Abstract
Asthma afflicts an estimated 339 million people globally and is associated with ill health, disability, and early death. Strong risk factors for developing asthma are genetic predisposition and environmental exposure to inhaled substances that may provoke allergic reactions. Asthma guidelines recommend identifying causal or trigger allergens with specific IgE (sIgE) testing after a diagnosis of asthma has been made. Allergy testing with sIgE targets subpopulations of patients considered at high risk, such as those with frequent exacerbations, emergency visits or hospitalizations, or uncontrolled symptoms. Specific recommendations apply to preschool children, school-age children, patients with persistent or difficult-to-control asthma, patients needing oral corticosteroids or high-dose inhaled steroids, patients seeking understanding and guidance about their disease, and candidates for advanced therapies (biologics, allergen immunotherapy). Allergen skin testing is common in specialized settings but less available in primary care. Blood tests for total and sIgE are accessible and yield quantifiable results for tested allergens, useful for detecting sensitization. Results are interpreted in the context of the patient’s clinical presentation, age, and relevant allergen exposures. Incorporating sIgE testing into asthma management adds objective information to identify specific allergies and can guide personalized treatment plans, which reinforce patient-doctor communication. Test results can also be used to predict exacerbations and response to therapies. Additional diagnostic information can be gleaned from (i) eosinophil count ≥300 μL, which significantly increases the odds of having exacerbations, and emerging eosinophil biomarkers (eg, eosinophil-derived neurotoxin), which can be measured in plasma or serum samples, and (ii) fractional exhaled nitric oxide (FeNO), with values ≥25 ppb regarded as the cutoff for diagnosis, evaluating inhaled corticosteroid response, and of probable response to anti-IgE, anti-IL4 and anti-IL5 receptor biologics. Referral to asthma/allergy specialists is warranted when the initial diagnosis is uncertain, and when asthma symptoms, impairment, or exacerbations are repeated or severe.
Introduction
Asthma afflicts an estimated 339 million people globally,Citation1 with 417,918 deaths and 24.8 million years of life lost to ill-health, disability or early death (disability-adjusted life years or DALYs).Citation2 “The strongest risk factors for developing asthma are a combination of genetic predisposition with environmental exposure to inhaled substances and particles that may provoke allergic reactions or irritate the airways”, according to the World Health Organization.Citation2 Sensitization to inhaled environmental allergens occurs in >80% of children and adolescents and in 60% of adults with asthma.Citation3 The National Institute for Health and Care Excellence (NICE) recommends identifying trigger allergens with specific IgE (sIgE) testing after a formal diagnosis of asthma has been made.Citation4 The US National Asthma Education and Prevention Program guidelines recommend evaluating the role of allergens, particularly indoor inhalant allergens, in patients with persistent asthma.Citation5 GINACitation6 and the European Respiratory Society/American Thoracic SocietyCitation7 (ERS/ATS) guidelines on severe asthma specifically recommend sIgE testing for relevant allergens if not already done as part of the assessment of comorbidities and phenotyping for those with severe asthma. Although the link between aeroallergen and asthma exacerbations has long been recognized, investigation of the underlying allergic triggers occurs infrequently for most patients with asthma.Citation8
The reasons for this oversight are numerous, involving national policies, health care systems, payers, providers, and patients ().Citation9 Guidelines consistently recommend skin prick testing (SPT) or sIgE testing for patients with asthma who are considered to be at riskCitation4,Citation5,Citation10–12 Yet guideline-recommended care may be inconsistent or absent in primary care settings,Citation8 where most patients with asthma are seen.Citation13 The increasing prevalence and complexity of allergic diseasesCitation13 has only added to the challenges faced by front-line providers in diagnosing and managing patients with asthma.
Box 1 Barriers to Implementation of Asthma Management Strategies from the Global Initiative for Asthma (GINA)
Allergy specialists are well prepared to manage such patients, yet access to allergy and asthma specialist treatment constituted the “greatest unmet need” according to a survey of primary care providers conducted jointly by the European Academy of Allergy and Clinical Immunology (EAACI) and the International Primary Care Respiratory Group (IPCRG).Citation14 A review of 246,116 patients with asthma seen from 2006 to 2017 in the UK found that <20% of those in the high-risk category received specialist referrals.Citation15 A more recent analysis of 207,557 patients in the UK Optimum Patient Care Research Database found that large numbers of patients with potentially severe asthma (8% or 16,409) were going unrecognized in primary care.Citation16 And a survey by ERS/EAACI of allergists, respiratory physicians, and generalists using sample case studies found that respiratory doctors and generalists were significantly less likely to correctly recognize allergic asthma than allergists.Citation17
Improving Guideline Adherence Through Cooperation
To encourage optimal asthma care focusing on the allergic component, we propose a pragmatic approach that relies on generalists and specialists to create local referral pathways, while acknowledging the current variability in practice and available resources. The ERS/EAACI survey led those organizations to recommend several measures designed to improve guideline adherence ().
Box 2 Recommendations from ERS/EAACI Investigations of Allergy/Asthma Care
Optimizing asthma care in primary care could help mitigate the escalation of symptoms and redirect scarce resources to the most complex cases. Factors associated with poor asthma control, such as lack of medication adherence, incorrect inhaler technique, and untreated comorbidities, can be appropriately managed in primary care.Citation19 NICE recommendations also emphasize the importance of personalized asthma treatment plans that support self-management and ensure that patients are receiving the best care for their current level of illness.Citation4 Using sIgE testing to supplement a history of allergic symptoms and aeroallergen exposure would further inform individualized asthma management and referral decisions. sIgE test results can also address the frequent questions patients ask about the possible allergic origin of their asthma.
In line with the educational needs that have been identified, this review highlights the patients with asthma who would benefit most from sIgE testing, describes the rationale for such testing, and recommends referral to asthma and allergy specialists when needed. As practicing physicians, we understand that implementing a holistic approach to asthma/allergy management may look very different from site to site. Still, we believe that the effort necessary to provide asthma/allergy care in both primary care and specialist settings will result in improved health for the patients we serve.
Who Should Be Tested?
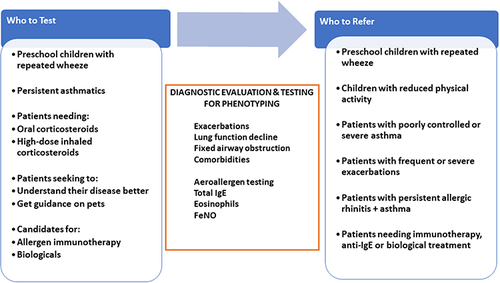
Not all patients with asthma have an allergic component, so allergy testing recommendations target subpopulations of patients with an asthma diagnosis and various risk factors. The definition of risk factors varies from guideline to guideline. Poor asthma control, which often foreshadows frequent exacerbations, unscheduled and emergency department visits or hospitalizations, has often been used to identify patients who might benefit from allergy testing.Citation20 Indeed, patients in the UK review were more likely to be referred to specialist care if they had emergency visits, hospitalizations, >3 courses of oral corticosteroids (OCS), >2 add-on medications, or a high-dose inhaled corticosteroid (ICS) prescription.Citation15 lists the patient subpopulations we recommend for sIgE testing.
Figure 1 Pragmatic approach to aeroallergen testing and referral to asthma specialists.
Preschool Children
We recommend sIgE testing for preschool age children with recurrent wheeze. The modified Asthma Predictive Index (mAPI) highlights the role of wheeze and early aeroallergen sensitization in the development of asthma ().Citation21 The mAPI uses ≥4 episodes of wheezing per year in the first 3 years of life, in combination with major or minor criteria, as the basis for predicting asthma development. Early sensitization to aeroallergens combined with continued exposure in the home are associated with asthma persistence, exacerbation and lung dysfunction.Citation3
Box 3 The Modified Asthma Predictive Index
The EAACI cites four reasons why preschool wheeze deserves attention:Citation22 1) Preschoolers have the highest rates of unscheduled medical visits for wheezing and asthma symptoms;Citation23 2) Episodes of wheezing, difficult breathing and coughing can lead to more limitations in everyday activities than for older children;Citation24 3) Early wheezing and cumulative lung injury from viral respiratory infections, such as rhinovirus and respiratory syncytial virus, may cause reduced lung function by age 6 years that persists into adulthood;Citation25 and 4) Preschoolers with persistent wheeze use a disproportionately high number of medications (bronchodilators and steroids). Moreover, preschool children with persistent wheeze and inhalant allergies are more likely to develop persistent asthma.Citation26
The INFANT (Individualized Therapy for Asthma in Toddlers) study found that in 300 preschool children (12 to 59 months old) with asthma who required daily controller therapy (Step 2), testing for aeroallergen sensitization identified those with a high probability of exacerbation.Citation27 Aeroallergen sensitization, but not exacerbation history or sex, predicted the probability of best response to daily ICS treatment, with treated individuals having more days of asthma control and fewer exacerbations. This result is consistent with a Th2 driven inflammatory response and with elevated eosinophil counts (≥300 μ/L).
Fitzpatrick et al identified five classes of preschool children with recurrent wheezing by analyzing data from five clinical trials of 1708 children ages 12–71 months old.Citation28 Annualized rates of exacerbations were highest for patients who had multiple sensitizations with reversible airflow limitation or sensitization with indoor pet exposure. Daily ICS treatment reduced their exacerbation rates, but did not help patients with minimal sensitization or sensitization plus tobacco smoke exposure.
The Preventing Asthma in High Risk Kids (PARK) study is an ongoing randomized controlled trial to determine if anti-IgE treatment with omalizumab will prevent the development or reduce the severity of asthma in 2- to 3-year-old children at high risk for persistent asthma due to aeroallergen sensitization.Citation29 Results have not yet been published.
School-Age Children
We recommend sIgE testing for school-age children with allergic symptoms and asthma. The TENOR study of children aged 6 to 12 years found a direct relationship between the number of allergens to which participants were sensitized and rates and severity of exacerbations, and associated deterioration in quality-of-life.Citation30 Of 438 subjects, 156 (35.6%) had two to three allergic triggers, 166 (37.8%) had four to five triggers, and 66 (15%) had six triggers at baseline. Children with a higher number of triggers had a longer duration of asthma and a higher prevalence of atopic dermatitis and allergic rhinitis (AR). Zoratti et al described parallel relationships between increasing numbers of sensitizations (nine to 14 positive sIgE results) and indicators of asthma severity (asthma symptoms, reduced lung function, and stepped medication therapy).Citation31
Published longitudinal studies suggest that children with AR are at high risk of becoming asthmatic and that allergen immunotherapy (AIT) may prevent the development of asthma.Citation32 Testing for allergic sensitization would identify these children so that referral to a specialist and AIT treatment could commence, ideally before polysensitization occurs.
A Cochrane review found that 4–6 weeks of pharmacologic treatment before school return might reduce peak autumn asthma exacerbations.Citation33 For example, Teach et al compared guideline-driven treatment to omalizumab or boost ICS before the fall season. They demonstrated a 50% reduction in fall exacerbations (from 21% to 11.3%) in the proportion of allergen-sensitized children with mild to severe asthma and total IgE >30 kU/mL who were treated with omalizumab rather than placebo before return to school.Citation34 Among participants with an exacerbation during the run-in phase, omalizumab was even more efficacious.
Persistent or Difficult-to-Control Asthmatics
Patients with asthma who continue to experience symptoms after diagnosis and during treatment should be re-evaluated. The International Consensus on (ICON) Pediatric Allergy notes that the definition of persistent varies among guidelines, acknowledging the influence of age, intermittent vs chronic symptoms, disease severity, and phenotypes.Citation35 The common denominator is a patient whose symptoms persist and are not well controlled. Although stepped-up medication may be appropriate, poor asthma control can also be due to worsening disease, allergic triggers, inadequate adherence, or comorbidities.Citation36,Citation37 Thus, re-evaluation requires more than simply prescribing more medication.
The NICE guidelines call for sIgE testing for patients with difficult-to-control asthma, which is defined as:
3 or more days a week with symptoms or
3 or more days a week with required use of a short-acting beta agonist (SABA) for symptomatic relief or
1 or more nights a week with awakening due to asthma.Citation4
Patients Needing Oral Corticosteroids or High-Dose Inhaled Corticosteroids
The medications used in Step 4 or 5 asthma treatment (specifically highdose ICSs or OCSs) can have consequential side effects and thus risks must be carefully balanced against benefits.Citation35 Efficacy for ICSs seems to plateau in medium- or low-dose ranges for most patients and outcomes. Increasing concerns about the life-long cumulative toxicity of OCS (even administered for very brief dosing periods but repeatedly) have been raised.Citation38 sIgE testing can identify aeroallergens that may be causing symptoms, however, allergen mitigation strategies get only conditional recommendations in the 2020 US asthma management guidelines.Citation39
Patients Seeking Guidance About Their Disease
As an incurable disease, asthma should be managed by “reducing exposure to known triggers if possible, relief of symptoms if there is airway narrowing, and reduction in airway inflammation by regular preventive treatment”, according to NICE, which also recommends a personalized treatment plan.Citation4 The plan can be used to engage patients in conversations about their disease and its management. Strict allergen avoidance for sensitized patients may not be practical or sustainable. In some cases, however, mitigation of allergic triggers can be effective as part of a comprehensive allergen control and asthma treatment plan.Citation40 summarizes studies that have evaluated allergen mitigation measures.
Patients in primary care who have demonstrated sensitizations to animals may decide not to have pets or to restrict their access to bedrooms. The same reasoning holds true for occupational allergic triggers and occupational allergic asthma.Citation47 For other specific conditions, referral to a specialist is warranted. For example, grass pollen immunotherapy has relieved symptoms associated with thunderstorm grass pollen asthma,Citation48 and GINA recommends AIT for patients with asthma and AR due to house dust mite.Citation6 These measures apply only to patients with mild or moderate disease, not to those with severe asthma.
Box 4 Studies Supporting Allergen Mitigation Measures
Candidates for Advanced Therapies
Advanced therapies include AIT and biologics administered by specialists. sIgE determination by SPT or in vitro testing is necessary before AIT to identify the target allergen. Before specialists prescribe biologics to manage severe asthma, the EAACI guidelines recommend characterization of the patient and disease with biomarkers, including sIgE, as a first step.Citation49 Multiple aeroallergen sensitizations (not total sIgE levels) and eosinophil count have been used to predict response to omalizumab therapy. In one study, multiple aeroallergen sensitizations were the best predictor of response to omalizumab; treated participants sensitized to ≥ 4 different groups of aeroallergens had a 51% reduction in the odds of a fall exacerbation of their asthma.Citation50 In another study of ICS vs placebo use, patients were grouped according to having low (<300/μL) or high (≥300/μL) baseline blood eosinophil counts.Citation51 Overall, omalizumab-treated patients had a 55% reduction in exacerbations compared with placebo-treated patients (P = 0.002). In patients with higher eosinophil counts (≥300/μL) and more severe asthma the reductions were even greater, suggesting that the benefit of omalizumab increases with the higher baseline eosinophil count.
Specific IgE Testing
Phenotyping
A phenotype constitutes the observable characteristics of a disease resulting from the interaction of individual genetics and the environment. Asthma exhibits phenotypic heterogeneity, with allergic sensitizations clinically relevant in some patients and not in others. Approximately 50% to 70% of patients with asthma have asthma that is characterized by allergic inflammation and comorbidities.Citation52–54 Understanding the underlying mechanisms of inflammation can help achieve optimal asthma control beyond symptom reliefCitation55 with the use of inflammatory biomarkers such as sIgE, blood eosinophils, and FeNO.Citation56,Citation57 Asthma/allergy management is moving from severity-based to phenotype-based care as evidence emerges supporting earlier aeroallergen diagnosis and intervention in very young children, and as AIT and biologics expand the number of patients who can be treated successfully by specialists.Citation58
Zoratti et al described five asthma phenotypes in children aged 6 to 17 years old using asthma and rhinitis severity/control (symptom days, controller use, night symptoms), lung physiology (bronchodilator response, FEV1), allergy (comorbidities, allergen sensitization determined by allergy skin testing, sIgE, and total IgE), and other criteria (FeNO and blood eosinophil count).Citation31 Eighty-five percent of the subjects demonstrated parallel relationships between increasing numbers of allergen sensitizations (one to 14 positive allergen skin testing/sIgE results out of a panel of 22) and indicators of asthma severity (asthma symptoms, exacerbations, lung function, and stepped medication therapy). The number of allergen sensitizations was the most important characteristic distinguishing the asthma phenotypes. Increasing allergic sensitizations accompanied by worsening symptoms, lung function and exacerbation risk likely represents progressive allergic asthma.
Phenotypic heterogeneity may also account for differing responses to asthma medications. This was true in the previously mentioned INFANT study.Citation27 Similarly, in adults ≥18 years old aeroallergen sensitization predicted the probability of best response to daily ICS, with responders having more days of asthma control and fewer exacerbations.Citation59,Citation60 In this study of adults with severe asthma, those with mildly allergic asthma and early age at onset responded less favorably to corticosteroid treatment than older patients with high levels of blood eosinophils and low lung function.
The Rationale for sIgE Testing
Charles H. Blakeley is credited with introducing SPT in 1865 by demonstrating that grass pollen applied to abraded skin generated the intense itching and inflammation experienced by hay fever sufferers.Citation61 The underlying physiologic mechanism for this reaction became clearer in the late 1960ʹs with the discovery of immunoglobulin E (IgE), and a variety of serologic assays became available in the ensuing decades.Citation62 compares the advantages of SPT and in vitro testing. SPT requires trained personnel, specialized techniques, allergen extracts, and safety precautions, so is rarely performed in primary care practices. Diagnostic allergen extracts are now subject to European Union regulation as medicinal products, which has also reduced the number of extracts available for skin testing. Adequate blood samples for sIgE testing can be obtained even from very young patients, those with extensive eczema, and those taking medications that may suppress skin reactions (eg, H1 antihistamines, antidepressants),Citation5 and sIgE testing carries no risk of systemic allergic reactions.
Box 5 Advantages of Skin or in vitro Testing
Most primary care providers have access to blood tests for allergen and sIgE,Citation14 which test for allergic immunity and yield quantifiable results for tested allergens. sIgE testing with an immunofluorescent assay measures allergen-specific IgE antibodies to specific antigens in a blood sample. A single blood sample can be used to test for a variety of indoor and outdoor allergens found in different geographic regions. Results for ImmunoCAP (a standard, validated sIgE laboratory test method) are reported in kUA/L (equivalent to IU/mL), a technical limit of quantitation of 0.1 kUA/L and sIgE concentrations ≥0.35 kUA/L considered by many to reflect clinically relevant sensitization. The sIgE test results should be interpreted in the context of the patient’s clinical presentation, age, relevant allergen exposures, and the sensitivity, specificity, and reproducibility of the laboratory test itself. sIgE can also identify molecular patterns that can be of great help in distinguishing primary from clinically irrelevant pan-allergen sensitizations,Citation62 as well as aiding in understanding the pollen and plant food syndromes.Citation63,Citation64 Negative sIgE test results suggest that additional investigation of the underlying causes of allergy-like symptoms is required. Patients who are sIgE-negative need not avoid allergens to which they are not sensitized or take anti-allergic medications that are likely ineffective (eg, H1 antihistamines).
In a cross-sectional analyses of primary care patients who visited the asthma/COPD service for the first time and had a pulmonologist-confirmed diagnosis of asthma, the positive predictive value (PPV) of a positive sIgE test for aeroallergens was 0.80, and was 0.88 for ICS-naïve patients.Citation65 The sensitivity of being sensitized was 0.73. Compare this with the sensitivity of spirometry for detecting asthma, which is reportedly 0.36 and its specificity which is reportedly 0.75.Citation66 These results suggest that a combination of sIgE testing and spirometry could assist in diagnosing asthma.
Total serum IgE levels are not allergen specific and are affected by many factors, including genetics and cigarette smoking, and are not a reliable indicator of allergen sensitizationCitation3 or response to omalizumab. Therefore, they should not be relied upon for asthma diagnosis. However, total IgE levels are sometimes helpful in explaining unexpected results. For example, elevated total IgE in combination with negative individual sIgE results may indicate that a relevant allergen has been overlooked. Elevated total IgE in the absence of clinical allergic symptoms may also point to a nonatopic etiology such as parasitic infection, and/or reflect a prominent comorbidity such as atopic dermatitis or chronic rhinosinusitis, which should be investigated and appropriately managed. Elevated total IgE is generally defined as serum levels >100 kU/L,Citation67 although various reference values for total IgE have been proposed.Citation67–69
Additional Diagnostic Information
The diagnosis of asthma incorporates essential elements of history, physical examination and investigation, including response to treatment. Blood eosinophil count, spirometry, and FeNO measurement can provide additional diagnostic information. In line with phenotyping, the EAACI guidelines use blood eosinophil count and FeNO measurement to differentiate eosinophilic asthma from allergic asthmaCitation49 ().
Box 6 Measurements Used by Specialists to Differentiate Asthma Phenotypes
Who Should Be Referred to an Allergy/Asthma Specialist?
In practice, many patients eligible for guideline-recommended referral never see a specialist. However, asthma referrals are a key component of optimal asthma management that need to be addressed in a primary care setting.Citation19 The UNTWIST study found that only 4% of 19,837 referral-eligible patients in England were actually referred, with a median waiting time of 880 days.Citation70 In a group of asthma patients with frequent exacerbations in Glasgow, 42% had no previous contact with an asthma specialist service despite meeting British Thoracic Society guidelines for referral. The Asthma Insight and Management survey of 2500 American patients with asthma and 309 physicians yielded similar results.Citation71 Only 22% of patients reported visiting an asthma specialist for usual care and 48% had never visited a specialist.
Our recommendations for referrals in describe patients who meet generally accepted guideline-referral criteria.Citation19 Asthma/allergy specialists provide help when the initial diagnosis is uncertain, when symptoms persist despite treatment, or when advanced therapies, such as AIT or biologics, are being considered. In the primary care setting a better understanding of the rationale behind sIgE testing in the appropriate population has the potential to improve the management of asthma before referral and to refer patients who are best managed by specialists.
Conclusion
Most patients with asthma are seen in the primary care setting, and most primary care providers have access to blood tests for sIgE. For selected clinical circumstances of patients with asthma, sIgE testing yields quantifiable results for tested allergens that can help guide asthma management in the context of the patient’s clinical presentation, age, and relevant allergen exposures. Test results can also be used to predict exacerbations and response to therapies, and to develop personalized treatment plans. sIgE test results have the potential to improve asthma management in primary care and to become the basis for referral to asthma/allergy specialists when symptoms persist or advanced therapies are being considered.
Abbreviations
AIT, allergen immunotherapy; AR, allergic rhinitis; EAACI, European Academy of Allergy and Clinical Immunology; GINA, Global Initiative for Asthma; IPCRG, International Primary Care Respiratory Group; IgE, specific immunoglobulin E; NICE, National Institute for Health and Care Excellence.
Disclosure
David Price has advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Thermofisher; consultancy agreements with Airway Vista Secretariat, AstraZeneca, Boehringer Ingelheim, Chiesi, EPG Communication Holdings Ltd, FIECON Ltd, Fieldwork International, GlaxoSmithKline, Mylan, Mundipharma, Novartis, OM Pharma SA, PeerVoice, Phadia AB, Spirosure Inc, Strategic North Limited, Synapse Research Management Partners S.L., Talos Health Solutions, Theravance and WebMD Global LLC; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Theravance and UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis, Thermofisher; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation programme, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline. Dr. Rodriguez del Rio reports personal fees from ALK Abello, grants and personal fees from Aimmune Therapeutics, grants from Merck, personal fees from GSK, personal fees from FAES, personal fees from Novartis, personal fees from Thermo Fisher, personal fees from LETI Pharma, personal fees from Allergy Therapeutics, personal fees from Miravo, outside the submitted work. Dr Liu reports consultant fees paid to his university employer from Thermo Fisher; Avillion and Labcorp; research grants paid to his university from ResMed Propeller Health, Revenio, and Avillion. Dr Casale reports consulting fees from Thermo Fisher Scientific, Genentech, Novartis, outside the submitted work. Dr. Pedersen reports personal fees from AstraZeneca, personal fees from ALK, personal fees from Thermo Fisher Scientific, outside the submitted work. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors thank Thermo Fisher Scientific for meeting support and writing assistance, and Sarah Staples, MA, ELS, for summarizing meeting discussions and assistance in manuscript preparation.
Additional information
Funding
References
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi:10.1016/S0140-6736(17)32154-2
- World Health Organization. Asthma. Available from: https://www.who.int/news-room/fact-sheets/detail/asthma. Accessed Dec 1, 2020.
- Liu A, Luskin A, Brown R, et al. The practical application of allergic trigger management to improve asthma outcomes: step 1: identify patients with allergic components of asthma. J Fam Pract. 2018;2018:S5–S13.
- The National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management (NG80); November 29, 2017. Available from: https://www.nice.org.uk/guidance/ng80. Assessed Mar 13, 2020.
- NAEPP, NAEaPP. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi:10.1016/j.jaci.2007.09.029
- Global Initiative for Asthma. GINA implementation toolbox; 2018. Available from: www.ginasthma.org. Accessed Mar 14, 2020.
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
- Yawn BP, Rank MA, Cabana MD, Wollan PC, Juhn YJ. Adherence to asthma guidelines in children, tweens, and adults in primary care settings: a practice-based network assessment. Mayo Clinic Proc. 2016;91(4):411–421. doi:10.1016/j.mayocp.2016.01.010
- Global Initiative for Asthma. Pocket guide for implementing asthma management strategies into health care; 2018. Available from: https://ginasthma.org/wp-content/uploads/2019/02/GINA-Implementation-Pocket-Guide-2019.pdf. Accessed March 13, 2020.
- Global Initiative for Asthma. Global strategy for asthma management and prevention; 2016. Available from: www.ginasthma.org. Accessed Mar 19, 2020.
- British Thoracic Society. BTS/SIGN British guideline on the management of asthma; 2016. Available from: https://www.brit-thoracic.org.uk/standards-of-care/guidelines/btssign-british-guideline-on-themanagement-of-asthma/. Accessed Jan 18, 2017.
- Lougheed MD, Leniere C, Ducharme FM, et al. Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults: executive summary. Can Respir J. 2012;19(6):e81–e88. doi:10.1155/2012/214129
- Demoly P, Chabane H, Fontaine JF, et al. Development of algorithms for the diagnosis and management of acute allergy in primary practice. World Allergy Organ. 2019;12(3):100022. doi:10.1016/j.waojou.2019.100022
- Agache I, Ryan D, Rodriguez MR, Yusuf O, Angier E, Jutel M. Allergy management in primary care across European countries – actual status. Allergy. 2013;68(7):836–843. doi:10.1111/all.12150
- Bloom CI, Walker S, Quint JK. Inadequate specialist care referrals for high-risk asthma patients in the UK: an adult population-based cohort 2006–2017. J Asthma. 2019;58(1):1–7.
- Ryan D, Heatley H, Heaney LG, et al. Potential severe asthma hidden in UK primary care. J Allergy Clin Immunol Pract. 2020:S2213–S2198. doi:10.1016/j.jaip.2020.11.053
- Mathioudakis AG, Tsilochristou O, Adcock IM, et al. ERS/EAACI statement on adherence to international adult asthma guidelines. Eur Respir Rev. 2021;30(161):161. doi:10.1183/16000617.0132-2021
- Cabrera M, Ryan D, Angier E, et al. Current allergy educational needs in primary care. Results of the EAACI working group on primary care survey exploring the confidence to manage and the opportunity to refer patients with allergy. Allergy. 2021;77(2):378–387. doi:10.1111/all.15084
- Price D, Bjermer L, Bergin DA, Martinez R. Asthma referrals: a key component of asthma management that needs to be addressed. J Asthma Allergy. 2017;10:209–223. doi:10.2147/JAA.S134300
- Teague WG, Iqbal A, Ding Y, Chipps BE, Zazzali JL. The added burden of allergen sensitization among children with severe or poorly controlled asthma. J Allergy Clin Immunol Pract. 2021;9(2):853–861.e855. doi:10.1016/j.jaip.2020.08.063
- Chang TS, Lemanske RF Jr, Guilbert TW, et al. Evaluation of the modified Asthma Predictive Index in high-risk preschool children. J Allergy Clin Immunol Pract. 2013;1(2):152–156. doi:10.1016/j.jaip.2012.10.008
- Ruszczyński M, Ambrożej D, Adamiec A, et al. Preschool wheezing and asthma in children: a systematic review of guidelines and quality appraisal with the AGREE II instrument. Pediatric Allergy Immunol. 2020. doi:10.1111/pai.13334
- Grigg J, Nibber A, Paton JY, et al. Matched cohort study of therapeutic strategies to prevent preschool wheezing/asthma attacks. J Asthma Allergy. 2018;11:309–321. doi:10.2147/JAA.S178531
- Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332(3):133–138. doi:10.1056/NEJM199501193320301
- Martinez FD, Drazen JM. Early-life origins of chronic obstructive pulmonary disease. New Engl J Med. 2016;375(9):871–878. doi:10.1056/NEJMra1603287
- Liu AH, Martinez FD. Chapter 2: natural history of allergic diseases and asthma. In: Leung DYM, Akdis C, Bacharier L, et al. editor. Pediatric Allergy: Principles and Practice. 3rd ed. Elsevier, Inc.; 2016.
- Fitzpatrick AM, Jackson DJ, Mauger DT, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol. 2016;138(6):1608–1618.e1612. doi:10.1016/j.jaci.2016.09.028
- Fitzpatrick AM, Bacharier LB, Jackson DJ, et al. Heterogeneity of mild to moderate persistent asthma in children: confirmation by latent class analysis and association with 1-year outcomes. J Allergy Clin Immunol Pract. 2020;8(8):2617–2627.e4. doi:10.1016/j.jaip.2020.02.032
- Phipatanakul W, Mauger DT, Guilbert TW, et al. Preventing asthma in high risk kids (PARK) with omalizumab: design, rationale, methods, lessons learned and adaptation. Contemp Clin Trials. 2020;100:106228. doi:10.1016/j.cct.2020.106228
- Chipps BE, Haselkorn T, Rosen K, Mink DR, Trzaskoma BL, Luskin AT. Asthma exacerbations and triggers in children in TENOR: impact on quality of life. J Allergy Clin Immunol Pract. 2018;6(1):169–176.e162. doi:10.1016/j.jaip.2017.05.027
- Zoratti EM, Krouse RZ, Babineau DC, et al. Asthma phenotypes in inner-city children. J Allergy Clin Immunol. 2016;138(4):1016–1029. doi:10.1016/j.jaci.2016.06.061
- Halken S, Larenas-Linnemann D, Roberts G, et al. EAACI guidelines on allergen immunotherapy: prevention of allergy. Pediatr Allergy Immunol. 2017;28(8):728–745. doi:10.1111/pai.12807
- Pike KC, Akhbari M, Kneale D, Harris KM. Interventions for autumn exacerbations of asthma in children. Cochrane Database Syst Rev. 2018;3(3):Cd012393. doi:10.1002/14651858.CD012393.pub2
- Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476–1485. doi:10.1016/j.jaci.2015.09.008
- Papadopoulos NG, Arakawa H, Carlsen KH, et al. International consensus on (ICON) pediatric asthma. Allergy. 2012;67(8):976–997. doi:10.1111/j.1398-9995.2012.02865.x
- Lommatzsch M, Virchow JC. Severe asthma: definition, diagnosis and treatment. Dtsch Arztebl Int. 2014;111(50):847–855. doi:10.3238/arztebl.2014.0847
- Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi:10.1016/j.jaci.2003.09.008
- Price D, Castro M, Bourdin A, Fucile S, Altman P. Short-course systemic corticosteroids in asthma: striking the balance between efficacy and safety. Eur Respir Rev. 2020;29(155):155. doi:10.1183/16000617.0151-2019
- National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. 2020 focused updates to the asthma management guidelines. Available from: https://www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines. Accessed Feb 12, 2021.
- Cipriani F, Calamelli E, Ricci G. Allergen avoidance in allergic asthma. Front Pediatr. 2017;5:103. doi:10.3389/fped.2017.00103
- Parikh K, Hall M, Kenyon CC, et al. Impact of discharge components on readmission rates for children hospitalized with asthma. J Pediatr. 2018;195:175–181.e172. doi:10.1016/j.jpeds.2017.11.062
- Murray CS, Foden P, Sumner H, Shepley E, Custovic A, Simpson A. Preventing severe asthma exacerbations in children: a randomised trial of mite impermeable bedcovers. Am J Respir Crit Care Med. 2017;196(2):150–158. doi:10.1164/rccm.201609-1966OC
- Rabito FA, Carlson JC, He H, Werthmann D, Schal C. A single intervention for cockroach control reduces cockroach exposure and asthma morbidity in children. J Allergy Clin Immunol. 2017;140(2):565–570. doi:10.1016/j.jaci.2016.10.019
- Kercsmar CM, Dearborn DG, Schluchter M, et al. Reduction in asthma morbidity in children as a result of home remediation aimed at moisture sources. Environ Health Perspect. 2006;114(10):1574–1580. doi:10.1289/ehp.8742
- Shirai T, Matsui T, Suzuki K, Chida K. Effect of pet removal on pet allergic asthma. Chest. 2005;127(5):1565–1571. doi:10.1378/chest.127.5.1565
- Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080. doi:10.1056/NEJMoa032097
- O’Hehir RE, Varese NP, Deckert K, et al. Epidemic thunderstorm asthma protection with five-grass pollen tablet sublingual immunotherapy: a clinical trial. Am J Respir Crit Care Med. 2018;198(1):126–128. doi:10.1164/rccm.201711-2337LE
- Henneberger PK, Patel JR, de Groene GJ, et al. The effectiveness of removal from exposure and reduction of exposure for managing occupational asthma: summary of an updated Cochrane systematic review. Am J Ind Med. 2020. doi:10.1002/ajim.23208
- Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–419. doi:10.1183/13993003.01359-2015
- Agache I, Akdis CA, Akdis M, et al. EAACI Biologicals Guidelines-Recommendations for severe asthma. Allergy. 2021;76(1):14–44. doi:10.1111/all.14425
- Sheehan WJ, Krouse RZ, Calatroni A, et al. Aeroallergen sensitization, serum IgE, and eosinophilia as predictors of response to omalizumab therapy during the fall season among children with persistent asthma. J Allergy Clin Immunol Pract. 2020;8(9):3021–3028.e3022. doi:10.1016/j.jaip.2020.03.051
- Casale TB, Chipps BE, Rosén K, et al. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy. 2018;73(2):490–497. doi:10.1111/all.13302
- Seys SF, Scheers H, Van den Brande P, et al. Cluster analysis of sputum cytokine-high profiles reveals diversity in T(h)2-high asthma patients. Respir Res. 2017;18(1):39. doi:10.1186/s12931-017-0524-y
- Peters MC, Mekonnen ZK, Yuan S, et al. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J Allergy Clin Immunol. 2014;133(2):388–394. doi:10.1016/j.jaci.2013.07.036
- Frøssing L, Silberbrandt A, Von Bülow A, Backer V, Porsbjerg C. The prevalence of subtypes of type 2 inflammation in an unselected population of patients with severe asthma. J Allergy Clin Immunol Pract. 2021;9(3):1267–1275. doi:10.1016/j.jaip.2020.09.051
- Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol. 2015;15:57–65. doi:10.1038/nri3786
- Robinson D, Humbert M, Buhl R, et al. Revisiting Type 2-high and Type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy. 2017;47:161–175. doi:10.1111/cea.12880
- Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi:10.1038/nm.2678
- Casale TB, Pedersen S, Rodriguez Del Rio P, Liu AH, Demoly P, Price D. The role of aeroallergen sensitization testing in asthma management. J Allergy Clin Immunol Pract. 2020;8(8):2526–2532. doi:10.1016/j.jaip.2020.07.004
- Wu W, Bang S, Bleecker ER, et al. Multiview cluster analysis identifies variable corticosteroid response phenotypes in severe asthma. Am J Respir Crit Care Med. 2019;199(11):1358–1367. doi:10.1164/rccm.201808-1543OC
- British Society for Immunology. Skin allergy test; 1865. Available from: https://www.immunology.org/skin-allergy-test-1865. Accessed June 13, 2021.
- Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, et al. EAACI molecular allergology user’s guide. Pediatr Allergy Immunol. 2016;27(Suppl 23):1–250. doi:10.1111/pai.12563
- Kleine-Tebbe J, Matricardi PM, Hamilton RG. Allergy work-up including component-resolved diagnosis: how to make allergen-specific immunotherapy more specific. Immunol Allergy Clin North Am. 2016;36(1):191–203. doi:10.1016/j.iac.2015.08.012
- Carlson G, Coop C. Pollen food allergy syndrome (PFAS): a review of current available literature. Ann Allergy Asthma Immunol. 2019;123:359–365. doi:10.1016/j.anai.2019.07.022
- Kocks JWH, Andringa HJH, van Heijst E, et al. Aeroallergen sensitization for detecting asthma in primary care: a diagnostic test accuracy study. Clin Exp Allergy. 2021;51(8):1080–1084. doi:10.1111/cea.13888
- Gjevre JA, Hurst TS, Taylor-Gjevre RM, Cockcroft DW. The American Thoracic Society’s spirometric criteria alone is inadequate in asthma diagnosis. Can Respir J. 2006;13(8):433–437. doi:10.1155/2006/198940
- Zetterström O, Johansson SG. IgE concentrations measured by PRIST in serum of healthy adults and in patients with respiratory allergy. A diagnostic approach. Allergy. 1981;36(8):537–547. doi:10.1111/j.1398-9995.1981.tb01871.x
- Carosso A, Bugiani M, Migliore E, Antò JM, DeMarco R. Reference values of total serum IgE and their significance in the diagnosis of allergy in young European adults. Int Arch Allergy Immunol. 2007;142(3):230–238. doi:10.1159/000097025
- Martins TB, Bandhauer ME, Bunker AM, Roberts WL, Hill HR. New childhood and adult reference intervals for total IgE. J Allergy Clin Immunol. 2014;133(2):589–591. doi:10.1016/j.jaci.2013.08.037
- Blakey JD, Gayle A, Slater MG, Jones GH, Baldwin M. Observational cohort study to investigate the unmet need and time waiting for referral for specialist opinion in adult asthma in England (UNTWIST asthma). BMJ Open. 2019;9(11):e031740. doi:10.1136/bmjopen-2019-031740
- Murphy KR, Meltzer EO, Blaiss MS, Nathan RA, Stoloff SW, Doherty DE. Asthma management and control in the United States: results of the 2009 asthma insight and management survey. Allergy Asthma Proc. 2012;33(1):54–64. doi:10.2500/aap.2011.32.3518
