Abstract
Background
Cysteinyl leukotrienes (CysLTs) are key mediators for bronchoconstriction, eosinophil recruitment and mucus production in the airways of asthmatic patients. To better understand the role of CysLTs in different asthma phenotypes, we compared the levels of arachidonic acid metabolites in relation to asthma control status and phenotypes in adult asthmatics on regular anti-asthma medications.
Methods
A total of 137 adult asthmatics (47 with aspirin-exacerbated respiratory disease [AERD] and 90 asthmatics with aspirin-tolerant asthma [ATA]) and 20 healthy controls were enrolled. Arachidonic acid metabolites in serum and urine were analyzed using LC-MS/MS methods, and clinical data, including asthma control status, exhaled NO (FeNO) and lung function tests, were collected.
Results
Urine LTE4 levels were significantly higher in AERD patients on inhaled corticosteroid-long-acting β2- agonist plus leukotriene receptor antagonist (LTRA) treatment than in ATA patients (P=0.001). No differences were found in the serum or urine levels of 15-HETE, TXB2, or PGF2α. High serum LTE4 levels were associated with lower FEV1% and uncontrolled status in AERD patients (P=0.006 and P=0.002, respectively), but not in ATA patients. Multivariate analysis demonstrated that blood eosinophil counts, FeNO levels and aspirin hypersensitivity were significant factors affecting urine LTE4 levels.
Conclusion
Despite LTRA treatment in AERD, the LTE4 levels remained high and showed close associations with blood eosinophilia, high FeNO levels and impaired disease control. Our real-world evidence indicates that control of asthma is not fully achieved by blocking the CysLT pathway with LTRA. Thus, introduction of treatment modalities targeting eosinophilia could be a better option for patients with high CysLTs.
Introduction
Asthma is a heterogeneous disease with many clinical phenotypes characterized by variable airflow obstruction, chronic airway inflammation and bronchial hyperresponsiveness. Asthma is usually controlled by the regular use of an inhaled corticosteroid (ICS) with or without a long-acting β2-agonist (LABA) or a leukotriene receptor antagonist (LTRA). Despite the standard asthma treatment, about 10% to 20% of patients still remain uncontrolled, often with frequent exacerbations.Citation1,Citation2
The pathophysiology of asthma is complex, and multicellular inflammatory processes including eosinophils, neutrophils, T helper cells, monocytes, mast cells, basophils and epithelial cells with various chemical mediators and cytokines are involved.Citation3 Cysteinyl leukotrienes (CysLTs), namely LTC4, LTD4 and LTE4, have been suggested to be important inflammatory mediators enhancing airway inflammation and uncontrolled asthma, especially for patients with aspirin-exacerbated respiratory disease (AERD). They are produced in a cascade manner via the arachidonic acid (AA) pathway by mast cells and eosinophils in the airways.Citation4
LTE4, a stable and reliable metabolite, has been detected in serum and urine, and used as an indicator for the activation of AA or CysLT pathways.Citation5 Higher urine levels of LTE4 (uLTE4) have been reported in patients with moderate to severe asthma as well as with AERD, in whom activation of the CysLT pathway is related to eosinophil recruitment into the airways,Citation6 and shows a negative correlation with FEV1.Citation7 Leukotriene receptor antagonists (LTRAs), including montelukast, zafirlukast and pranlukast, have been developed to reduce CysLTs-mediated airway inflammation in asthmatics.Citation8 CysLTs are known to cause bronchoconstriction, mucosal edema and mucus secretion.Citation9 Moreover, they are involved in airway remodeling via activating epithelial cells. Previous studies have revealed the role of CysLTs in airway remodeling via TGF-β1 productionCitation10 and enhancing TGF-β1 response.Citation11 The clinical efficacy of LTRAs in adult asthmatics has been reported in some studies, in which addition of LTRAs improves asthma control status in adult asthmatics whose symptoms remain uncontrolled despite the use of ICSs.Citation12 However, some asthmatics do not respond adequately to LTRAs, even together with ICS and LABA, and the detection of high LTE4 levels in urine or serum has been suggested to enrich the responders. To date, there have been few studies to examine whether airway inflammatory responses remain uncontrolled with use of LTRAs in adult patients with AERD or aspirin-tolerant asthma (ATA).
The primary objective of the present study was to examine the levels of AA metabolites according to LTRA medication as well as anti-inflammatory medication including ICS, and to find whether the LTRA treatment could suppress the levels of LTE4. Furthermore, we evaluated whether the levels of AA metabolites are associated with asthma control status and the degree of eosinophilic inflammation in a real-world clinical setting involving patients with AERD and those with ATA.
Methods
Study Design and Study Subjects
This study was a prospective cross-sectional and noninterventional real-world study. We enrolled 47 patients with AERD, 90 patients with ATA and 20 normal healthy controls (HC) at Ajou University Hospital in Suwon, South Korea. Asthma was diagnosed according to the GINA by the allergy specialists. Exclusion criteria for enrollment were as follows: 1) asthmatics who had ever been treated with type 2 biologics, including omalizumab, mepolizumab, reslizumab and dupilumab, within 130 days of enrollment, 2) current smokers or ex-smokers who quit smoking within 30 days of enrollment; and 3) asthmatics whose controller medications were changed within 7 days of enrollment.
Study subjects were recruited according to current maintenance medications, especially LTRA as well as ICS-LABA. In addition, they were grouped by NSAID/aspirin hypersensitivity because the levels of AA metabolites are known to be different in patients with AERD compared to those with ATA.Citation5 The ATA group was divided into 2 subgroups: ATA with ICS-LABA and ATA with ICS-LABA plus LTRA. In the AERD group, all the AERD patients enrolled had been taking ICS-LABA plus LTRA (). The doses of ICS were grouped into low, medium and high doses as defined in the Global Initiative for Asthma guideline (GINA) guideline.Citation13
Figure 1 Study subject group enrolled in the present study.
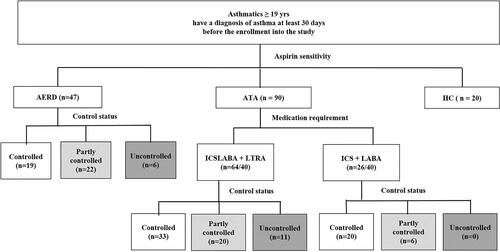
AERD was defined by a typical clinical history (recurrent exacerbation of upper or lower respiratory reactions after ingestion of aspirin/NSAIDs) and/or a positive response to the lysine-aspirin bronchial provocation test (Lys-ASA BPT).Citation14 The Lys-ASA BPT was performed with increasing doses of Lys-ASA solution up to 300 mg/mL using the method previously reported.Citation15 The result of the Lys-ASA BPT was considered “positive” if FEV1% was decreased by more than 20% after the challenge. Subjects who showed negative results to the Lys-ASA BPT or denied any changes in upper or lower respiratory tract symptoms after ingestion of aspirin/NSAIDs were defined as ATA.
To evaluate the levels of AA metabolites according to asthma control status, study subjects were grouped by asthma control status (well controlled, partly controlled or uncontrolled status) based on the GINA guideline. Well-controlled asthma was defined if patients experienced daytime symptoms and reliever use less than twice a week without night awakening or activity limitation in the past 4 weeks. Uncontrolled asthma was defined if asthmatics showed ACT ≤ 20Citation8,Citation16 or ACQ-6 ≥ 1.5.Citation17 Severe asthma was defined according to the American Thoracic Society Workshop.Citation18 All subjects gave written informed consent at the time of enrolment, and the study was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-BMR-SUR-15-498). This study was conducted in accordance with the Declaration of Helsinki.
Collection of Clinical Data and Samples
Peripheral venous blood, sputum and urine samples were collected from the subjects between 8:00 AM and 11:00 AM when the patients were maintained on controller medications including ICS-LABA and LTRAs. Blood eosinophil counts, sputum eosinophil counts and total immunoglobulin E (IgE) levels were analyzed. The serum and urine levels of metabolites, including LTE4, 15-hydroxyeicosatetraenoic acid (15-HETE), 11-dehydro thromboxane B2 (TXB2) and prostaglandin F2α (PGF2α), were quantified using liquid chromatography-tandem mass spectrometry (LC-MS/MS). LTE4-d5, 15(S)-HETE-d8, TXB2-d4 and 8-iso PGF2α-d4 were used as internal standards. Chromatographic separation was performed using a Waters Acquity UPLC system (Waters) with a Hypersil GOLD column (2.1 x 100 mm, 1.9 μm: ThermoFisher Scientific, San Jose, CA, USA). Data were obtained using an API5500 triple quadrupole mass spectrometer (AB Sciex, Framingham, MA, USA) equipped with an ESI source. For the quantitative determination of creatinine in urine samples, 10 μL of the urine sample were applied to the Creatinine Parameter Assay Kit (R&D Systems, Minneapolis, MN, USA).Citation19 Pulmonary function test, fractional exhaled nitric oxide (FeNO) measurement, questionnaires survey using the asthma control test (ACT), and asthma quality of life (AQLQ) and asthma control questionnaire (ACQ-6: ACQ mean of 6 individual item scores) were performed on the same day of enrollment.
Measurement of Serum and Urine Levels of Metabolites
Serum and urine samples stored at −70°C were thawed and analyzed by LC-MS/MS. Chromatographic separations of metabolites in urine and serum were performed with a Hypersil GOLD (2.1 X 100 mm, 1.9 μm: ThermoScientific, San Jose, CA, USA). The eluent was introduced into an AB Sciex, API5500 Triple Quadrupole mass spectrometer. The following deuterated internal standards were used: LTE4-d5 for LTE4, 15(S)-HETE-d8 for 15-HETE, 11-dehydro TXB2-d4 for 11-dehydro TXB2 and 8-iso PGF2α-d4 for PGF2α (Cayman Chemical Company, Ann Arbor, MI, USA). In urine samples, creatinine was also quantified to normalize to the actual concentrations of each biomarker and 1,3-dimethyl-2-imidazolidinone (Sigma-Aldrich, St. Louis, MO, USA) and then used as the internal standard for creatinine.
Statistical Analysis
Continuous variables were compared using Student’s t-test, and Pearson’s chi-squared or Fisher’s exact test were used for categorical variables. Analysis of variance was performed for comparisons among the 3 groups. General linear regression analysis was performed to adjust for the confounding factors age and sex, and to compare metabolite levels between the groups. Pearson’s correlation analysis identified associations among continuous variables. All computations were performed using SPSS software, version 22.0 (IBM Corp., Armonk, NY, USA). GraphPad Prism 5.0 software (GraphPad Inc., San Diego, CA, USA) was used for the production of graphs.
Results
Clinical Characteristics and Asthma Control Status of the Study Subjects
summarizes the demographic information of all the study subjects on the day of enrollment. ICSs that were prescribed to the study subjects were beclomethasone, budesonide and fluticasone. The proportion of low-, medium or high-dose ICS users was not significantly different between patients with AERD and ATA (low-dose: 25.5% vs 15.6%, medium-dose: 57.4% vs 59.4%, high-dose: 17.0% vs 25.0%; P>0.05 for all). LTRAs that were used in the study subjects were montelukast (10mg/day) and pranlukast (450mg/day). The AERD group showed higher blood eosinophil counts but lower levels of total IgE compared to the ATA group (P=0.011 and P=0.029, respectively), while no significant differences were observed in lung function parameters. There were no significant differences in asthma control status between the AERD and ATA groups. The ACT, ACQ or AQLQ scores showed no significant differences between the AERD and ATA groups (ACT:21.15 ± 3.11 vs 20.40 ± 4.06, ACQ: 4.60 ± 4.95 vs 6.32 ± 5.74, AQLQ: 172.72 ± 37.04 vs 165.82 ± 38.84; P>0.05 for all). Inflammatory parameters or lung functions showed no significant differences between ATA patients with ICS/LABA and with ICS/LABA plus LTRA. However, a higher proportion of well-controlled asthmatics, as defined by the GINA guideline, was observed in ATA patients with ICS/LABA than with ICS/LABA plus LTRA (P=0.031). The ACT and AQLQ scores were significantly higher in ATA patients with ICS-LABA than with ICS-LABA plus LTRA (ACT: 21.77 ± 3.18 vs 19.84 ± 4.26, P=0.041,AQLQ: 186.96 ± 23.61 vs 157.23 ± 40.63; P<0.001 for both). The ACQ score showed no significant difference (4.680 ± 6.04 vs 6.97 ± 5.53, P = 0.092) between the 2 groups. Regarding the comorbidities, chronic rhinosinusitis was more common in AERD than in ATA patients (40 [85.1%] in AERD and 35 [38.9%] in ATA patients, P<0.001). There were no significant differences in prevalence of gastroesophageal reflux disease and obesity between patients with AERD and those with ATA (4 [8.5%] vs 7 [7.8%], P=0.881; 12 [25.5] vs 32 [35.6], P=0.254).
Table 1 Clinical Characteristics of the Total Study Subjects
Serum and Urine Levels of LTE4, 15-HETE, TXB2 and PGF2α in the Study Subjects
In HCs, the serum levels of LTE4, 15-HETE, TXB2, PGF2α and LTE4/PGF2α ratio were found to be 0.03 ± 0.06 ng/mL, 0.97 ± 0.97 ng/mL, 0.26 ± 0.70 ng/mL, 0.07 ± 0.15 ng/mL and 0.09 ± 0.19 (mean ± SD), respectively; the urine levels of LTE4, 15-HETE, TXB2, PGF2α and LTE4/PGF2α ratio were 469.11 ± 1390.33 pg/mg creatinine, 412.06 ± 1423.53 pg/mg creatinine, 381.92 ± 703.50 pg/mg creatinine, 781.11 ± 454.50 pg/mg creatinine and 0.22 ± 0.48 ().
Figure 2 The serum and urine levels of lipid mediators in the 3 study groups. (A) LTE4. (B) 15-HETE. (C) TXB2. (D) PGF2α. (E) LTE4/PGF2α.
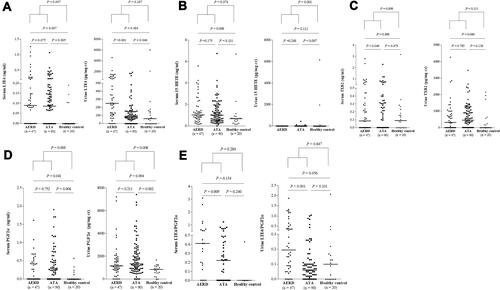
The serum levels of LTE4, LTE4/PGF2α ratio and the urine levels of LTE4, PGF2α and LTE4/PGF2α ratio showed significantly different among groups of AERD, ATA, and HC (ANOVA, P=0.014, P=0.016, P=0.007, P=0.010, and P<0.001, respectively) (). The urine levels of LTE4, and the serum and urine levels of LTE4/PGF2α ratio were significantly higher in the AERD group than in the ATA group (urine: LTE4, 539.25 ± 789.53 pg/mg creatinine vs 161.57 ± 328.94 pg/mg creatinine, serum: LTE4/PGF2α, 0.62 ± 0.81 vs 0.39 ± 0.46, urine: LTE4/PGF2α, 0.39 ± 0.46 vs 0.12 ± 0.21; P<0.001, P=0.009 and P<0.001, respectively), while marginal differences were noted in the serum levels of TXB2; no significant differences were noted in the levels of 15-HETE (). Subsequently, we further analyzed the association of the levels of LTE4 and LTE4/PGF2α ratio with clinical parameters. Receiver operating characteristic analysis (Figure S1) revealed that the urine levels of LTE4 and LTE4/PGF2α ratio could discriminate AERD patients from ATA patients (P<0.001, AUC=0.746 and P<0.001, AUC=0.747, respectively). Significant positive correlations were observed between the serum and urine levels of LTE4, but no correlation was found between the serum and urine levels of other metabolites (Figure S2).
Serum and Urine Levels of LTE4 in Asthmatics According to LTRA Medication
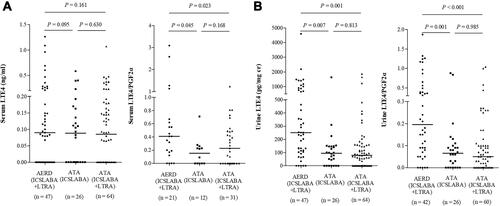
The serum and urine levels of LTE4 and LTE4/PGF2α ratio were found to be 0.15 ± 0.23 ng/mL, 0.43 ± 0.59, 324.44 ± 600.14 pg/mg creatinine and 0.23 ± 0.36 in asthmatics who had been taking ICS/LABA plus LTRA. The serum levels of LTE4/PGF2α ratio and the urine levels of LTE4, and LTE4/PGF2α ratio showed significantly different among groups (ANOVA, P=0.031, P=0.001, and P<0.001, respectively) as shown in . The urine levels of LTE4, and the serum and urine levels of LTE4/PGF2α ratio were significantly higher in the AERD patients than in the ATA patients who were on LTRA medication (P=0.001, P=0.023 and P<0.001, respectively) ( and ). Neither serum nor urine levels of LTE4 or LTE4/PGF2α ratio showed any significant differences in the ATA group between ATA patients on ICS-LABA and on ICS-LABA plus LTRA (P>0.05 for all). Considering the higher proportion of well-controlled asthmatics in the ATA group with ICS-LABA use, multivariate linear regression analysis was performed to adjust for asthma control status. After adjusting for asthma control status (regardless of GINA guideline asthma control status, ACT, ACQ and AQLQ score), neither serum or urine levels of LTE4 or LTE4/PGF2α ratio showed any significant differences according to LTRA medication in ATA patients (P>0.05 for all). There were no differences in the serum or urine levels of 15-HETE, TXB2 or PGF2α between the AERD and ATA groups according to asthma control status or clinical parameters.
Figure 3 The serum (A) and urine (B) levels of LTE4 in asthmatics according to LTRA medication.
Serum and Urine LTE4 Levels in Asthmatics According to Asthma Control Status and Severity
Although the serum and urine levels of LTE4 and LTE4/PGF2α ratio were not significantly different by ANOVA, uncontrolled AERD patients showed significantly higher serum and urine levels of LTE4 compared to controlled or partly controlled AERD patients (). The serum levels of 15-HETE and TXB2 were significantly higher in uncontrolled AERD patients than in controlled or partly controlled patients (P=0.002 and P=0.011, respectively), while no differences were noted in the serum levels of PGF2α (data not shown). When asthma control status was classified according to ACQ scores, the AERD patients with ACQ ≥ 1.5 showed significantly higher serum levels of LTE4 and LTE4/PGF2α ratio compared to controlled AERD patients (P=0.025 and P=0.011, respectively) (Figure S3).
Figure 4 The levels of LTE4 in patients according to asthma control status in AERD (A) and ATA (B) patients.
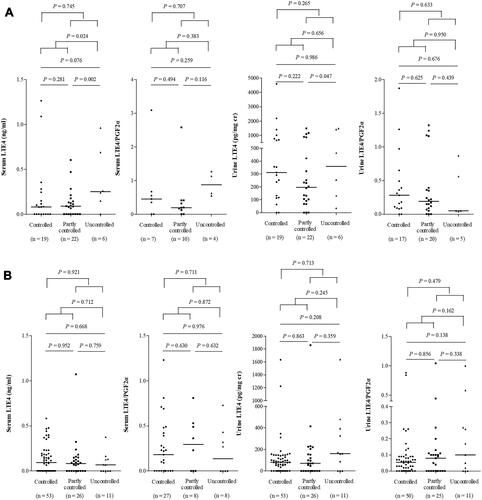
No differences in the serum or urine levels of LTE4 or LTE4/PGF2α ratio were observed in ATA patients according to asthma control status as defined by the GINA guideline, ACT ≤ 20 or ACQ-6 ≥ 1.5 (). However, ACQ scores significantly associated with the urine levels of LTE4 after adjusting for age and sex using multivariate linear regression analysis in ATA patients (P=0.032, B=11.931, Exp[B]=151,979.07).
There were no significant differences in the serum or urine levels of LTE4, 15-HETE, TXB2 PGF2α or LTE4/PGF2α ratio between non-severe asthma and severe asthma (data not shown).
Correlation Between LTE4 Levels and Type 2 Inflammatory Markers
The serum and urine levels of LTE4 and LTE4/PGF2α ratio were found to be significantly higher in patients with blood eosinophil counts ≥300/µL (serum, P=0.001 and P=0.020 respectively; urine, P<0.001 for each) (). There were weak but significant positive associations of the serum and urine levels of LTE4 with blood eosinophil counts and FeNO levels (serum: r=0.426, P<0.001, and r=0.243, P=0.005, respectively; urine: r=0.386, P<0.001, and r=0.356, P<0.001, respectively) (), These correlations were seen in both AERD and ATA patients (AERD: serum, r=0.446, P=0.002, and P=0.168; urine, r=0.389, P=0.007 and r=0.524, P<0.001; ATA: serum, r=0.367, P<0.001, and r=0.263, P=0.014; urine, r=0.274, P=0.009 and P=0.566, respectively).
Figure 5 The levels of LTE4 in patients with eosinophilic asthma and non-eosinophilic asthma.
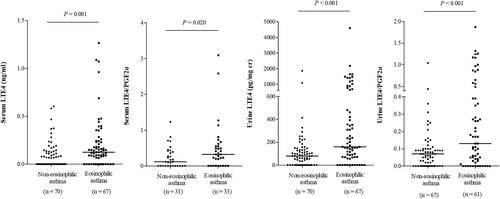
Figure 6 Correlation between the levels of LTE4 and clinical parameters ((A) eosinophil markers, (B) pulmonary function).
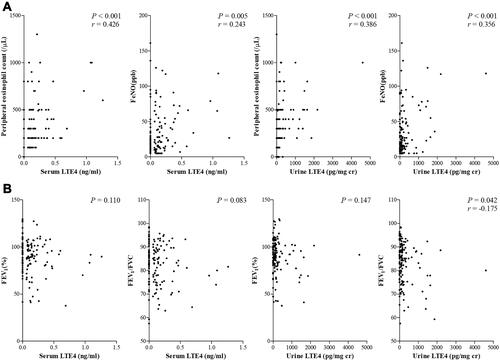
Patients with chronic rhinosinusitis (CRS) had significantly higher serum and urine levels of LTE4 and LTE4/PGF2α ratio than those without (serum: P=0.001 and P=0.018, respectively; urine: P=0.001 and P<0.001, respectively) (data not shown). No significant associations were found between the serum and urine levels of LTE4 and lung function parameters, including FEV1, FVC and MMEF (), in all asthmatics.
However, when study subjects were classified into the high (≥the median level of LTE4) vs low (<the median level of LTE4) LTE4 groups, significantly lower predicted values of FEV1, FVC and MMEF were noted in subjects with high LTE4 within the AERD group (P=0.006, P=0.015 and P=0.016, respectively) (Tables S1 and S2). Also, blood eosinophil counts were significantly higher in the high LTE4 group than in the low LTE4 group (P<0.05). In addition, to investigate factors affecting the serum and urine levels of LTE4, multivariate analyses of aspirin sensitivity, presence of CRS, asthma control status by the GINA guideline, blood eosinophil counts, FeNO levels age and sex were performed. Blood eosinophil count was the only factor significantly affecting the serum levels of LTE4 (P<0.001, B=0.000336, Exp[B]=1.000336) (Table S3). Aspirin sensitivity, blood eosinophil counts and FeNO levels significantly affected the urine levels of LTE4 (P=0.009, B=257.246, Exp[B]=5.26E+111; P<0.001, B=0.674, Exp[B]=1.961, and P=0.001, B=4.444, Exp[B]=85.094, respectively) (Table S4).
Discussion
This is the first study to demonstrate that LTE4 levels remain high and show a strong association with blood eosinophil counts, FeNO levels and asthma control status in asthmatic patients despite maintenance medications with ICS-LABA plus LTRA. First, we showed that, in a subpopulation of AERD patients with high LTE4 levels, airway inflammation could not be fully suppressed by maintenance medications according to current treatment guidelines, ie ICS-LABA plus LTRA, suggesting that CysLTs-mediated inflammation does not solely drive disease severity in these patients. Secondly, high LTE4 levels in ATA patients did not show association with lung function, and the proportion of well-controlled patients was lower in patients treated with ICS-LABA plus LTRA when compared to those treated with ICS-LABA alone. Thirdly, in both AERD and ATA patients, high LTE4 levels were linked with persistent eosinophilia. Based on these findings, we propose that earlier eosinophilia-targeting treatment could be a better option than LTRA when a combination of ICS-LABA does not lead to disease control.
ICS-LABA is known to be the mainstay of treatment options for asthma.Citation13 LTRA provides an additive benefit for asthmatics whose asthma is not adequately controlled with ICS alone or ICS-LABA, which is derived from the limited capability of corticosteroids to inhibit the leukotriene synthesis pathway.Citation8,Citation20–Citation23 Clinical studies on the efficacy of LTRA treatment have been discrepant, and targeting of the LTRA treatment to asthmatic patients with high LTE4 levels has been suggested to improve the efficacy. The present study performed in a real-world clinical setting clearly demonstrated that the serum and urine levels of LTE4 were high in a considerable number of adult asthmatics, and the higher in the AERD group than in the ATA group despite ICS-LABA plus LTRA medications, suggesting that LTRA medication does not modulate the levels of LTE4.Citation5 LTRA, a selective antagonist of the CysLT1 receptor, is known to directly block the action of CysLTs, but not their synthesis. However, it has been speculated that the synthesis of CysLTs could be reduced in the chronic use of LTRAs.Citation24 LTD4 up-regulates IL-13 and its receptor, and IL-13 production up-regulates the synthesis of CysLTs and then the expression of the CysLT1 receptor.Citation25 Furthermore, the anti-inflammatory activity of LTRAs could decrease the influx of inflammatory cells in the lungs. Accordingly, the cross-talk between CysLTs and type 2 cytokines could result in the self-perpetuating circuit of airway inflammation.Citation20 Blocking of CysLT1 receptor could interrupt this and secondarily down-regulate the levels of LTE4. However, this kind of modulation of the levels of CysLTs by LTRA was not supported by our findings.
Human eosinophils are the main source of CysLTs, and CysLTs exert autocrine signals regulating IL-4 secretion and activating eosinophils.Citation26 Recent studies suggest that CysLTs also induce innate immune responses via stimulating release of thymic stromal lymphopoietin (TSLP) and IL-33 from type 2 alveolar cells and epithelial cells.Citation14,Citation27,Citation28 Therefore, TSLP/IL-33 and CysLTs synergistically activate group 2 innate lymphoid cells, provoking IL-5 release and persistent eosinophilic inflammation.Citation29 The present study showed that there were significant associations between the serum/urine levels of LTE4 and blood eosinophil counts/FeNO levels, with a negative correlation between uLTE4 and FEV1/FVC, indicating a close link between LTE4 and type 2/eosinophilic airway inflammation.
The strength of the present study is that this study was performed in a real-world clinical setting, which also has its limitations in design. Although the critical factors that may affect the levels of LTE4 were controlled (eg asthmatics who used biologics or showed recent asthma exacerbation were excluded, LTRA users were separately analyzed), other factors that could affect the levels of LTE4 including medications for comorbidities, ICS adherence or diet pattern were not completely controlled. Because of the cross-sectional study design, further studies are needed to confirm our results. Altogether, our findings support earlier reports that a subgroup of patients with asthma, both in the ATA and AERD groups, is not adequately controlled with a combination of ICS-LABA plus LTRA.Citation30 In addition, targeting LTRA treatment to patients with high LTE4 levels does not necessarily improve the efficacy, since persistent eosinophilia, lung inflammation and progressive lung function decline were seen in patients with high LTE4 despite LTRA treatment along with ICS-LABA. Another plausible explanation for the poor efficacy of LTRA treatment may be that an unidentified LTE4 receptor could mediate airway inflammation in asthma and resist currently available LTRAs.Citation31,Citation32 However, no such recent evidence exists to support this interpretation.
In conclusion, our results emphasize the need for other therapeutic options in asthmatic patients with high LTE4 levels, such as targeting eosinophils, TSLP or IL-33, and suggest the role of CysLTs as a major driver of eosinophilic airway inflammation and remodeling.Citation33
Abbreviations
AA, arachidonic acid; ACQ, asthma control questionnaire; ACT, asthma control test; AERD, aspirin-exacerbated respiratory disease; AQLQ, asthma quality of life; ATA, aspirin-tolerant asthma; CysLTs, Cysteinyl leukotrienes; FeNO, Fractional exhaled NO; GINA, Global Initiative for Asthma guideline; IgE, immunoglobulin E; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LC-MS/MS, liquid chromatography-tandem mass spectrometry; LTRA, leukotriene receptor antagonist; Lys-ASA BPT, lysine-aspirin bronchial provocation test; PGF2α, prostaglandin F2α; TSLP, thymic stromal lymphopoietin; TXB2, 11-dehydro thromboxane B2; 15-HETE, 15-hydroxyeicosatetraenoic acid.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Fanyi Jiang, Katerina Pardali, Outi Vaarala and Joo-Youn Cho for their cooperation and contribution to this research.
Disclosure
The authors declare that they have no competing interests.
Additional information
Funding
References
- Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377(10):965–976. doi:10.1056/NEJMra1608969
- Fajt ML, Wenzel SE. Development of new therapies for severe asthma. Allergy Asthma Immunol Res. 2017;9(1):3–14. doi:10.4168/aair.2017.9.1.3
- Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011;242(1):205–219. doi:10.1111/j.1600-065X.2011.01030.x
- Lee JH, Jung CG, Park HS. An update on the management of aspirin-exacerbated respiratory disease. Expert Rev Respir Med. 2018;12(2):137–143. doi:10.1080/17476348.2018.1417843
- Ban GY, Cho K, Kim SH, et al. Metabolomic analysis identifies potential diagnostic biomarkers for aspirin-exacerbated respiratory disease. Clin Exp Allergy. 2017;47(1):37–47. doi:10.1111/cea.12797
- Gauvreau GM, Parameswaran KN, Watson RM, O’byrne PM. Inhaled leukotriene E4, but not leukotriene D4, increased airway inflammatory cells in subjects with atopic asthma. Am J Respir Crit Care Med. 2001;164(8):1495–1500. doi:10.1164/ajrccm.164.8.2102033
- Green S, Malice M, Tanaka W, Tozzi C, Reiss T. Increase in urinary leukotriene LTE4 levels in acute asthma: correlation with airflow limitation. Thorax. 2004;59(2):100–104. doi:10.1136/thorax.2003.006825
- Reiss TF, Dockhorn RJ, Shingo S, Seidenberg B, Edwards TB; for the Montelukast Clinical Research Study Group. Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma. Arch Intern Med. 1998;158:1213–1220. doi:10.1001/archinte.158.11.1213
- Hallstrand TS, Henderson WR. An update on the role of leukotrienes in asthma. Curr Opin Allergy Clin Immunol. 2010;10(1):60–66. doi:10.1097/ACI.0b013e32833489c3
- Bosse Y, Thompson C, McMahon S, Dubois C, Stankova J, Rola‐Pleszczynski M. Leukotriene D4‐induced, epithelial cell‐derived transforming growth factor β1 in human bronchial smooth muscle cell proliferation. Clin Exp Allergy. 2008;38(1):113–121.
- Altraja S, Jaama J, Altraja A. Proteome changes of human bronchial epithelial cells in response to pro-inflammatory mediator leukotriene E4 and pro-remodelling factor TGF-β1. J Proteomics. 2010;73(6):1230–1240. doi:10.1016/j.jprot.2010.02.017
- Montuschi P, Peters-Golden ML. Leukotriene modifiers for asthma treatment. Clin Exp Allergy. 2010;40(12):1732–1741. doi:10.1111/j.1365-2222.2010.03630.x
- Global strategy for asthma management and prevention; 2020 [ cited July 10, 2020]. Available from: http://ginasthma.org/gina-reports/. Accessed October 5, 2021.
- Woo SD, Luu QQ, Park HS. NSAID-exacerbated respiratory disease (NERD): from pathogenesis to improved care. Front Pharmacol. 2020;11:1147. doi:10.3389/fphar.2020.01147
- Park H. Early and late onset asthmatic responses following lysine‐aspirin inhalation in aspirin‐sensitive asthmatic patients. Clin Exp Allergy. 1995;25(1):38–40. doi:10.1111/j.1365-2222.1995.tb01000.x
- Crimi C, Campisi R, Noto A, et al. Comparability of asthma control test scores between self and physician-administered test. Respir Med. 2020;170:106015. doi:10.1016/j.rmed.2020.106015
- Juniper EF, Bousquet J, Abetz L, Bateman ED; Goal Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100(4):616–621. doi:10.1016/j.rmed.2005.08.012
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
- Ban GY, Youn DY, Ye YM, Park HS. Increased expression of serine palmitoyl transferase and ORMDL3 polymorphism are associated with eosinophilic inflammation and airflow limitation in aspirin-exacerbated respiratory disease. PLoS One. 2020;15(10):e0240334. doi:10.1371/journal.pone.0240334
- Peters-Golden M, Henderson WR. Leukotrienes. N Engl J Med. 2007;357(18):1841–1854. doi:10.1056/NEJMra071371
- Sandrini A, Ferreira IM, Gutierrez C, Jardim JR, Zamel N, Chapman KR. Effect of montelukast on exhaled nitric oxide and nonvolatile markers of inflammation in mild asthma. Chest. 2003;124(4):1334–1340. doi:10.1378/chest.124.4.1334
- Ilowite J, Webb R, Friedman B, et al. Addition of montelukast or salmeterol to fluticasone for protection against asthma attacks: a randomized, double-blind, multicenter study. Ann Allergy Asthma Immunol. 2004;92(6):641–648. doi:10.1016/S1081-1206(10)61430-5
- Laviolette M, Malmstrom K, Lu S, et al. Montelukast added to inhaled beclomethasone in treatment of asthma. Am J Respir Crit Care Med. 1999;160(6):1862–1868. doi:10.1164/ajrccm.160.6.9803042
- Cai C, Yang J, Hu S, Zhou M, Guo W. Relationship between urinary cysteinyl leukotriene E4 levels and clinical response to antileukotriene treatment in patients with asthma. Lung. 2007;185(2):105–112. doi:10.1007/s00408-006-0001-8
- Espinosa K, Bossé Y, Stankova J, Rola-Pleszczynski M. CysLT1 receptor upregulation by TGF-β and IL-13 is associated with bronchial smooth muscle cell proliferation in response to LTD4. J Allergy Clin Immunol. 2003;111(5):1032–1040. doi:10.1067/mai.2003.1451
- Baptista-dos-Reis R, Muniz VS, Neves JS. Multifaceted roles of cysteinyl leukotrienes in eliciting eosinophil granule protein secretion. Biomed Res Int. 2015;2015:848762. doi:10.1155/2015/848762
- Lund SJ, Portillo A, Cavagnero K, et al. Leukotriene C4 potentiates IL-33–induced group 2 innate lymphoid cell activation and lung inflammation. J Immunol. 2017;199(3):1096–1104. doi:10.4049/jimmunol.1601569
- Liu T, Barrett NA, Kanaoka Y, et al. Type 2 cysteinyl leukotriene receptors drive IL-33–dependent type 2 immunopathology and aspirin sensitivity. J Immunol. 2018;200(3):915–927. doi:10.4049/jimmunol.1700603
- Jonckheere AC, Bullens DM, Seys SF. Innate lymphoid cells in asthma: pathophysiological insights from murine models to human asthma phenotypes. Curr Opin Allergy Clin Immunol. 2019;19(1):53–60. doi:10.1097/ACI.0000000000000497
- Buchheit KM, Laidlaw TM. Update on the management of aspirin-exacerbated respiratory disease. Allergy Asthma Immunol Res. 2016;8(4):298. doi:10.4168/aair.2016.8.4.298
- Paruchuri S, Tashimo H, Feng C, et al. Leukotriene E4–induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med. 2009;206(11):2543–2555. doi:10.1084/jem.20091240
- Laidlaw TM, Boyce JA. Cysteinyl leukotriene receptors, old and new; implications for asthma. Clin Exp Allergy. 2012;42(9):1313–1320. doi:10.1111/j.1365-2222.2012.03982.x
- Choi Y, Lee Y, Park HS. Which factors associated with activated eosinophils contribute to the pathogenesis of aspirin-exacerbated respiratory disease? Allergy Asthma Immunol Res. 2019;11(3):320. doi:10.4168/aair.2019.11.3.320
