Abstract
Purpose
Treatment of severe eosinophilic asthma (SEA) has been revolutionized by the development of monoclonal antibodies targeting underlying immunological pathways of eosinophilic asthma. Two of the most frequently used antibodies in clinical practice are mepolizumab, targeting interleukin (IL) 5 and benralizumab, targeting the IL5 receptor alpha. The comparative treatment efficacy of these antibodies remains unclear, particularly regarding long-term outcomes.
Patients and Methods
In this multicenter, retrospective study, we included 123 patients treated with mepolizumab and 64 patients treated with benralizumab for 12 months at one of three study sites in Germany. Data were collected at baseline and after 6 and 12 months of therapy. Endpoints were changes in pulmonary function (PF), exacerbation rate, oral corticosteroid (OCS) use and dose, asthma control test (ACT) score and fractional exhaled nitric oxide (FeNO) levels.
Results
Both mepolizumab and benralizumab led to significant improvements in PF with an increase in median forced expiratory volume (FEV1) after 12 months from 59% to 74% for mepolizumab and 63% to 72% for benralizumab. Treatment also led to significant improvements in ACT scores after 12 months (mepolizumab: 13 [interquartile range (IQR) 9–17] to 19 [IQR 15–23]; benralizumab: 12 [IQR 9–16] to 22 [IQR 16–25]) as well as a reduction of mean OCS dose (mepolizumab 8 mg [IQR 5–12.5 mg] median prednisolone equivalent at baseline to 5 mg [IQR 3–7.5 mg]; benralizumab 7.5 mg [IQR 5–15 mg] to 5 mg [IQR 2–10 mg]). The exacerbation rates were reduced significantly, irrespective of the treatment. Overall, changes were similar after 6 and 12 months of therapy.
Conclusion
Both mepolizumab and benralizumab are highly effective in the long-term treatment of SEA, with no clinically relevant differences in outcomes after 12 months of therapy. In both groups, improvements were similar after 6 and 12 months of therapy, underlining the feasibility of early treatment evaluation.
Introduction
Asthma is a chronic airway disease with variable clinical presentations and phenotypes. Severe asthma is defined as uncontrolled if symptoms persist despite high-dose inhaled corticosteroids (ICS) combined with inhaled bronchodilators and other additive controllers (ie, montelukast) or if patients are dependent on oral corticosteroid (OCS) therapy.Citation1,Citation2 Severe eosinophilic asthma (SEA), which accounts for at least a third of all cases of severe asthma, is a distinct phenotype of severe asthma characterized by eosinophilic airway inflammation and eosinophilia in both peripheral blood and sputum.Citation3–Citation5 SEA is part of the type 2 high group of asthma phenotypes and is mainly caused by an innate lymphoid cell type 2 (ILC2)-driven inflammation with increased secretion of pro-inflammatory interleukins (IL)-4, -5 and -13.Citation6,Citation7 Patients suffering from SEA often experience severe exacerbations and hospitalizations, and thus bear a high burden of morbidity and health-care associated costs.Citation8,Citation9 Moreover, SEA can cause lung remodeling and airway hyperinflation, leading to persistent decreases in pulmonary function even outside of exacerbations.Citation10
In the last decade, several monoclonal antibodies (mAbs) which target these cytokines were approved for SEA. Currently, two mAbs targeting IL5 (mepolizumab and reslizumab) and one targeting the IL5 receptor alpha (IL5Rα, benralizumab) are commercially available.Citation11,Citation12 For mepolizumab and benralizumab, a reduction of exacerbation rate, improvement of pulmonary function and OCS requirements were demonstrated.Citation13,Citation14 However, it remains unclear whether there is a significant difference between these antibodies in terms of clinical outcomes. So far, comparisons have exclusively been indirect, combining and reanalyzing the results of the individual Phase III trials, and have led to contradicting results.Citation15–Citation20 In this retrospective multicenter study, we compared treatment with mepolizumab and benralizumab over 12 months in patients with SEA.
Patients and Methods
Aim, Design and Setting
In this multicenter, retrospective analysis, patients with SEA treated with the IL5 antibody mepolizumab or the IL5Rα antibody benralizumab were compared in regard to clinical efficacy (asthma control, pulmonary function (PF), exacerbation rate and OCS use and dosage) over a 12-months period. All patients were treated between February 2016 and December 2019 in severe asthma outpatient clinics at three university hospitals in Germany (Mainz, Munich and Hannover). This study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of the Hannover Medical School (9624_BO_K_2021). All patients provided written informed consent prior to initiation of mAb therapy allowing the use of their data for scientific purposes. Data were pseudonymized and the study relied exclusively on information collected as part of routine care.
Inclusion Criteria and Treatment
All patients suffered from physician-diagnosed SEA and did not achieve adequate asthma control despite medium to high-dose inhaled glucocorticoids and a long-acting β2-agonist plus an optional second or third controller and/or additional OCS therapy. Therefore, subcutaneous add-on therapy with either mepolizumab 100 mg once every 4 weeks or benralizumab 30 mg once every 4 weeks for the first 3 doses and once every 8 weeks for subsequent doses was initiated. The choice of antibody was made by the treating physician. Patients were included in the present analysis if they had received mAb therapy for 12 months without interruption at one of the study sites. Patients who had previously received anti-IL5, anti-IL5Rα, anti-IgE or anti-IL4/13 therapy were only eligible for this study if there was a wash-out phase of 6 months or longer between therapies. Adjustments of asthma medication and OCS therapy during antibody treatment were performed by the treating physicians based upon the patients’ clinical situation.
Data Collection
Data of three time points were included in the study: firstly, data from a screening visit to assess anti-IL5/anti-IL5rα eligibility within 3 months prior to mAb therapy (called baseline, BL). Secondly, data from a time point 6 months (± 90 days) after initiation of mAB therapy (timepoint 1, T1) and thirdly, data from a timepoint 12 months (± 90 days) after initiation of mAB therapy (timepoint 2, T2). If multiple visits occurred during the ± 90-day interval for a timepoint, the latest suitable visit was used. Time to T1 and T2 were calculated from the actual date of mAb therapy initiation, not from the date of the BL screening visit.
Routine follow-up included measurement of exhaled nitric oxide (FeNO), differential blood cell count as well as forced expiratory volume in 1 second (FEV1), functional vital capacity (FVC), residual volume (RV) and total lung capacity (TLC). All PF parameters were measured as absolute values and percentage of predicted values (ie, FEV1%). PF was routinely conducted using spirometry or body plethysmography standardized to ERS/ATS guidelines.Citation21 The degree of asthma control was evaluated using the asthma control test (ACT).Citation22 Changes in medication and ACT scores were assessed at each visit. Exacerbation rate per year was assessed at baseline and after 12 months. Exacerbations were defined as an acute aggravation of asthma symptoms requiring de novo OCS or an increase in OCS dose for at least 3 days.Citation23 Smoking status and atopic and eosinophilic phenotype-related comorbidities like allergies, nasal polyposis, atopic dermatitis and chronic sinusitis were assessed at baseline. Among patients showing a RV ≥ 130% of predicted at baseline, we performed a subgroup analysis to assess the impact of pulmonary hyperinflation on treatment efficacy.Citation24
Statistical Analysis
Statistical analyses and figure preparation were performed using IBM SPSS v27™ (IBM SPSS Statistics, Armonk, NY) and STATA version 13 (State Corp LP, USA). Categorical variables are stated as numbers (n) and percentages (%). Depending on distribution, continuous variables are shown as mean ± standard deviation (SD) or median with interquartile ranges (IQR) unless indicated otherwise. Standard distribution was assessed using the Kolmogorov–Smirnov-test. For group comparisons, Chi-square test, two-sided paired t-test, Wilcoxon rank-sign or Mann–Whitney-U-test were used, as appropriate. All reported p-values are two-sided. P-values <0.05 were considered statistically significant.
Results
Data from 187 patients with SEA who had completed at least 1 year of treatment with mepolizumab or benralizumab were included in the analysis. Of these patients, 123 (66%) were treated with mepolizumab and 64 (34%) with benralizumab. Baseline characteristics for the treatment groups were similar in terms of sex, age, exacerbation rate, ACT scores and prevalence of comorbidities ().
Table 1 Baseline Characteristics
The changes in PF, ACT scores, exacerbation rates and laboratory values within groups from BL to T1 and T2 as well as the intergroup comparison of the degree of change (delta) from BL to T1 and T2 are summarized in . At the 6-month follow-up, both groups showed significant improvements in PF, particularly regarding increased FEV1 and FVC as well as reduced RV and TLC compared to baseline, without significant differences between groups. After 12 months, we observed further PF improvements in patients treated with mepolizumab, whereas PF remained stable in patients treated with benralizumab ( and –).
Table 2 Treatment Outcomes Over Time
Figure 1 (A–F) Time course of FEV1% (A), RV % (B), FVC % (C), ACT (D), FeNO (E), eosinophils (F), exacerbations per year (G) and systemic steroid dose (H).
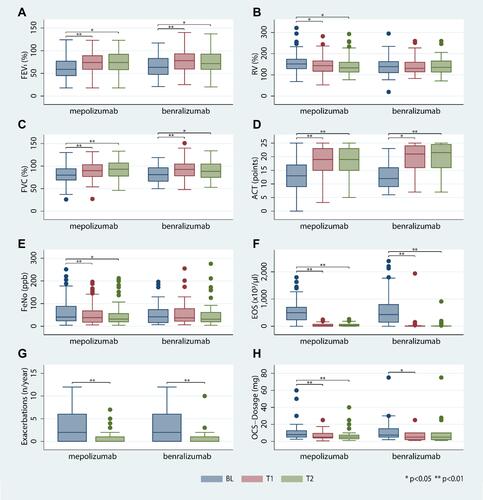
There were significant reductions in RV% at both time points in patients receiving mepolizumab, but not in patients receiving benralizumab. To assess the impact of baseline pulmonary hyperinflation on PF outcomes, we included 93 (76%) mepolizumab patients and 37 (58%) benralizumab patients with RV% of ≥130% of predicted for subgroup analysis. Significantly more patients with mepolizumab showed pulmonary hyperinflation (p=0.033). In this subgroup, we observed a significant reduction of RV% among mepolizumab recipients (BL 160%, IQR 146–183% to T1 154%, IQR 130%-170%, p<0.001), but not among benralizumab recipients (BL 153%, IQR 142–173% to T1 150%, IQR 129%-194%, p=0.203).
FeNO medians decreased from BL to T1 and T2 in both treatment groups (). However, due to a strong skew in FeNO values in the benralizumab group not matching a standard distribution, FeNO means only decreased significantly in the mepolizumab group (mepolizumab mean FeNO parts per billion (ppb) BL 60,3 to T2 48.1, p=0.002; benralizumab FeNO ppb BL 52,9 to T2 53,5, p=0.781). P-values for median reductions were therefore calculated using the Wilcoxon test.
Asthma control assessed by ACT improved significantly within the first 6 months of treatment ( and ). The median ACT score showed further improvement in patients receiving benralizumab after 12 months, but not in those receiving mepolizumab. The median improvement of ACT from BL to 12 months was more robust in patients receiving benralizumab (+9 points) than in those receiving mepolizumab (+5 points). After 12 months, the mean ACT was 19 (mepolizumab) and 22 (benralizumab (p=0.053)). In both groups the total number of eosinophils decreased significantly from BL to T1 without significant difference between treatment groups. This reduction persisted during the subsequent 6 months ().
The exacerbation rate decreased significantly in both groups during the year of anti-IL5/anti-IL5Rα therapy (). Sixty-eight percent of patients in either group reported no asthma exacerbations at all (IQR 0–1 exacerbations/year for both groups). In the year prior to antibody therapy, patients in either group had reported a median of 2 exacerbations per year (IQR 0–6 exacerbations/year, p<0.001 for the decrease in both groups). Applying the criteria for anti-IL5/anti-IL5Rα response defined by Drick et al,Citation23 we classified 87 (71%) mepolizumab patients and 49 (77%) benralizumab patients as treatment responders (p=0.251).
At baseline, 74 (60%) mepolizumab patients and 36 (56%) benralizumab patients received OCS therapy. The median OCS dosage decreased significantly from baseline in both groups: from 7.5 mg and 8 mg prednisone equivalent in patients receiving mepolizumab and benralizumab, respectively (mepolizumab IQR 5–12.5 mg, benralizumab IQR 5–15 mg, no significant intergroup differences) to a median dosage of 5 mg in both groups (mepolizumab IQR 4–9.5 mg, benralizumab IQR 2–10 mg, difference between baseline and T1 p<0.001/p=0.014 for mepolizumab and benralizumab, respectively). OCS therapy could be discontinued in 54% of mepolizumab patients and 50% of benralizumab patients (p = 0.964) who had received OCS at BL. There was no significant difference in OCS dosage decrease between groups ( and ).
Table 3 Steroid Reduction of Patients with OCS Therapy at Baseline (n=110) Compared to 6 Months (T1) and 12 Months (T2)
Discussion
In this retrospective multi-center real-life study, we compared the clinical outcomes of 1 year of mepolizumab therapy in 123 patients and benralizumab therapy in 64 patients. In summary, we found that both treatments caused significant improvements in both asthma control and PF after 6 and 12 months of therapy. Anti-IL5 and anti-IL5Rα therapy led to reduction of overall OCS therapy as well as median OCS dosage among OCS-dependent patients. The improvements after 6 months were consistent with those following 12 months of therapy.
Our findings are in line with the reports by Bourdin et al and Cabon et al, who found reslizumab, mepolizumab and benralizumab to be equally effective regarding improvement of PF and reduction of exacerbations in metanalyses of the respective licensing trials.Citation15,Citation16 Our data also support observations by recent smaller studies focused on the clinical outcomes and underlying immunological changes following anti-IL5 or anti-IL5Rα therapy.Citation7,Citation25–Citation28 However, our findings stand in contrast to the indirect comparison performed by Busse et al, who had seen an advantage for mepolizumab in terms of asthma control and reduction of exacerbations.Citation17 It is important to note, however, that Bourdin et al, Cabon et al, and Busse et al re-analyzed existing phase-3 drug trial data by using the respective control groups as references to perform an indirect comparison between treatment groups. Aside from the difficulties arising from the heterogeneity of study populations and study protocols, requiring complex statistical methods to allow for comparison, any study population in a licensing trial is carefully selected and may not adequately reflect the real-life patient population.Citation15 By contrast, our study used data from three high-volume asthma outpatient clinics, representing a more realistic patient sample and allowing for direct comparison of the efficacy of mepolizumab and benralizumab in the relevant patient cohort. Notably, both groups showed similar baseline distributions of sex, age and asthma-related comorbidities.
Patients in the mepolizumab group displayed higher RV and TLC values at baseline, potentially indicative of more lung hyperinflation. We did not observe differences in prevalence of tobacco use and consecutive chronic obstructive pulmonary disease (COPD) that could explain pulmonary hyperinflation. However, given that mepolizumab patients trended towards having lower ACT scores and higher OCS doses at baseline, patients in the mepolizumab group might have suffered from on average more severe asthma at baseline. These statistically non-significant trends as well as the minor PF differences may be explained by the fact that mepolizumab was the first antibody of its class to reach the market: many of the mepolizumab patients with the worst baseline parameters were among the earliest in our data set, likely having begun therapy at a comparatively later and more severe disease stage than patients started on either mAb in later years. Additionally, due to the retrospective nature of this study, the earlier availability of mepolizumab also led to an overrepresentation among our patient collective and thus a disbalance in group sizes.
We found a more pronounced reduction in RV in mepolizumab-treated patients both in all patients and in the subgroup of patients with clinically important pulmonary hyperinflation at baseline, as marked by an RV% of >130% of predicted.Citation24 However, the biological relevance of these differences remains unclear, particularly since patients’ subjective condition as measured by the exacerbation rate as well as the ACT score improved similarly in both patient groups. In either group, the majority of patients reached an ACT score of 16 or greater, representing a controlled or at least “partially controlled” asthma.Citation29 Additionally, the absence of a statistically significant RV reduction among benralizumab patients may be explained by the smaller group size, as benralizumab patients also trended towards a lower median RV at T1 and T2.
Since Asthma is a chronic disease, treatment efficacy has to be measured in long-term improvement as well as short-term amelioration of symptoms. The time-frames used by the various licensing trials have varied widely, from 32 weeks in the MENSA trial assessing the use of mepolizumab to 56 weeks in the benralizumab CALIMA trial.Citation30,Citation31 However, given the frequent association of exacerbations with seasonal respiratory infections in winter, an assessment of clinical efficacy and particularly of exacerbation reduction should include the peak infection season.Citation32 Therefore, we chose to include a full year in our retrospective analysis. However, we found that peak clinical efficacy was already reached after 6 months of therapy, with only minor further improvements in the subsequent period. This underlines the recommendations in the current GINA and NVL guidelines that treatment responders to anti-IL5/IL5Rα therapy can be determined within the first 4 to 6 months of therapy, while also calling into question the recommendation for further 6 months of therapy if the treatment response is uncertain at this point.Citation1,Citation23 It is also important to note that the majority of patients in our study can be classified as responders to their respective therapy both clinically and according to the criteria defined by Drick et al.Citation23 The majority of anti-IL5/IL5Rα non-responders treated at our study sites were likely switched to other forms of therapy at the 6-month follow-up or even earlier, and were thus not included in this study. The treatment results we present can therefore be seen as ideal outcomes for treatment responders, but may not reflect all patients.
Limitations
Our study is limited by its retrospective design and the disbalance in group sizes, potentially favoring the larger mepolizumab group regarding statistical significances. This particularly affected the FeNO values, where a strong skew of data points in the benralizumab group likely precluded statistical significance. Moreover, while the multi-centricity of this study enhances the generalizability of this study overall, one caveat is the use of different spirometers at the three study sites. While all spirometers were standardized according to ERS/ATS guidelines,Citation21 we cannot exclude some variability in PF results between study sites. However, as patients did not switch between study sites in the course of this study, all PF results remain internally consistent. In addition, as we intended to evaluate long-term treatment outcomes of benralizumab and mepolizumab, we included only patients who had completed a full year of antibody therapy. Our analysis may therefore overestimate the efficacy of either antibody, as patients who benefited are more likely to have completed a full year of therapy. However, this issue is partially offset by the fact that this applies to both treatment groups, and thus does not hamper the comparison between both antibodies. We did not collect data on adverse events, as prior studies by our group and others had not observed any relevant adverse events or an increased rate of adverse events compared to placebo.Citation23,Citation25,Citation33,Citation34 Moreover, any patient experiencing serious adverse events would most likely not have completed a full year of mAb therapy, and thus would not have been included in this study.
Conclusion
Overall, our study complements the findings of meta-analyses by Bourdin et al and Cabon et al with real-life data.Citation15,Citation16 Both antibodies were overall equally efficient in the long-term treatment of SEA and led to significant improvements in PF, exacerbation rates and asthma control as well as reduction of OCS dependency. Treatment efficacy could feasibly be assessed after 6 months of therapy.
Abbreviations
ACT, asthma control test; ATS, American Thoracic Society; BL, baseline; COPD, chronic obstructive pulmonary disease; ERS, European Respiratory Society; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; FVC, functional vital capacity; ICS, inhaled corticosteroids; IL, interleukin; IL5Rα, interleukin 5 receptor alpha; ILC2, innate lymphoid cell type 2; IQR, interquartile range; mAbs, monoclonal antibodies; OCS, oral corticosteroids; PF, pulmonary function; RV, residual volume; SD, standard deviation; T1, timepoint 1; T2, timepoint 2; TLC, total lung capacity.
Disclosure
Katrin Milger reports personal fees/speaker honoraria from AstraZeneca, GSK, Novartis, Sanofi, outside the submitted work. Nikolaus Kneidinger reports personal fees from AstraZeneca outside the submitted work. Stephanie Korn reports grants, personal fees from AstraZeneca, grants, personal fees from GSK, personal fees from Novartis, personal fees from Sanofi, personal fees from Teva, during the conduct of the study. Roland Buhl received grants to his institution and/or personal fees from Boehringer Ingelheim, GSK, Novartis, and Roche, as well as personal fees from AstraZeneca, Berlin-Chemie, Chiesi, Cipla, Sanofi, and Teva, outside the submitted work. Tobias Welte and/or his institution received grants and advisory/lecture/clinical trial fees from AstraZeneca, Basilea, Biotest, Bayer, Boehringer, Berlin Chemie, GSK, Infectopharm, MSD, Novartis, Pfizer, Roche, AstraZeneca, Basilea, Biotest, Bayer, Boehringer, Gilead, GSK, Janssen, Novartis, Pfizer, Roche, all outside the submitted work. Hendrik Suhling reports personal fees/speaker honoraria from AstraZeneca, GSK, Novartis and Sanofi, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- Global Initiative for Asthma. Global strategy for asthma management and prevention. 2020.
- Lommatzsch M, Virchow JC. Severe asthma: definition, diagnosis and treatment. Dtsch Arztebl Int. 2014;111(50):847–855. doi:10.3238/arztebl.2014.0847
- Nagasaki T, Sato K, Kume N, et al. The prevalence and disease burden of severe eosinophilic asthma in Japan. J Asthma. 2019;56(11):1147–1158. doi:10.1080/02770903.2018.1534967
- Tran TN, Zeiger RS, Peters SP, et al. Overlap of atopic, eosinophilic, and TH2-high asthma phenotypes in a general population with current asthma. Ann Allergy Asthma Immunol. 2016;116(1):37–42. doi:10.1016/j.anai.2015.10.027
- Albers FC, Price RG, Smith SG, Yancey SW. Mepolizumab efficacy in patients with severe eosinophilic asthma receiving different controller therapies. J Allergy Clin Immunol. 2017;140(5):1464–1466.e4. doi:10.1016/j.jaci.2017.06.010
- Domingo C. Overlapping effects of new monoclonal antibodies for severe asthma. Drugs. 2017;77(16):1769–1787. doi:10.1007/s40265-017-0810-5
- Landi C, Cameli P, Vantaggiato L, et al. Ceruloplasmin and oxidative stress in severe eosinophilic asthma patients treated with Mepolizumab and Benralizumab. Biochim Biophys Acta Proteins Proteom. 2021;1869(2):140563. doi:10.1016/j.bbapap.2020.140563
- Chastek B, Korrer S, Nagar SP, et al. Economic burden of illness among patients with severe asthma in a managed care setting. J Manag Care Spec Pharm. 2016;22(7):848–861. doi:10.18553/jmcp.2016.22.7.848
- Kerkhof M, Tran TN, Soriano JB, et al. Healthcare resource use and costs of severe, uncontrolled eosinophilic asthma in the UK general population. Thorax. 2018;73(2):116–124. doi:10.1136/thoraxjnl-2017-210531
- Brightling CE, Gupta S, Gonem S, Siddiqui S. Lung damage and airway remodelling in severe asthma. Clin Exp Allergy. 2012;42(5):638–649. doi:10.1111/j.1365-2222.2011.03917.x
- Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi:10.1056/NEJMoa1403291
- Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled Phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi:10.1016/S0140-6736(16)31324-1
- Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150(4):789–798. doi:10.1016/j.chest.2016.03.032
- Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019;50(4):975–991. doi:10.1016/j.immuni.2019.03.018
- Bourdin A, Husereau D, Molinari N, et al. Matching-adjusted indirect comparison of benralizumab versus interleukin-5 inhibitors for the treatment of severe asthma: a systematic review. Eur Respir J. 2018;52(5):1801393. doi:10.1183/13993003.01393-2018
- Cabon Y, Molinari N, Marin G, et al. Comparison of anti-interleukin-5 therapies in patients with severe asthma: global and indirect meta-analyses of randomized placebo-controlled trials. Clin Exp Allergy. 2017;47(1):129–138. doi:10.1111/cea.12853
- Busse W, Chupp G, Nagase H, et al. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. J Allergy Clin Immunol. 2019;143(1):190–200.e20. doi:10.1016/j.jaci.2018.08.031
- Henriksen DP, Bodtger U, Sidenius K, et al. Efficacy, adverse events, and inter-drug comparison of mepolizumab and reslizumab anti-IL-5 treatments of severe asthma – a systematic review and meta-analysis. Eur Clin Respir J. 2018;5(1):1536097. doi:10.1080/20018525.2018.1536097
- Casale TB, Pacou M, Mesana L, Farge G, Sun SX, Castro M. Reslizumab compared with benralizumab in patients with eosinophilic asthma: a systematic literature review and network meta-analysis. J Allergy Clin Immunol Pract. 2019;7(1):122–130.e1. doi:10.1016/j.jaip.2018.08.036
- Mauger D, Apter AJ. Indirect treatment comparisons and biologics. J Allergy Clin Immunol. 2019;143(1):84–86. doi:10.1016/j.jaci.2018.11.005
- Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi:10.1164/rccm.201908-1590ST
- Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi:10.1016/j.jaci.2003.09.008
- Drick N, Seeliger B, Welte T, Fuge J, Suhling H. Anti-IL-5 therapy in patients with severe eosinophilic asthma - clinical efficacy and possible criteria for treatment response. BMC Pulm Med. 2018;18(1):119. doi:10.1186/s12890-018-0689-2
- O’Donnell DE, Laveneziana P. Physiology and consequences of lung hyperinflation in COPD. Eur Respir Rev. 2006;15(100):61–67. doi:10.1183/09059180.00010002
- Cameli P, Bergantini L, d’Alessandro M, et al. A comprehensive evaluation of mepolizumab effectiveness in a real-life setting. Int Arch Allergy Immunol. 2020;181(8):606–612. doi:10.1159/000507996
- Bergantini L, d’Alessandro M, Cameli P, et al. Personalized approach of severe eosinophilic asthma patients treated with mepolizumab and benralizumab. Int Arch Allergy Immunol. 2020;181(10):746–753. doi:10.1159/000508936
- Vantaggiato L, Perruzza M, Refini RM, et al. Mepolizumab and benralizumab in severe eosinophilic asthma: preliminary results of a proteomic study. Lung. 2020;198(5):761–765. doi:10.1007/s00408-020-00379-6
- Bergantini L, d’Alessandro M, Cameli P, et al. Regulatory T cell monitoring in severe eosinophilic asthma patients treated with mepolizumab. Scand J Immunol. 2021;94:e13031. doi:10.1111/sji.13031
- Korn S, Both J, Jung M, Hübner M, Taube C, Buhl R. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107(6):474–479. doi:10.1016/j.anai.2011.09.001
- FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi:10.1016/S0140-6736(16)31322-8
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi:10.1056/NEJMoa1403290
- Szefler SJ, Raphiou I, Zeiger RS, Stempel D, Kral K, Pascoe S. Seasonal variation in asthma exacerbations in the AUSTRI and VESTRI studies. ERJ Open Res. 2019;5(2):00153–2018. doi:10.1183/23120541.00153-2018
- Busse WW, Bleecker ER, FitzGerald JM, et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir Med. 2019;7(1):46–59. doi:10.1016/S2213-2600(18)30406-5
- Jackson DJ, Korn S, Mathur SK, et al. Safety of eosinophil-depleting therapy for severe, eosinophilic asthma: focus on benralizumab. Drug Saf. 2020;43(5):409–425. doi:10.1007/s40264-020-00926-3
