Abstract
Epinephrine is a life-saving medication used to treat systemic allergic reactions including anaphylaxis. Epinephrine autoinjectors (EAIs) are expensive and worldwide availability is limited. Epinephrine prefilled syringes and epinephrine kits are potentially lower-cost alternatives to EAIs. Advantages, disadvantages, and costs of available products are discussed. The socioeconomic factors impacting access to EAIs are described.
Introduction
Epinephrine is the first-line treatment for systemic allergic reactions (SARs) to foods, insect stings or bites, medications, and other allergens. The early use of epinephrine in SARs can be life-saving; delayed use has been associated with death.Citation1–Citation3 Cox et al updated the World Allergy Organization (WAO) grading system for SARs as summarized in to clarify the early signs and symptoms of an SAR and to encourage early use of epinephrine.Citation4 The term “SAR” applies to all grades with the term “anaphylaxis” also appropriate for grade 4 or 5 reactions.
Table 1 Proposed modification of the 2010 World Allergy Organization grading system
Epinephrine autoinjectors (EAIs) were developed in the 1970s and were first approved by the US Food and Drug Administration (FDA) in the United States in 1987 with the EpiPen® (Mylan, Canonsburg, PA, USA). EAIs available in the USA include: EpiPen; epinephrine injection, United States Pharmacopeia autoinjector, generic (Mylan); epinephrine injection, USP autoinjector (Impax Generics, Hayward, CA, USA); and Auvi-Q® (Kaléo, Richmond, VA, USA).
The annual direct costs in year 2010 in the USA for EAIs are estimated to be $294 million, accounting for about 25% of the $1.2 billion annual cost to treat SARs including anaphylaxis.Citation5,Citation6 The average wholesale price (AWP) of each EAI is included in , except for the Auvi-Q. Accurate wholesale pricing for the Auvi-Q is not available as it is distributed through a single specialty pharmacy network. The complexity of drug pricing is beyond the scope of this article. Costs for the EpiPen were relatively stable until Mylan acquired this product from Merck (Kenilworth, NJ, USA) in 2007. The AWP since that time for two EpiPens has increased 545% from $113.27 to $730.33.Citation4 This price increase persists even after accounting for inflation ().Citation7,Citation8 Although the out-of-pocket expenses for individual subjects may have decreased since the public outcry about EpiPen costs in 2016, the effective date of the most recent AWP available is May 16, 2016 and does not reflect the impact of price cuts or patient assistance programs.Citation7
Figure 1 AWP for the EpiPen® 2001–2016.
Abbreviation: AWP, average wholesale price.
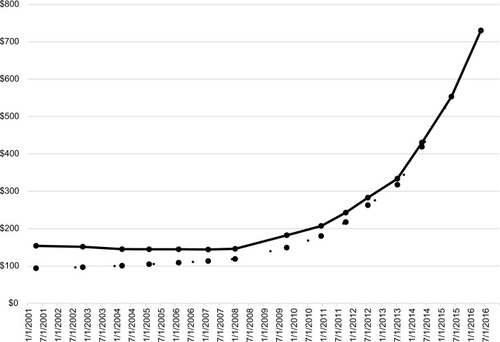
Table 2 Average wholesale prices for epinephrine autoinjectors in the USA
Use of the AWP is controversial, but it is often used as a proxy for societal cost in cost-effectiveness analyses.Citation9 An economic analysis published in 2011 utilized the 2006–2007 AWP of the EpiPen to estimate the annual cost of EAIs for food-induced SARs.Citation10 According to the International Society for Pharmacoeconomics and Outcomes Research good research practices guidelines from 2010:
Pharmaceutical prices used in the vast majority of cost-effectiveness analyses are either based on AWPs in the USA or government-negotiated prices in Europe. The former are not only imperfect measures of actual prices paid (e.g., ignoring discounts and rebates), but may also greatly overestimate societal opportunity costs because of the implicit inclusion of producer surplus created through patent-protected monopoly pricing.Citation11
In summary, AWP is used as an approximation for societal drug costs, despite its limitations. The United States Department of Veterans Affairs (VA) Health Economics Resource Center (HERC) discusses the challenge of determining medication costs for research purposes. The HERC states, “We recommend using 121% of the drug costs reported in the Federal Supply Schedule, 152% of the VA cost, or 64% of AWP. To find the cost of a generic label prescription drug, we recommend using 27% of AWP.”Citation9 Federal Supply Schedule prices are publicly available.Citation12
Several factors limit the ability of newer products to garner and maintain a better market share versus Mylan’s EpiPen. First, the EpiPen has name recognition. Second, training for the use of each device is different. To illustrate, the epinephrine injection, USP autoinjector from Impax Generics requires the removal of two caps rather than just one. Although learning to use a new device can be challenging, novel design elements can improve safety and usability. An example is that the Auvi-Q has unique features including its rectangular shape intended to fit into a pocket, a retractable needle, and voice instructions. Third, insurance coverage differs for each EAI. According to the Managed Markets Insight and Technology database, the EpiPen has unrestricted access for 61% of commercial lives among 4624 commercial health plans.Citation13 In contrast, Auvi-Q has unrestricted access for “19% of commercial lives in all locations” among these same commercial health plans.Citation8 Even though Auvi-Q is not covered by many commercial insurance plans, the manufacturer offers it with $0 copayment to all commercially insured and Medicaid patients through a specialty pharmacy network distributor. Fourth, EAIs are rated “BX” by the FDA indicating that “data that have been reviewed by the Agency are insufficient to determine therapeutic equivalence” and when ordered may not be substituted by a pharmacist, one for another.Citation14
The impact of copayments, coupons, and patient assistance programs on prices that subjects pay at the pharmacy counter requires further research. Pourang et al found that copayments did not affect the likelihood of an EAI being dispensed once it was prescribed in the Kaiser Permanente Health Maintenance Organization.Citation15 However, while the authors indicate that nearly 30% of copayments exceeded $30, they did not consider higher copayments. Data are not available on prescription:dispense ratios for subjects with exceedingly high copayments or for uninsured and underinsured subjects who may pay retail prices. More than 50% of EpiPen prescriptions are abandoned or not filled when the cost exceeds $300 for a 2-pack.Citation5 Two generic EAIs manufactured by Mylan have an AWP of $375. Two generic EAIs manufactured by Impax Generics have an AWP of $494.01. Patients with any commercial insurance plan or Medicaid receive Auvi-Q for $0 copayment through the specialty pharmacy network, ASPN Pharmacies LLC (200 Park Avenue, Suite 300, Florham Park, NJ 07932, 973-295-3289). All uninsured subjects who make <$100,000 annually have no copayment for Auvi-Q; uninsured subjects whose incomes exceed $100,000 pay no more than $360 for Auvi-Q.
Socioeconomic factors impact access to EAIs. Children from high-income versus low-income homes are 8.35 times more likely to be prescribed EAIs.Citation16 Medicaid-enrolled children are less likely to receive EAIs prior to arrival at an emergency department.Citation17 In another study, Caucasian versus non-Caucasian children were more likely to receive epinephrine early during an SAR.Citation18 Early use of epinephrine was defined as epinephrine administered before arrival to the emergency department. Owning an EAI greatly increased the odds of early epinephrine treatment (odds ratio 12.67, 95% CI: 4.46–35.96). The authors did not assess insurance status but indicate that this finding suggests that there might be an economic influence on access to EAIs.Citation18 Fleming et al examined the out-of-pocket costs for medications associated with food allergy and found higher costs for Caucasian and higher-income subjects.Citation18 They hypothesize that Medicaid-enrolled children may have lower out-of-pocket costs, that is, lower copayments. To reduce or eliminate insurance copayments, Fromer suggests that epinephrine be classified as a preventive medicine by the US Preventive Services Task Force (USPSTF).Citation5
Decision analysis software (TreeAge Pro, Williamstown, MA, USA) has been used to evaluate the cost of generic EAIs versus the EpiPen using a model that tracked spending for individual subjects over 20 years, with the assumption that each subject needs two 2-packs yearly, one each for home and school or work.Citation19 The cost for the EpiPen over a 20-year model duration totals $58,667 (95% CI: $57,745–$59,588) versus $45,588 for the generic EAI (95% CI: $44,873–$46,304). The model also incorporates other food allergy-related costs, such as specialist visits, grocery costs, and loss of work time for parents of food-allergic children. These costs are assumed to be the same for all subjects regardless of the type of EAI prescribed.
The price of EAIs also affects school districts and communities. The Michigan legislature mandated that all public schools stock EAIs. It estimated the cost for two EAI 2-packs, one adult and one pediatric, at $140, while the “recently reported costs for commercial sources” was $1200, according to the authors of the article. The annual calculated cost to Michigan public schools based on these two cost estimates ranges from $565,460 to $4,846,800.Citation20
A 2007 WAO survey of its House of Delegates indicates that EAIs are available in 59% of 44 countries surveyed.Citation21 Those without EAIs employed other methods for the self-administration of epinephrine.Citation22 These include the use of ampules of epinephrine 1:1000 (1 mg/mL) with an empty 1 cc syringe to be drawn up as needed or prefilled syringes containing various amounts of epinephrine. Both options are much less expensive than EAIs; for example, a vial of epinephrine 1:1000 (1 mg/mL) (Hospira, Lake Forest, IL, USA) had an AWP of $2.52 and retail price of ~$12 in 2016.Citation7 Epinephrine ampules may not be available in all countries. Both options also allow for tailored dosing of epinephrine, above or below the standard 0.15 or 0.3 mg doses contained in most FDA-approved EAIs. This may be beneficial for children weighing <15 kg (33 pounds) or for large or obese subjects. Of note, in November 2017, the FDA approved an infant version of the Auvi-Q, Auvi-q 0.1mg, for children weighing 7.5–15 kg (16.5–33 pounds). It has a shorter needle and a smaller dose of epinephrine (0.1 mg versus 0.15 mg contained in other “junior” products).
Market forces appear to influence the cost of EAIs. For example, some US companies are offering low-cost alternatives. Symjepi™ (Adamis, San Diego, CA, USA) is an epinephrine prefilled syringe (EPS) that contains 0.3 mg of epinephrine, with a user-friendly design. It was approved by the FDA in June 2017 for subjects 30 kg (66 pounds) or more and is expected to be available at a lower cost than the current EAIs. A “junior” version is expected to follow. The concept of prefilled syringes is not new. The Ana-Kit® (Hollister-Stier Laboratories, Spokane, WA, USA) consisted of a syringe filled with 1 mL of epinephrine 1:1000 (1 mg/mL) housed in a protective case for subcutaneous injection before it was removed from the US market.Citation23,Citation24 The Ana-Kit syringe had 0.1 mL graduations so that smaller doses could be administered depending on the subject’s age. The instructions recommended the following doses: “Adults and children over 12 years: 0.3 mL; 6–12 years: 0.2 mL; 2–6 years: 0.15 mL; infants to 2 years: 0.05–0.1 mL.”Citation25 An appropriate dose could be administered by pushing the syringe plunger until it stopped. A second dose could be administered as appropriate, after rotating the rectangular plunger ¼ turn to the right, to line up with a rectangular slot in the syringe.Citation25 An advantage of the prefilled Symjepi syringe is that it is housed in a dark blue plastic encasement to protect the epinephrine from ultraviolet light degradation. Epinephrine degrades with exposure to ultraviolet light, oxygen in ambient air, and excessive heat.Citation26–Citation28 EPSs stored at room temperature in a pencil box maintain acceptable US Pharmacopeia concentrations (90%–115% of label claim), pH, and sterility for 3 months.Citation22 The stability is limited to 2 months in high-temperature and low-humidity climates.Citation22,Citation29 In addition, some physicians and other health care professionals provide subjects with prefilled syringes wrapped in aluminum foil.Citation28,Citation30 These can be transported in a crush-resistant eyeglass case.
Snap Medical Industries (Dubin, OH, USA) has developed EpinephrineSnap-V® (a kit containing a vial of epinephrine 1:1000 [1 mg/mL] and empty syringes) and the EpinephrineSnap convenience kit (a kit containing an ampule of epinephrine 1:1000 [1 mg/mL] and empty syringes). The Focus Health Group, located in Knoxville, TN, USA, has been contracted to commercialize these products. The AWP of this product is $156Citation7 although information from company officials states that the average price of EpinephrineSnap is $80 and the EpinephrineSnap-V is $130, with discounted group purchasing organization pricing available (Personal communication, November 1, 2017). Use of these options may not be as practical as is an EAI. The reason is that an epinephrine ampule or vial with an empty syringe requires more skill to properly draw up the epinephrine and administer it under emergency circumstances. Parents of individuals with a history of an SAR take longer to draw up epinephrine from an ampule (average 142±13 seconds) compared to emergency department nurses (29±0.09 seconds; p<0.05).Citation31 The epinephrine dose drawn up by parents also ranged 40-fold as compared to twofold for emergency department nurses.Citation31 The EpinephrineSnap products are geared toward use by emergency medical technicians and other health care professionals rather than individual subjects.
The rising cost of EAIs has made self-administered epinephrine potentially unavailable to some subjects. There are no data on deaths attributed to inability to afford EAIs. Lower-cost alternatives such as EPSs and epinephrine kits are entering the US market. EAIs have advantages, such as ease of use, but they are expensive. More research is needed on the complexity of drug pricing and on the optimal methods to determine individual and societal costs. Classifying EAIs as USPSTF preventive medicines could improve access by reducing or eliminating copayments.
Acknowledgments
The authors did not receive compensation nor was the content of the article influenced in any way. Adamis Pharmaceuticals paid publication fees for the articles in this special issue on anaphylaxis.
Disclosure
The authors report no conflicts of interest in this work.
References
- BockSAMunoz-FurlongASampsonHAFatalities due to anaphylactic reactions to foodsJ Allergy Clin Immunol2001107119119311150011
- SampsonHAMendelsonLRosenJPFatal and near-fatal anaphylactic reactions to food in children and adolescentsN Engl J Med199232763803841294076
- PumphreyRWhen should self-injectible epinephrine be prescribed for food allergy and when should it be used?Curr Opin Allergy Clin Immunol20088325426018560302
- CoxLSSanchez-BorgesMLockeyRFWorld allergy organization systemic allergic reaction grading system: is a modification needed?J Allergy Clin Immunol Pract2017515862.e5528065342
- FromerLPrevention of anaphylaxis: the role of the epinephrine auto-injectorAm J Med2016129121244125027555092
- DunnJDSclarDAAnaphylaxis: a payor’s perspective on epinephrine autoinjectorsAm J Med20141271 SupplS45S5024384137
- Red Book Online ® System (electronic version) Truven Health AnalyticsGreenwood Village, Colorado, USA Available from: http://www.micromedexsolutions.com/Accessed December 25, 2016
- PepperANWestermann-ClarkELockeyRFThe high cost of epinephrine autoinjectors and possible alternativesJ Allergy Clin Immunol Pract201753665668.e128215605
- Determining the cost of pharmaceuticals for a cost-effectiveness analysis Available from: https://www.herc.research.va.gov/include/page.asp?id=pharmaceutical-costsAccessed November 1, 2017
- PatelDAHoldfordDAEdwardsECarrollNVEstimating the economic burden of food-induced allergic reactions and anaphylaxis in the United StatesJ Allergy Clin Immunol20111281110115.e521489610
- GarrisonLPJrMansleyECAbbottTA3rdBresnahanBWHayJWSmeedingJGood research practices for measuring drug costs in cost-effectiveness analyses: a societal perspective: the ISPOR Drug Cost Task Force report–Part IIValue Health201013181319883405
- Pharmaceutical Pricing for Federal Supply Schedule and National Contracts. United States Department of Veterans Affairs Office of Acquisition and LogisticsAvailable via United States Department of Veterans Affairs Office of Acquisition and Logistics Available from: https://www.va.gov/oal/business/fss/pharmPrices.aspAccessed November 3, 2017
- Managed Markets Insight and Technology Database Available from: https://formularylookup.comAccessed November 1, 2017
- FoodUSDrug Administration Orange Book Preface Available from: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/ucm079068.htmAccessed August 26, 2016
- PourangDBatechMSheikhJSamantSKaplanMAnaphylaxis in a health maintenance organization: International Classification of Diseases coding and epinephrine auto-injector prescribingAnn Allergy Asthma Immunol20171182186190.e127890557
- CoombsRSimonsEFotyRGStiebDMDellSDSocioeconomic factors and epinephrine prescription in children with peanut allergyPaediatr Child Health201116634134422654545
- HuangFChawlaKJarvinenKMNowak-WegrzynAAnaphylaxis in a New York City pediatric emergency department: triggers, treatments, and outcomesJ Allergy Clin Immunol20121291162168e1e322018905
- FlemingJTClarkSCamargoCAJrRuddersSAEarly treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalizationJ Allergy Clin Immunol Pract201531576225577619
- ShakerMBeanKVerdiMEconomic evaluation of epinephrine auto-injectors for peanut allergyAnn Allergy Asthma Immunol2017119216016328634019
- SteffensCClementBFalesWChehadeAEHPutmanKSworREvaluating the cost and utility of mandating schools to stock epinephrine auto-injectorsPrehosp Emerg Care201721556356628414559
- SimonsFELack of worldwide availability of epinephrine autoinjectors for outpatients at risk of anaphylaxisAnn Allergy Asthma Immunol200594553453815945555
- KerddonfakSManuyakornWKamchaisatianWSasisakulpornCTeawsomboonkitWBenjaponpitakSThe stability and sterility of epinephrine prefilled syringeAsian Pac J Allergy Immunol2010281535720527517
- BrownerBD PAGuptonCLAllergic reactions and envenomationsEmergency Care and Transportation of the Sick and Injured8th edSudbury, MA, USAJones and Bartlett Publishers2002
- AuerbachPMedicine for the Outdoors: The Essential Guide to First Aid and Medical Emergencies, Allergic Reaction5th edPhiladelphia, PA, USAMosby, an affiliate of Elsevier Inc.2009
- Ana-kit drug information Available from: http://www.kiessig.com/drugs/druginfo.aspx?id=1207Accessed November 27, 2017
- EPIPEN (epinephrine injection, USP), Auto-Injector 0.3 mg, EPIPEN Jr (epinephrine injection, USP) Auto-Injector 0.15 mgMorgantown, WVMylan Inc.2016
- RachidOSimonsFERawas-QalajiMLewisSSimonsKJEpinephrine doses delivered from auto-injectors stored at excessively high temperaturesDrug Dev Ind Pharm201642113113525997362
- ParishHGBowserCSMortonJRBrownJCA systematic review of epinephrine degradation with exposure to excessive heat or coldAnn Allergy Asthma Immunol20161171798727221065
- Rawas-QalajiMSimonsFECollinsDSimonsKJLong-term stability of epinephrine dispensed in unsealed syringes for the first-aid treatment of anaphylaxisAnn Allergy Asthma Immunol2009102650050319558009
- WasermanSAvillaEBen-ShoshanMRosenfieldLAdcockABGreenhawtMEpinephrine autoinjectors: new data, new problemsJ Allergy Clin Immunol Pract2017551180119128888248
- SimonsFESCXGKJSEpinephrine for the out-of-hospital (first-aid) treatment of anaphylaxis in infants: is the ampule/syringe/needle method practical?J ALlergy Clin Immunol200110861040104411742286
- United States Department of Labor Bureau of Labor Statistics Consumer Price Index Available from: https://www.bls.gov/cpi/Accessed December 4, 2017
- PassalacquaGBaena-CagnaniCEBousquetJGrading local side effects of sublingual immunotherapy for respiratory allergy: speaking the same languageJ Allergy Clin Immunol2013132939823683513
- SampsonHAMunoz-FurlongACampbellRLSecond symposium on the definition and management of anaphylaxis: summary report - Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposiumJ Allergy Clin Immunol200611739139716461139
