistone chromosomal proteins HMG-1 and HMG-2 in sera of patients with juvenile rheumatoid arthritisArthritis Rheum1990339137813832403401
- KokkolaRSundbergEUlfgrenAKHigh mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitisArthritis Rheum200246102598260312384917
- PulleritsRJonssonIMVerdrenghMHigh mobility group box chromosomal protein 1, a DNA binding cytokine, induces arthritisArthritis Rheum20034861693170012794838
- PopovicKEkMEspinosaAIncreased expression of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in skin lesions of patients with lupus erythematosusArthritis Rheum200552113639364516255056
- AnderssonACovacuRSunnemarkDPivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosisJ Leukoc Biol20088451248125518644848
- RobinsonAPCaldisMWHarpCTGoingsGEMillerSDHigh-mobility group box 1 protein (HMGB1) neutralization ameliorates experimental autoimmune encephalomyelitisJ Autoimmun201343324323514872
- AndrassyMVolzHCIgweJCHigh-mobility group box-1 in ischemia-reperfusion injury of the heartCirculation2008117253216322618574060
- LieszADalpkeAMracskoEDAMP signaling is a key pathway inducing immune modulation after brain injuryJ Neurosci201535258359825589753
- HoppeGTalcottKEBhattacharyaSKCrabbJWSearsJEMolecular basis for the redox control of nuclear transport of the structural chromatin protein Hmgb1Exp Cell Res2006312183526353816962095
- TangDKangRLiveseyKMEndogenous HMGB1 regulates autophagyJ Cell Biol2010190588189220819940
- AnderssonUTraceyKJHMGB1 is a therapeutic target for sterile inflammation and infectionAnnu Rev Immunol20112913916221219181
- BonaldiTTalamoFScaffidiPMonocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretionEMBO J200322205551556014532127
- LuBNakamuraTInouyeKNovel role of PKR in inflammasome activation and HMGB1 releaseNature2012488741367067422801494
- AntoineDJJenkinsREDearJWMolecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicityJ Hepatol20125651070107922266604
- LamkanfiMSarkarAVande WalleLInflammasome-dependent release of the alarmin HMGB1 in endotoxemiaJ Immunol201018574385439220802146
- LamkanfiMEmerging inflammasome effector mechanismsNat Rev Immunol201111321322021350580
- TangDKangRChehCWHMGB1 release and redox regulates autophagy and apoptosis in cancer cellsOncogene201029385299531020622903
- VolpKBrezniceanuMLBösserSIncreased expression of high mobility group box 1 (HMGB1) is associated with an elevated level of the antiapoptotic c-IAP2 protein in human colon carcinomasGut200655223424216118352
- GongHZulianiPKomuravelliAFaederJRClarkeEMAnalysis and verification of the HMGB1 signaling pathwayBMC Bioinformatics201011Suppl 7S1021106117
- JiaoYWangHCFanSJGrowth suppression and radiosensitivity increase by HMGB1 in breast cancerActa Pharmacol Sin200728121957196718031610
- WangLLMengQHJiaoYHigh-mobility group boxes mediate cell proliferation and radiosensitivity via retinoblastoma-interaction-dependent and -independent mechanismsCancer Biother Radiopharm201227532933522655796
- NegriniSGorgoulisVGHalazonetisTDGenomic instability – an evolving hallmark of cancerNat Rev Mol Cell Biol201011322022820177397
- GiavaraSKosmidouEHandeMPYeast Nhp6A/B and mammalian Hmgb1 facilitate the maintenance of genome stabilityCurr Biol2005151687215649368
- PolanskáEDobšákováZDvořáčkováMFajkusJŠtrosMHMGB1 gene knockout in mouse embryonic fibroblasts results in reduced telomerase activity and telomere dysfunctionChromosoma2012121441943122544226
- LiaoDJohnsonRSHypoxia: a key regulator of angiogenesis in cancerCancer Metastasis Rev200726228129017603752
- van BeijnumJRBuurmanWAGriffioenAWConvergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1)Angiogenesis2008111919918264787
- LiuLYangMKangRHMGB1-induced autophagy promotes chemotherapy resistance in leukemia cellsLeukemia2011251233120927132
- EllermanJEBrownCKde VeraMMasquerader: high mobility group box-1 and cancerClin Cancer Res200713102836284817504981
- HanfordLEEnghildJJValnickovaZPurification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE)J Biol Chem200427948500195002415381690
- OhndorfUMRouldMAHeQPaboCOLippardSJBasis for recognition of cisplatin-modified DNA by high-mobility-group proteinsNature1999399673770871210385126
- ZongWXDitsworthDBauerDEWangZQThompsonCBAlkylating DNA damage stimulates a regulated form of necrotic cell deathGenes Dev200418111272128215145826
- UlloaLOchaniMYangHEthyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammationProc Natl Acad Sci U S A20029919123511235612209006
- LiangXChavezARSchapiroNEEthyl pyruvate administration inhibits hepatic tumor growthJ Leukoc Biol200986359960719584311
- LotzeMTDeMarcoRADealing with death: HMGB1 as a novel target for cancer therapyCurr Opin Investig Drugs200341214051409
