Abstract
The goal of allergic rhinitis (AR) management is to achieve satisfactory symptom control to ensure good quality of life. Most patients with AR are currently treated with pharmacotherapy. However, knowledge gaps on the use of pharmacotherapy still exist among physicians, particularly in the primary care setting, despite the availability of guideline recommendations. Furthermore, it is common for physicians in the secondary care setting to express uncertainty regarding the use of new combination therapies like intranasal corticosteroid plus antihistamine combinations. Inadequate treatment leads to significant reduction of quality of life that affects daily activities at home, work, and school. With these concerns in mind, a practical consensus statement was developed to complement existing guidelines on the rational use of pharmacotherapy in both the primary and secondary care settings.
Introduction
The prevalence of allergic rhinitis (AR) is increasing worldwide in both children and adults.Citation1 It is associated with comorbidities like asthma, atopic dermatitis/eczema, allergic conjunctivitis, rhinosinusitis, sleep disturbance and eustachian tube dysfunction.Citation2 AR does not lead to fatal outcomes but its effects on patient morbidity, quality of life and productivity add significant costs to health-care systems.
In Malaysia, the reported prevalence of AR is approximately 7%.Citation3 The country’s humid climate facilitates the growth of house dust mites, which drives persistent AR in Malaysia, though intermittent AR is also reported. House dust mites, cockroaches and animal furs are commonly identified as predominant allergens for nasal allergies in Malaysia.Citation4 In persistent AR, symptoms occur more than 4 days a week and more than 4 consecutive weeks, while symptoms in intermittent AR occur less than 4 days a week and less than 4 consecutive weeks.Citation5 Severity is classified according to the effect of AR on daily activities and sleep: sleep is not disturbed and daily activities at work or school are not affected in mild AR, unlike moderate to severe AR.Citation5
Due to the high prevalence of AR in all age groups, patients seek treatment from physicians in various disciplines, mainly pediatricians, allergists, otorhinolaryngologists, family medicine specialists and general practitioners (GPs). Pharmacists and GPs are commonly the first point of contact for any patient with symptoms of nasal allergy. It is thus important for primary care providers to be able to effectively manage AR.
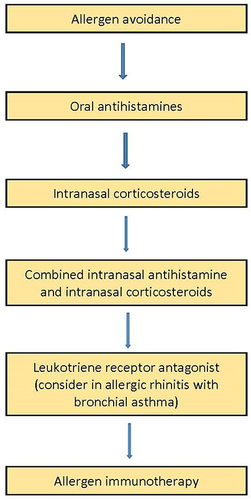
A real-world survey of children treated for allergic rhinitis revealed 37.9% had mild intermittent AR, 9.2% moderate/severe intermittent AR, 47.6% mild persistent AR and 5.2% moderate/severe persistent AR.Citation6 The survey reported that 75% chose pharmacotherapy and only 19.5% opted for allergen immunotherapy. Notably, oral antihistamines were the predominant treatment in 57.6%, intranasal corticosteroids (INCS) in 53%, and combined oral antihistamines with INCS in 35.0% of patients.Citation6 This emphasizes the role of pharmacotherapy as the mainstay treatment for the different types and severity of AR. As all pharmacotherapy is safe at the recommended dose, the treatment can be given as long as it is required.Citation7 Following the initiation of each drug or treatment, response is reassessed at 2 weeks and decision will be made to maintain, step up or step down. The treatment strategy by pharmacotherapy for AR is shown in . Nonetheless, knowledge gaps have been identified between recommended treatments for AR and actual clinical practice in a study in the Southeast Asian countries.Citation8 Although 80% of GPs are aware of international guidelines, majority of primary care providers in Malaysia do not prescribe INCS as one of the main therapies in AR.Citation8 Moreover, the selection and personalization of pharmacotherapy to control symptoms and improve quality of life can be challenging in uncontrolled and severe AR.Citation9
A simplified treatment guide is necessary to address these shortcomings and rationalize the use of pharmacotherapy to treat AR, especially within the Malaysian landscape where treatment gaps have been recognized.Citation8 Virtual expert panel meetings of pediatricians, allergists, otorhinolaryngologists and a pulmonologist were held on 11th April 2021 and 8th August 2021 to deliberate on the recommended pharmacological treatments of AR in Malaysian adults and children. Experts subsequently performed an iterative review, in accordance with the Rudmik and Smith methodology,Citation10 which was designed to ensure the completeness of cited literature and appropriateness of recommendations. This consensus statement is a result of the review process and is aimed at providing a practical reference for physicians in the primary- and secondary-care settings. It can complement existing guideline recommendations and is not intended to dictate individualized patient care or define the standard of care for AR.
The Role of Oral Antihistamines
Oral antihistamines have been used for decades, especially by otolaryngologists, primarily for the treatment of AR. This class of medication is indicated in a variety of conditions, thus making these agents readily accessible. Histamine is a naturally occurring messenger, important in neural transmission and maintenance of the natural physiological state via its four types of receptors, H1–4. In general, the H1 receptor is predominantly found in the central nervous system (CNS), H2 receptors in the gastric parietal cells, H3 receptors in both CNS and cardiovascular system and finally H4 receptors on inflammatory cells such as neutrophils, eosinophils, basophils, and mast cells.Citation11 Due to the diverse distribution of histamine receptors and their myriad effects, antihistamines have a broad mechanism of action.
Oral antihistamines that are widely available in the market mostly target H1 and H2 receptors. Within this class are the old and new generation antihistamines, primarily distinguished by their ability to cross the blood brain barrier (BBB).Citation11 H1-antihistamines were previously thought to be histamine antagonists, but recent research found that their mechanism of action is more that of an H1 inverse agonist, whereby they downregulate H1 receptors and stabilize mast cells from degranulation.Citation12
Due to concerns of their adverse effects, first generation oral antihistamines are not recommended as a treatment for AR.Citation13 Reported adverse effects include sedation or somnolence, dizziness, orthostatic hypotension, tachycardia, palpitations, syncope, QT prolongation, ventricular arrhythmias, torsade de pointes leading to sudden cardiac arrest, blurred vision, dry eyes and mouth, urinary retention, constipation, and erectile dysfunction.Citation11,Citation13–16 When taken at night, first-generation antihistamines, despite having sedative effects, disrupt the sleep cycle, resulting in a shorter rapid eye movement stage, and increasing the latency towards restful sleep.Citation11,Citation13 Consequently, patients often experience hungover the next morning with impaired attention and residual sleepiness.Citation11,Citation13 First-generation antihistamines use in children is correlated with poor school performance and bad or low grades in examination.Citation13 In contrast to the first generation, the use of second generation oral antihistamines is recommended in patients with allergic rhinitis. The characteristics of the commonly used second generation oral antihistamines in AR are listed in .Citation17–22
Table 1 Characteristics of Commonly Used Second-Generation Oral Antihistamines
Irrespective of the plasma peak of each individual second-generation oral antihistamines, there is marked variability in the duration of the effect influenced by the volume of distribution, the presence of active metabolites over a period of time and affinity of binding to plasma proteins.Citation23 A prolonged pharmacological effect is expected when there is higher affinity to plasma proteins. The considerations for the use of oral antihistamines are summarized in .
Box 1 Practical Considerations for the Use of Oral Antihistamines
Summary of Evidence
Second-generation oral antihistamines have diminished ability to cross the BBB, making them non-sedating, in addition to being antimuscarinic, antiserotoninergic and anti α-adrenergic.Citation11–14 Unlike their predecessors, this class of drugs was subject to intense clinical investigations via randomized controlled trials, which established their superior safety profile and efficacy.Citation13 Examples of these second-generation antihistamines include, but are not limited to, bilastine, cetirizine, desloratadine, fexofenadine, levocetirizine and loratadine.Citation11 Majority of the second-generation antihistamines demonstrated good efficacy, faster onset of action, and excellent safety profiles compared with their predecessors.Citation14 Due to its exceptional profile and efficacy, the second-generation antihistamines are the drugs of choice, for airline and military pilots, together with those who perform multiple tasks and operate machinery.Citation11,Citation13 Even at high doses, there was no impairment of cognition, alertness, and memory associated with second-generation antihistamines use.Citation11 Nonetheless, it is worth mentioning that not all second-generation antihistamines possess similar attributes. The use of cetirizine has been associated with sedative effect and all patients must be counselled about such effect prior to prescription.Citation24,Citation25
Place in Primary Care
Oral antihistamines are recommended as a first-line therapy for mild-to-moderate seasonal and mild perennial AR. Maintenance of therapy may be required especially during periods of allergen exposure. A step-up approach to INCS alone or in combination with oral antihistamines should be considered in refractory patients following 2–4 weeks of treatment.
Place in Secondary Care
First-line therapy for mild-to-moderate seasonal and mild perennial AR and as add on combination therapy with INCS for moderate-to-severe AR.
Safety Concerns and Compliance Issues (Adults/Children)
Only two second-generation antihistamines were found to have caused cardiac arrhythmias, prolonged QT intervals and torsade de pointes, namely astemizole and terfenadine.Citation11,Citation13,Citation26 Both drugs have since been removed from the international market. All subsequent second-generation antihistamines have undergone rigorous testing at recommended and higher dosages for cardiac safety, as well as for drug interactions and effects on vulnerable populations like the elderly and young.Citation11,Citation13,Citation27
Recommendation (Adults/Children)
Second-generation oral antihistamines play key role in the treatment of AR due to their superiority over their predecessors. Yet, medical practitioners continue to use first-generation oral antihistamines, primarily due to its cheaper cost than second-generation antihistamines. Driving the shift from first-generation to second-generation oral antihistamines depends on the affordability of the latter and practitioners’ greater awareness of the former’s detrimental adverse effects.
Benefit versus Harm
Various studies have not shown any adverse effects in overdoses up to 30 times the recommended dose for second generation oral antihistamines.Citation11 Antihistamines are excreted in breastmilk and although not known to be harmful, manufacturers generally advise that antihistamines should be avoided during this period.
The Role of Intranasal Corticosteroids with/Without Intranasal Antihistamines
AR is characterized by an influx of inflammatory cells and mediators into the nasal mucosa after allergen exposure. The response is biphasic, encompassing an early phase of nasal pruritis, itching, rhinorrhea, and a late phase with predominant nasal congestion. INCS reduce the production of inflammatory enzymes and cytokines together with inhibition of lymphocyte proliferation, and thus useful in treating both early and late inflammatory response.Citation28 The onset of action is after a few hours, but the clinical improvement may not be experienced immediately and the full effect only apparent after a few days or weeks.Citation28,Citation29 The characteristics of the most used INCS in AR are listed in .Citation28
Table 2 Characteristics of Commonly Used Intranasal Corticosteroids
Summary of Evidence
Treatment with INCS relieves early and late-phase clinical symptomsCitation30 with few exacerbations. This is achieved by downregulating the recruitment and activation of inflammatory cells, increasing degradation of neuropeptides and reducing epithelial cell activity, vascular permeability and chemokine secretions.Citation31 INCS have a high receptor binding affinity to cells in the nasal mucosa with negligible systemic absorption resulting in excellent safety profiles.Citation32 The anti-inflammatory effect develops over several hours or days while the vasoconstrictive effect is observed as quickly as 2–20 minutes after administration, though, there are significant variability between each individual INCS.Citation33 A fraction of the dose that makes its way into the gastrointestinal tract is bio-transformed during the first liver passage before entering systemic circulation.Citation33
Intranasal antihistamines (INA) exert their anti-inflammatory effects on mediators including histamine, leukotrienes, cytokines, and chemokines. These effects are seen at clinically relevant concentrations of the topical drug compared with oral antihistamines, which require higher concentrations than the usual levels achieved by routine dosing. The high local concentration allows for additional pharmacological effects such as mast cell stabilization, inhibition of chemokines release, inflammatory cell chemotaxis and migration, and decreased influx of inflammatory cells in the mucosa to be expressed, anti-inflammatory actions that usually were not seen with the use of oral antihistamines.Citation34 A comparative study investigating the outcomes of treatment between INA and oral antihistamines found INA was preferable in patients with bothersome nasal congestion due to its rapid onset of action.Citation35 In contrast, oral antihistamines were preferable in young children, especially those at risk of developing asthma, those with poor compliance and those bothered by histamine-associated local symptoms of itching or red and watery eyes. Both INCS and INA have been shown to reduce the overall symptoms of AR.Citation36
While INCS alone can be used for all categories of AR in general, INCS-INA combination may be used for those not responding to INCS monotherapy.Citation5,Citation37 Though the treatment using combined INCS and oral antihistamines is no different to INCS alone, the INCS-INA combination therapy has been demonstrated to be superior to INCS alone.Citation37–39 The clinical impact of INCS-INA is significantly higher than that of INCS and oral antihistamines, as INA has a synergistic effect on INCS.Citation39
INCS are generally easy to administer but may be challenging for disabled or younger patients. At present, there are two different delivery mechanisms available, one of which is a side press system that is more user-friendly for younger patients. The absorption of an INCS can be altered by the condition of the nasal mucosa. Nonetheless, the preferred INCS should be selected based on patient’s choice and physician’s assessment of compliance.Citation40,Citation41
Place in Primary Care
Treatment with INCS can be initiated in mild perennial AR or moderate-to-severe AR for both seasonal and perennial types (). Factors that should be considered when treating AR with INCS or INCS-INA combination are symptomatic control, patient compliance, patient concerns, ease of administration of nasal spray, nasal conditions, adverse effects, age and pregnancy. Duration of INCS treatment should be for the period that the patient is symptomatic or exposed to the allergen. It is advisable that patients requiring long-term use of INCS should undergo further examination, which can include endoscopic nasal examination to exclude other pathology such as septal deviation, nasal granulomatous disease, and nasal polyps. If patient’s symptoms persist or do not improve with treatment, primary care practitioners should refer patients for specialist assessment.
Box 2 Practical Considerations for the Use of Intranasal Corticosteroids with/Without Intranasal Antihistamines
Place in Secondary Care
Patients may consult secondary care practitioners for initial treatment of their AR or upon referral by primary care practitioners. Secondary care practitioners include pediatricians, pulmonologists, allergists and otorhinolaryngologists. While INCS play a main role in the management of AR, INCS-INA is recommended if symptoms persist despite INCS treatment. The combination INCS-INA is also recommended for patients with moderate-to-severe AR and patients with lower airway hyperreactivity or recurrent complications arising from uncontrolled AR. Concurrent or alternative treatment with other modalities is recommended for patients who do not adequately respond to regular use of INCS-INA, patients with poor tolerance and patients with poor treatment adherence.
Safety Concerns and Compliance Issues (Adults/Children)
Lack of patient compliance to regular INCS or INCS-INA therapy remains a constant challenge in the management of AR.Citation7 Reasons for lack of compliance are patients’ own forgetfulness, improvement in symptoms, unpleasant odor or taste, and fear of the bad effects of steroids. Health education is necessary at the onset of treatment to explain the importance of compliance for disease control and on the safety profile of INCS/INCS-INA. In patients who report adequate control of symptoms with the intermittent use of INCS/INCS-INA, it would be necessary to revisit patient’s symptoms, frequency of medication use and clinical findings before determining if the patient should change to an alternative treatment modality. In patients who remain non-compliant to INCS/INCS-INA and report inadequate symptom control despite satisfactory counselling, other treatment modalities should be considered.
Adverse effects with INCS are uncommon and limited to local effects. These include sneezing, burning, epistaxis, nasal dryness, crusting, throat irritation and bitter aftertaste. The incidence of these adverse reactions is comparable to those observed with placebo.Citation35 Systemic absorption is minimal. Mucosal atrophy, as evaluated by nasal biopsy, was not seen after one year of fluticasone or mometasone useCitation42,Citation43 or after 5.5 years of budesonide use.Citation44 No studies have conclusively shown that growth in children was affected with the continued use of INCS for a year.Citation45,Citation46 A meta-analysis by Valenzuela et al has shown that INCS was not associated with a significant risk of elevated intraocular pressure or posterior subcapsular cataract; but there was a significant risk in the presence of glaucoma.Citation47 Adverse effects of INCS-INA are limited to dryness and bitter taste in the throat. Advice to gargle with water might be helpful after using INCS-INA nasal spray to lessen the bitter taste.
It is advisable to prescribe INCS in children based on the minimum age and dose recommendation for each INCS (). At present, budesonide is the only INCS classified under US Food and Drug Administration (FDA) pregnancy category B.
Recommendation (Adults/Children)
INCS are superior to oral antihistamines for the relief of nasal symptoms like obstruction, rhinorrhea, nasal itch, and postnasal drip.Citation29,Citation48 INCS also improve ocular symptoms and lower airway symptoms when there is concomitant allergic conjunctivitis and asthma. There is no added benefit of routinely combining INCS with an oral antihistamine or a leukotriene receptor antagonist (LTRA) in AR.Citation49,Citation50 Correct intranasal spray technique and adherence to prescribed dosage and dosing frequency are necessary to achieve the desired benefit from INCS or INCS-INA. Optimal effects of INCS are seen after 1–2 weeks of constant use. To sustain symptomatic control in persistent AR, maintenance therapy with INCS or INCS-INA over a period of months to years is necessary.
Benefit versus Harm
The benefits of INCS and INCS-INA in AR outweigh the potential adverse effects. INCS should be avoided in patients with glaucoma.
The Role of Systemic Corticosteroids
Systemic corticosteroids were first introduced for the treatment of arthritisCitation51 and gradually gained popularity in the management of hay fever. Previously, it was not uncommon for seasonal AR to be treated with a short course of oral steroids or depot injection of corticosteroids. Due to adverse events associated with systemic corticosteroids, their use has gradually reduced with the introduction of INCS for the treatment of AR.Citation52 The potential adverse effects with their long term or recurrent use are infections, myopathies, osteoporosis, aseptic necrosis of the femur, skin thinning, hyperglycemia, weight gain, fluid retention, cushingoid appearance, neuropsychiatric disorders, cataract, glaucoma, and hypertension.
Synthetic corticosteroids, which include prednisone, prednisolone, methylprednisolone, dexamethasone, hydrocortisone, cortisone, and fludrocortisone, are used for their immunosuppressive, anti-proliferative and anti-inflammatory effects. Plasma cortisol levels are reduced after a single intramuscular injection of corticosteroids with its maximum effect seen at 3 days and its effect lasting up to 3 weeks.Citation53
Through their trans-repression and trans-activation effect, corticosteroids reduce the synthesis of cytokines, affect recruitment, localization, protein synthesis and survival of inflammatory cells such as eosinophils, inhibit expression of adhesion molecules, and involved in remodelling.Citation53 In AR, systemic corticosteroids reduce the influx of eosinophils and levels of eosinophil mediators (major basic protein, eosinophil cationic protein and eosinophil-derived neurotoxin) in nasal secretions during the late phase response. The considerations for the use of systemic corticosteroids are summarized in .
Box 3 Practical Considerations for the Use of Systemic Corticosteroids
Summary of Evidence
Oral corticosteroids produce a dose-related reduction in certain symptoms of AR. The use of low doses of oral corticosteroid was able to significantly reduce nasal blockage, drainage and eye symptoms but not itching, rhinorrhea and sneezing.Citation54 Laursen et al showed no difference between daily oral prednisolone for 3 weeks versus a single intramuscular betamethasone injection for the relief of nasal and eye symptoms in AR. However, plasma cortisol level measured at 3 weeks was significantly reduced after the daily administration of oral prednisolone.Citation55
The beneficial effects of oral or depot steroids on AR symptoms and quality of life are significant when compared with placebo and oral antihistamines. Systemic corticosteroids and INCS have similar efficacy in controlling symptoms of AR, though eye symptoms respond better with systemic corticosteroids.Citation56
Despite certain benefits of systemic corticosteroids in the treatment of AR, international guidelines strongly recommend against their use due to concerns of adverse effects.Citation57
Place in Primary Care
The use of systemic steroids for AR is not recommended in the primary care setting. If the patient does not respond to the recommended first-line treatment for AR, the patient should be referred to the secondary care practitioner.
Place in Secondary Care
Systemic corticosteroids should not be prescribed for AR routinely. In patients suffering from very severe and therapy-resistant symptoms, other treatment options should be utilized before resorting to a short course of systemic corticosteroids. The exception is patients with concomitant acute exacerbation of bronchial asthma or AR with nasal polyposis, where short course of oral prednisolone can be given for 2 to 5 days, in mild-to-moderate exacerbations.
Recommendation
Systemic corticosteroids are not recommended in AR and are only considered a potential treatment option in exceptional cases.
Benefit versus Harm
The risk of adverse effects with the use of systemic corticosteroids outweighs their benefits.
The Role of Oral and Intranasal Decongestants
Decongestants stimulate α-adrenergic receptors leading to vasoconstriction of the blood vessels in the nasal mucosa, paranasal sinuses, and upper respiratory tract. These drugs reduce the volume of edematous mucosal tissue.Citation58,Citation59 Decongestants can be administered by per oral or topical intranasal route. They have rapid onset of action in decreasing the symptom of nasal obstruction and last up to 12 hours.Citation60 Oral decongestants produce more common adverse effects compared with topical intranasal sprays such as dryness of nasal mucosa, increased blood pressure, headache, dizziness, nausea, vomiting, insomnia, restlessness, anxiety, intracranial bleed, stroke, arrhythmias, myocardial infarction, urinary dysfunction and psychosis.Citation61 Decongestants are contraindicated in patients with heart disease, hypertension, benign prostatic hypertrophy, thyroid disease and diabetes. Prolonged use (more than 10 days) may cause the development of rhinitis medicamentosa (rhinopathia medicamentosa). Hence, decongestants should not be taken for longer than a week.Citation62,Citation63 Decongestant use in pregnancy is generally avoided due to the possibility of vasoconstriction of the uterine arteries, causing reduction of fetal blood supply.Citation64 The considerations for the use of oral and intranasal decongestants are summarized in .
Box 4 Practical Considerations for the Use of Oral and Intranasal Decongestants
Summary of the Evidence
Thongngarm et al conducted a randomized controlled trial to investigate the effectiveness of oxymethazoline plus INCS for the treatment of chronic rhinitis. They saw that nasal symptom and quality of life scores were significantly improved in the intervention group.Citation65 A similar study by Baroody et al reported that the total nasal symptom score of combination treatment (fluticasone furoate and oxymetazoline) once daily over 4 weeks was lower compared with placebo or oxymetazoline alone.Citation66 When acoustic rhinometry was compared between the groups at the end of 4 weeks of treatment, the combination produced a significantly higher nasal volume compared with both placebo and oxymetazoline alone.Citation66 On the contrary, a systematic review and meta-analysis by Khattiyawittayakun et al with 1071 participants found no difference between combined therapy of decongestant with INCS and INCS alone in the outcomes of total nasal symptom scores, nasal congestion scores, and the Rhinoconjunctivitis Quality of Life Questionnaire score. Moreover, there were no differences on objective tests for nasal patency after 1 week.Citation67
Place in Primary Care
Short-term topical and systemic decongestants may be used to treat nasal obstruction in severe AR.
Place in Secondary Care
Short-term add-on treatment to oral antihistamine and INCS in patients with severe AR to accelerate relief of symptoms, particularly nasal obstruction.
Safety Concerns and Compliance Issues (Adults/Children)
Oral and topical intranasal decongestants should be used with caution in adults with heart disease, hypertension, benign prostatic hypertrophy, thyroid disease, and diabetes. Decongestants should not be taken for more than 1 week.
Recommendation (Adults/Children)
Oral decongestants are contraindicated in young children and pregnancy. Topical decongestant is an option in adults and older children.
Benefit versus Harm
Due to the adverse effects, the risks of oral decongestants must be weighed against their potential benefits.
The Role of Leukotriene Receptor Antagonists
The role of leukotrienes in the pathogenesis of AR was apparent from the high levels of cysteinyl leukotrienes (CysLTs) found in the secretions taken from patients with persistent rhinitis.Citation68 It has also been revealed that the intensity of leukotrienes-induced inflammation is a hundred-fold greater than that of histamines. The leukotrienes-induced inflammation has been linked directly with nasal congestion and obstruction. When exposed to allergens, cystenyl leukotrienes (CysLTs) are released by the nasal mucosa during both early and late phase reactions, thus inducing symptoms of AR like nasal and airway congestions.Citation3,Citation68,Citation69 The considerations for the use of leukotriene receptor antagonists are summarized in .
Box 5 Practical Considerations for the Use of Leukotriene Receptor Antagonists
Summary of Evidence
Montelukast, an LTRA, has been used for AR treatment for more than 3 decades following approval by the US FDA.Citation70 Due to its high affinity and high selectivity for CysLT receptors, montelukast is beneficial in the control of allergic symptoms.Citation70 The improvement obtained with the use of montelukast includes all cardinal symptoms (including nasal and ocular) of AR, quality of sleep, and quality of life in general. In patients aged 15 years and older who had seasonal AR, the use of montelukast reduced 13% of peripheral blood eosinophil counts compared with placebo.Citation3,Citation71,Citation72 LTRA is recommended for the control of asthma and rhinitis in many guidelines including GINA (Global Initiative for Asthma), PRACTALL (Practicing Allergology) and ARIA (Allergic rhinitis and its impact on asthma) following the reports of the efficacy of LTRA in the treatment of AR since 1990s.Citation3,Citation71–75
Although montelukast provides good cost-benefit value, it is nonetheless less effective than INCS.Citation71,Citation72
A systematic review and meta-analysis compared the use of INCS, LTRA and antihistamines for the treatment of AR.Citation76 LTRAs were equivalent to antihistamines for nasal symptoms but were less effective than antihistamines in the quality of life. Although the combination of LTRAs with antihistamines was better for nasal symptoms than either monotherapy, the combination was not significant for the improvement of quality of life.
Another important factor when considering montelukast is its safety, especially in elderly. Since there are no studies on the safety of montelukast in pregnancy, it should not be prescribed in pregnant mothers.Citation3 Montelukast should also be prescribed with caution in nursing mothers as it is not known whether montelukast is eliminated via human breast milk.Citation3,Citation71,Citation72
Place in Primary Care
Montelukast can be used as an adjunctive therapy in moderate to severe AR with refractory symptoms.
Place in Secondary Care
Montelukast is prescribed as a second-line treatment following INCS treatment failure in cases of AR with asthma. It may be prescribed as an add-on therapy to the combination of INCS and oral antihistamine if there is inadequate response. It may also be considered for AR with night-time symptoms and severe nasal block.
Safety Concerns and Compliance Issues (Adults/Children)
Due to the possibility of inducing neuropsychiatric symptoms like agitation, hallucinations, suicidality, the US FDA mandated the inclusion of a boxed warning that specified montelukast should be reserved for patients with AR who do not response or tolerate other treatment options.Citation71 The adverse effects associated with overdose of montelukast include abdominal pain, somnolence, thirst, headache, vomiting, psychomotor hyperactivity, and less frequently, convulsion.Citation70
Recommendation (Adults/Children)
Montelukast may be used in adults and children above 2 years. Recent evidence suggests that when used in combination with oral antihistamines, montelukast produces significant control of the overall symptoms of AR.Citation77 Montelukast is useful in combination with antihistamines for the reduction of nasal symptoms.
Benefit versus Harm
It is beneficial as an add-on therapy in uncontrolled AR but the impact of higher cost must be considered.
The Role of Biologics
Biologics have been used to treat moderate-to-severe allergic asthma and chronic urticaria with good outcomes. Globally, regulatory bodies have not approved the use of biologics as a treatment of AR except for Japan where approval was obtained since 2019.Citation55,Citation78,Citation79 Thus far, there are no biologics currently approved for the treatment of AR in Malaysia.
Biologics differs according to their mechanisms of action. Omalizumab is an anti-immunoglobulin E (IgE) monoclonal antibody which selectively binds to free IgE and decreases its availability for expression at IgE receptors on mast cells, basophils, and dendritic cells.Citation79–82 Other biologics like mepolizumab, and benralizumab bind to interleukin (IL)-5 receptor to induce eosinophil and basophil apoptosis.Citation83 A relatively new biologic is dupilumab, which targets IL-4. Only omalizumab is used in children under 12 years of age while other biologics are prescribed in the older age groups. The considerations for the use of biologics are summarized in .
Box 6 Practical Considerations for the Use of Biologics
Summary of Evidence
Most trials conducted on biologics as a treatment for AR used omalizumab. Studies have shown the use of omalizumab as a single agent or combined with immunotherapy to treat patients with seasonal or perennial AR was superior to placebo. Omalizumab therapy was well tolerated without any significant adverse effects. The application of omalizumab in patients with moderate to severe AR improved nasal symptoms score, eye symptoms score and quality of life with concomitant reduction of IgE levels and use of rescue medication, regardless of the type of allergens.Citation84–86 Thus, omalizumab was suited for use in polysensitized allergic patients. Omalizumab treatment significantly improved both asthma and rhinitis quality of life questionnaire scores.Citation80 Omalizumab in combination with immunotherapy improved AR symptoms and reduced immunotherapy-derived systemic allergic reactions.Citation57,Citation87–92 Omalizumab pretreatment in patients receiving accelerated immunotherapy demonstrated reduction of systemic and respiratory-related reactions, which makes it use potentially a good application to prevent immunotherapy-derived anaphylaxis.Citation89,Citation92 Irrespective of the allergen used in immunotherapy, combination therapy with omalizumab was superior to immunotherapy alone for the reduction of use of rescue medication, symptoms load and number of symptomatic days which may prove beneficial for polysensitized patients undergoing buildup dosing for immunotherapy.Citation87 Likewise, the combination of immunotherapy with omalizumab was superior to omalizumab alone.Citation88
A trial has evaluated the efficacy of omalizumab in patients with concomitant moderate to severe asthma and persistent AR while another study has evaluated omalizumab as an adjunct to subcutaneous injection immunotherapy.Citation57,Citation80,Citation93 There were reductions in symptomatic improvements and quality of life measures in both studies. International guidelines recommend the use of biologics like omalizumab for the treatment of asthma in patients with concomitant AR when conventional medical therapy failed and if there is a clear IgE-dependent allergic component.Citation57,Citation94 Similar recommendation was made for the use of other biologic such as anti-IL-5, which has yielded positive results in the treatment of asthma and other atopic diseases.Citation94 Despite the potential benefits, cost is a major limiting factor.Citation95 Adverse effects reported with use of biologics are anaphylaxis, oropharyngeal pain, increased blood creatine phosphokinase, myalgia, and localized injection site reactions.Citation96
Place in Primary Care
Biologics are not recommended for use at the primary care level.
Place in Secondary Care
Biologics are not approved for the treatment of uncomplicated AR. Special consideration is given to patients with allergic asthma and comorbid AR, who are refractory to conventional pharmacotherapy.
Safety Concerns and Compliance Issues (Adults/Children)
The use of biologics, except for omalizumab, is restricted to patients aged 12 years and above. Adverse events commonly associated with biologics are local reactions at injection sites and rarely anaphylaxis.
Recommendation (Adults/Children)
Omalizumab may be considered as an add-on therapy to immunotherapy for the prevention of immunotherapy-induced adverse effects. It is well tolerated in adults and children.
Benefit versus Harm
Omalizumab is effective and well tolerated for the treatment of nasal symptoms in AR patients. Its use is limited due to the high cost.
The Role of Immunotherapy
By inducing long-term remission following treatment cessation in most patients with AR, immunotherapy is the only treatment that alters the disease course and progression.Citation97–99 Immunotherapy effectively improves symptoms, quality of life and medication use.Citation100–102 Two types of allergen-based immunotherapy supplied in clinical practice are subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT). The administration of SCIT requires weekly dosing based on an incremental schedule from initiation, followed by a maintenance phase of a 4–6 weekly injections for a total treatment duration of 3–5 years. The administration of SLIT involves giving the medication either as a dissolvable tablet (under the tongue) or an aqueous or liquid extract. Compared with SCIT, SLIT is self-administered by patients or caregivers. Only SLIT-tablets are approved by US FDA and other regulatory bodies while the SLIT-drops (liquid extract) are used off-label. The considerations for the use of immunotherapy are summarized in .
Box 7 Practical Considerations for the Use of Immunotherapy
Summary of Evidence
SCIT is effective for both seasonal and perennial rhinitis; the former is caused by pollens (grass, tree) while the latter due to house dust mite and cat fur.Citation103–106 Due to concerns of systemic adverse effects, immunotherapy should only be administered in a controlled environment by trained personnel with access to resuscitation services.Citation73 Thirteen confirmed fatalities have been reported since 2008 associated with the administration of SCIT.Citation107
An alternative to SCIT for the treatment of AR with or without seasonal asthma is SLIT.Citation101 SLIT is safer and well tolerated with fewer risk of systemic adverse effects.Citation108–112 The effects can be curtailed by prescribing a concomitant oral antihistamine for the first 2 weeks or longer following the administration of SLIT. Both grass pollen and house dust mites SLIT are commonly used. As a safety precaution, the first dose of SLIT should be taken under the observation of trained personnel with access to resuscitation facilities. Uncontrolled asthma has been reported to be associated with severe systemic reactions after both SCIT and SLIT administration.Citation113 Observation is required for up to 1–2 hours following administration.Citation72 After the first dose, SLIT is self-administered daily at home. There is strong evidence to support the role of immunotherapy to prevent the progression of seasonal rhinitis to asthma and development of new sensitizations.Citation114–116 Both SCIT and SLIT are effective and safe for use in children and adults with additional long-term clinical advantage over the short-term benefits of pharmacotherapy.Citation108 There is no difference in adherence rates between SCIT and SLIT, with an overlapping range of 18% to over 90%.Citation117–120 The main contributing factors for the lack of adherence are experience of adverse effects, poor efficacy, forgetfulness and inconvenience.Citation121–124
Place in Primary Care
Immunotherapy is not recommended for use at the primary care level.
Place in Secondary Care
Immunotherapy is recommended for moderate to severe seasonal or perennial AR confirmed by positive skin prick test and/or elevated allergen-specific IgE, refractory to optimal medical therapy of INCS and oral antihistamine. Depending on patient’s preference, either SCIT or SLIT may be prescribed.
Safety Concerns and Compliance Issues (Adults/Children)
SCIT is associated with a risk of localized reactions at injection site and anaphylaxis while SLIT is associated with local site reactions of swelling and itchiness around oral cavity and throat.
Recommendation (Adults/Children)
The administration of both SCIT and SLIT are efficacious and well tolerated in children and adults with AR.
Benefit versus Harm
The long-term benefit of immunotherapy outweighs the associated risks of anaphylaxis and local reactions. A minimum of 5-year symptom-free period or longer may be attained following 3 years of administration.
The Role of Intranasal Sodium Cromoglycate
Sodium cromoglycate (SC) is a mast cell stabilizer, which prevents the release of inflammatory mediators like histamine from mast cells. SC, a bis-cromone compound, has no intrinsic bronchodilator, antihistaminic or anti-inflammatory activity.Citation125 Through stabilization of mast cells, SC blocks allergic reactions like allergen-induced bronchospasm, which led to its approval by the US FDA for the treatment of asthma.Citation125,Citation126 Since then, SC has been increasingly used for the treatment of other conditions involving mast cell mechanism, including AR, allergic conjunctivitis and mastocytosis.Citation127
SC is derived from the plant Amni visnaga and traditionally used for its spasmolytic properties by the ancient Egyptians. SC has been demonstrated to impede the function of chloride channels, which serves to regulate cell volume and prevents extracellular calcium influx into the cytoplasm of the mast cells. SC reduces the release of inflammatory mediators by inhibiting the degranulation of the sensitized mast cells. It has also been revealed that SC has an anti-inflammatory property unrelated to mast cell activation, which impedes inflammatory mediators, specifically macrophages, eosinophils, monocytes, and platelets activating factor.Citation128 The considerations for the use of intranasal sodium cromoglycate are summarized in .
Box 8 Practical Considerations for the Use of Intranasal Sodium Cromoglycate
Summary of Evidence
SC is effective compared with placebo for the treatment of AR, with greater efficacy in seasonal AR compared with perennial AR. Its effectiveness increases with higher dosage and dosing frequency.Citation129
A randomized controlled trial by Lejeune et al demonstrated that 4% SC administered four times a day for a duration of 4 weeks significantly improved nasal symptom score compared with placebo when used in patients with house-dust mite allergy. There was a significant and specific reduction of neutrophil influx in the early phase reaction.Citation130 Additionally, the study demonstrated intranasal SC reduces the platelet activating factor.Citation130
Nonetheless, SC is less effective than INCS. Compared with intranasal mometasone furoate and levocabastin specifically, nasal SC was less effective in all nasal symptoms, global evaluation of efficacy, eosinophil cationic protein concentration and nasal inspiratory flow measurement.Citation131
Overall, intranasal SC is safe in children and pregnant women.Citation15,Citation132 SC is available as a 4% nasal spray solution with a short half-life, requires dosing of three to six times per day.Citation133 SC has an excellent safety profile, with no major adverse effects reported. Minor adverse effects of SC include nasal irritation, burning, sneezing, epistaxis, and unpleasant taste.Citation133
Place in Primary Care
Intranasal SC is not recommended for AR treatment in the primary care setting.
Place in Secondary Care
Intranasal SC is recommended as treatment for rhinorrhea, sneezing and itchiness in pregnant women (no teratogenic effects have been reported) or in patients who cannot tolerate INCS.Citation125 It is also recommended as an add-on therapy for symptom-specific treatment such as sneezing and nasal congestion.
Safety Concerns and Compliance Issues (Adults/Children)
SC is generally well tolerated with minor local effects.
Recommendation (Adults/Children)
SC is recommended in children above 2 years of age and in pregnant women.
Benefit versus Harm
SC is beneficial as a protective measure in patients with known allergen and in patients with mild to moderate symptoms. It reduces nasal symptoms such as nasal blockage, rhinorrhea, sneezing, and nasal pruritus. SC is disadvantaged by its short half-life, which requires a dosing frequency of 4 times a day.
The Role of Intranasal Anticholinergics
The role of anticholinergic lies with its reduction of parasympathetic tone, which aims to lessen secretion and rhinorrhea.Citation134 Anticholinergic medications have a rapid onset of action, yet frequent dosing is required to achieve optimal effect. To date, ipratropium bromide (IB) is the only commercially available intranasal anticholinergic agent.Citation135 In patients with AR, the parasympathetic pathway is stimulated once allergens enter the nose, causing the release of acetylcholine, which subsequently causes hypersecretion of muscarinic receptors in the nasal mucous gland.Citation136 As IB is a cholinergic receptor antagonist, it prevents hypersecretion of nasal mucous glands by blocking the interaction of acetylcholine on muscarinic receptors.Citation136 The considerations for the use of intranasal anticholinergics are summarized in .
Box 9 Practical Considerations for the Use of Intranasal Anticholinergics
Summary of Evidence
Intranasal IB is effective in decreasing the severity and duration of the rhinorrhea but has no effects on other nasal symptoms. To date, there is no published meta-analysis regarding the usage of topical IB. Cytology studies involving nasal scrapings performed after intranasal IB revealed no notable changes in the number of eosinophils, basophils, and neutrophils.Citation137 IB is more effective when combined with an INCS than as a monotherapy.Citation138 IB is easy and safe to administer in children with allergic-rhinitis-related rhinorrhea.Citation139 IB has a rapid onset of action but short half-life that requires administration of six times a day. Its absorption has been reported to be less than 10%.Citation140
Adverse effects associated with IB include nasal dryness, irritation, epistaxis, and burning sensation. Systemic anticholinergic effects reported in overdose cases include agitation and decrease gastrointestinal motility.Citation140 Although IB has an excellent safety profile, it must be used cautiously in patients with prostate hypertrophy and narrow-angle glaucoma.Citation141 There are no studies of IB use in pregnancy.
Place in Primary Care
Intranasal IB is not recommended in the primary care setting.
Place in Secondary Care
Intranasal IB is recommended as an adjunct treatment in patients with uncontrolled rhinorrhea.
Safety Concerns and Compliance Issues (Adults/Children)
IB is safe in both adults and children. There are no studies are available in pregnant women. Its common adverse effects are minor local effects including nasal irritation, epistaxis and burning sensation.
Recommendation (Adults/Children)
IB can be prescribed in children above 12 years of age but is not recommended in pregnant women.
Benefit versus Harm
IB is only useful for excessive rhinorrhea and requires frequent administration, up to six times daily due to its short half-life.
Conclusions
Most AR patients can be treated effectively by pharmacological intervention at primary and secondary care settings. The effective control of symptoms ensures patient satisfaction and improves compliance and adherence to treatment which are key to obtain superior outcome and good quality of life. With a clear understanding of the benefit of each specific pharmacological agent, primary care practitioners can manage most AR patients circumventing the patients from having to consult specialists at secondary care. Patients with refractory or complicated AR require escalation to secondary care level where appropriate specific pharmacological treatment can be initiated.
Disclosure
Jeevanan Jahendran reports personal fees from Inova Pharmaceuticals (S’pore) Pte. Ltd. (M’sia Branch), Viatris, Menarini Singapore Pte Ltd, Reckitt Benckiser (Malaysia) Sdn. Bhd., and (M) Sdn Bhd, outside the submitted work. Ralph Mösges reports personal fees from Menarini Asian Pacific, during the conduct of the study, and received personal fees from ALK, grants from ASIT biotech, personal fees from Allergopharma, Allergy Therapeutics, grants and personal fees from Bencard, grants from Leti, grants, personal fees and non-financial support from Lofarma, non-financial support from Roxall, grants and personal fees from Stallergenes, grants from Optima, personal fees from Friulchem, personal fees from Hexal, Servier, Klosterfrau, non-financial support from Atmos, personal fees from Bayer, non-financial support from Bionorica, personal fees from FAES, GSK, MSD, Johnson & Johnson, Meda, personal fees and non-financial support from Novartis, non-financial support from Otonomy, personal fees from Stada, and UCB, non-financial support from Ferrero, grants from BitopAG, Hulka, personal fees from Nuvo, grants from Ursapharm, personal fees from Menarini, Mundipharma, Pohl-Boskamp, grants from Inmunotek. The authors report no other potential conflicts of interest in this work.
Acknowledgments
A. Menarini Singapore Pte Ltd offered logistical support, which included medical editing support, for the development of this consensus statement.
References
- Passali D, Cingi C, Staffa P, Passali F, Muluk NB, Bellussi ML. The international study of the allergic rhinitis survey: outcomes from 4 geographical regions. Asia Pac Allergy. 2018;8(1):e7. doi:10.5415/apallergy.2018.8.e7
- Cingi C, Gevaert P, Mösges R, et al. Multi-morbidities of allergic rhinitis in adults: European Academy of Allergy and Clinical Immunology task force report. Clin Transl Allergy. 2017;7(1):17. doi:10.1186/s13601-017-0153-z
- Katelaris CH, Lai CKW, Rhee CS, et al. Nasal allergies in the Asian-Pacific population: results from the allergies in Asia-Pacific survey. Am J Rhinol Allergy. 2011;25(Suppl 1):S3–S15. doi:10.2500/ajra.2011.25.3674
- Ho TM, Murad S, Kesavapillai R, Singaram SP. Prevalence of allergy to some inhalants among rhinitis patients in Malaysia. Asian Pac J Allergy Immunol. 1995;13(1):11–16.
- Brozek JL, Bousquet J, Agache I, et al. Allergic rhinitis and its impact on asthma guidelines – 2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi:10.1016/j.jaci.2017.03.050
- Tosca MA, Marseglia GL, Ciprandi G, et al. The treatment of allergic rhinitis in asthmatic children and adolescents: practical outcomes from the real-world “ContoL’Asma” study. Eur Ann Allergy Clin Immunol. 2021;53(03):143–145. doi:10.23822/EurAnnACI.1764-1489.171
- Bousquet J, Schunemann HJ, Togias A, Bachert C, Erhola M, Hellings PW. Next-generation allergic rhinitis and its impact on asthma (ARIA) guidelines for allergic rhinitis based on grading of recommendations assessment development and evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol. 2020;145(1):70–80.e3. doi:10.1016/j.jaci.2019.06.049
- Abdullah B, Snidvongs K, Recto M, Poerbonegoro NL, Wang Y. Primary care management of allergic rhinitis: a cross-sectional study in four ASEAN countries. Multidiscip Respir Med. 2020;15:726. doi:10.4081/mrm.2020.726
- Licari A, Castagnoli R, Tosca MA, Marseglia G, Ciprandi G. Personalized therapies for the treatment of allergic rhinitis. Exp Rev Prec Med Drug Dev. 2019;4(5):275–281. doi:10.1080/23808993.2019.1681896
- Rudmik L, Smith TL. Development of an evidence based review with recommendations using an online iterative process. Int Forum Allergy Rhinol. 2011;1(6):431–437. doi:10.1002/alr.20095
- Simons FER, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011;128(6):1139–1150. doi:10.1016/j.jaci.2011.09.005
- May JR, Dolen WK. Management of allergic rhinitis: a review for the community pharmacist. Clin Ther. 2017;39(12):2410–2419. doi:10.1016/j.clinthera.2017.10.006
- Fein MN, Fischer DA, O’Keefe AW, Sussman GL. CSACI position statement: newer generation H1-antihistamines are safer than first-generation H1-antihistamines and should be the first-line antihistamines for the treatment of allergic rhinitis and urticaria. Allergy Asthma Clin Immunol. 2019;15(1):61. doi:10.1186/s13223-019-0375-9
- Morgan MM, Khan DA, Nathan RA. Treatment for allergic rhinitis and chronic idiopathic urticaria: focus on oral antihistamines. Ann Pharmacother. 2005;39(12):2056–2064. doi:10.1345/aph.1E638
- Small P, Keith PK, Kim H. Allergic rhinitis. Allergy Asthma Clin Immunol. 2018;14(Suppl 2):51. doi:10.1186/s13223-018-0280-7
- Dykewicz MS, Wallace DV, Amrol DJ, et al. Rhinitis 2020: a practice parameter update. J Allergy Clin Immunol. 2020;146(4):721–767. doi:10.1016/j.jaci.2020.07.007
- Bilastine. Full prescribing information. A. Menarini; 2021.
- Cetirizine. Full prescribing information. GlaxoSmithKline. Available from: https://www.mims.com/malaysia/drug/info/zyrtec?type=full. Accessed April 14, 2022.
- Desloratidine. Full prescribing information. Organon. Available from: https://www.mims.com/malaysia/drug/info/aerius?type=full. Accessed April 14, 2022.
- Fexofenadine. Full prescribing information. Available from: https://www.mims.com/malaysia/drug/info/fexofenadine?mtype=generic. Accessed April 14, 2022.
- Levocetirizine. Full prescribing information. Organon. Available from: https://www.mims.com/malaysia/drug/info/levocetirizine?mtype=generic. Accessed April 14, 2022.
- Loratadine. Full prescribing information. Organon. Available from: https://www.mims.com/malaysia/drug/info/loratadine?mtype=generic. Accessed April14, 2022.
- Tiligada E, Ennis M. Histamine pharmacology: from Sir Henry Dale to the 21st century. Br J Pharmacol. 2020;177(3):469–489. doi:10.1111/bph.14524
- Singh Randhawa A, Mohd Noor N, Md Daud MK, Abdullah B. Efficacy and safety of bilastine in the treatment of allergic rhinitis: a systematic review and meta-analysis. Front Pharmacol. 2022;12:731201. doi:10.3389/fphar.2021.731201
- Snidvongs K, Seresirikachorn K, Khattiyawittayakun L, Chitsuthipakorn W. Sedative effects of levocetirizine: a systematic review and meta-analysis of randomized controlled studies. Drugs. 2017;7(2):175–186. doi:10.1007/s40265-016-0682-0
- Scadding GK, Kariyawasam HH, Scadding G, et al. BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis (Revised Edition 2017; First edition 2007). Clin Exp Allergy. 2017;47(7):856–889. doi:10.1111/cea.12953
- Kawauchi H, Yanai K, Wang D-Y, Itahashi K, Okubo K. Antihistamines for allergic rhinitis treatment from the viewpoint of nonsedative properties. Int J Mol Sci. 2019;20(1):213. doi:10.3390/ijms20010213
- Benninger MS, Ahmad N, Marple BF. The safety of intranasal steroids. Otolaryngol Head Neck Surg. 2003;129(6):739–750. doi:10.1016/j.otohns.2003.10.001
- Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ. 1998:317:1624–1629.
- Marple BF. Targeting congestion in allergic rhinitis: the importance of intranasal corticosteroids. Allergy Asthma Proc. 2008;29(3):232–240. doi:10.2500/aap.2008.29.3110
- Meltzer EO, Ratner PH, McGraw T. Oral phenylephrine HCl for nasal congestion in seasonal allergic rhinitis: a randomized, open-label, placebo-controlled study. J Allergy Clin Immunol Pract. 2015;3(5):702–708. doi:10.1016/j.jaip.2015.05.007
- Mygind N, Nielsen LP, Hoffmann H, et al. Mode of action of intranasal corticosteroids. J Allergy Clin Immunol. 2001;108(1):16–25. doi:10.1067/mai.2001.115561
- Rot P, Rapiejko P, Jurkiewicz D. Intranasal steroid therapy – EPOS 2020. Otolaryngol Pol. 2020;74(3):41–44. doi:10.5604/01.3001.0014.2449
- Lieberman P. Intranasal antihistamines for allergic rhinitis: mechanism of action. Allergy Asthma Proc. 2009;30(4):345–348. doi:10.2500/aap.2009.30.3263
- Chipps BE, Harder JM. Antihistamine treatment for allergic rhinitis: different routes, different outcomes? Allergy Asthma Proc. 2009;30(6):589–594. doi:10.2500/aap.2009.30.3287
- Kaliner M, Berger WE, Ratner PH, Siegel CJ. The efficacy of intranasal antihistamines in the treatment of allergic rhinitis. Ann Allergy Asthma Immunol. 2011;106(2):6–11. doi:10.1016/j.anai.2010.08.010
- Feng A, Deng C, Li L, et al. Efficacy of intranasal antihistamine in the treatment of allergic rhinitis: a meta-analysis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;49(10):832–838.
- Du K, Qing H, Zheng M, Wang X, Zhang L. Intranasal antihistamines is superior to oral H1 antihistamines as an add-on therapy to intranasal corticosteroids for treating allergic rhinitis. Ann Allergy Asthma Immunol. 2020;125(5):589–596.e3. doi:10.1016/j.anai.2020.06.038
- Seresirikachorn K, Chitsuthipakorn W, Kanjanawasee D, Khattiyawittayakun L, Snidvongs K. Effects of H1 antihistamine addition to intranasal corticosteroid for allergic rhinitis: a systemic review and meta-analysis. Int Forum Allergy Rhinol. 2018;8(10):1083–1092. doi:10.1002/alr.22166
- Neffen H, Wingertzah MA. Ciclesonide, a hypotonic intranasal corticosteroid. Allergy Asthma Proc. 2010;31(31 Suppl 1):S29–S37. doi:10.2500/aap.2010.31.3348
- Sharpe SA, Sandweiss V, Tuazon J, et al. Comparison of the flow properties of aqueous suspension corticosteroid nasal sprays under differing sampling conditions. Drugs Div Ind Pharm. 2003;29:1005–1012.
- Holm AF, Fokkens WJ, Godthelp T, Mulder PG, Vroom TM, Rijntjes E. A 1-year placebo-controlled study of intranasal fluticasone propionate aqueous nasal spray in patients with perennial allergic rhinitis: a safety and biopsy study. Clin Otolaryngol Allied Sci. 1998;23(1):69–73. doi:10.1046/j.1365-2273.1998.00096.x
- Minshall W, Ghaffar O, Cameron L, et al. Assessment by nasal biopsy of long-term use of mometasone furoate aqueous nasal spray (Nasonex) in the treatment of perennial rhinitis. Otolaryngol Head Neck Surg. 1998;118(5):648–654. doi:10.1177/019459989811800514
- Pipkorn U, Pukander J, Suonpaa J, Makinen J, Lindqvist N. Long-term safety of budesonide nasal aeroson: a 5.5-year follow-up study. Clin Allergy. 1988;18(3):253–259. doi:10.1111/j.1365-2222.1988.tb02867.x
- Gradman J, Caldwell MF, Wolthers OD. A 2-week, crossover study to investigate the effect of fluticasone furoate nasal spray on short-term growth in children with allergic rhinitis. Clin Ther. 2007;29:1738–1747.
- Mener DJ, Shargorodsky J, Varadhan R, Lin SY. Topical intranasal corticosteroids and growth velocity in children: a meta-analysis. Int Forum Allergy Rhinol. 2015;5(2):95–103. doi:10.1002/alr.21430
- Valenzuela C, Liu JC, Vila PM, Simon L, Doering M, Lieu JEC. Intranasal corticosteroids do not lead to ocular changes: a systematic review and meta-analysis. Laryngoscope. 2019;129(1):6–12. doi:10.1002/lary.27209
- Vervloet D, Charpin D, Desfougeres JL. Intranasal fluticasone once daily compared with once-daily cetirizine in the treatment of seasonal allergic rhinitis: results of a multicentre, double-blind study. Clin Drug Investig. 1997;13(6):291–298. doi:10.2165/00044011-199713060-00001
- Ratner PH, Van Bavel JH, Martin BG, et al. A comparison of the efficacy of fluticasone propionate aqueous nasal spray and loratidine, alone and in combination, for the treatment of seasonal allergic rhinitis. J Fam Pract. 1998;47(2):118–125.
- Di Lorenzo G, Pacor ML, Pellitteri ME, et al. Randomized placebo-controlled trial comparing fluticasone aqueous nasal spray in monotherapy, fluticasone plus cetirizine, fluticasone plus montelukast and cetirizine plus montelukast for seasonal allergic rhinitis. Clin Exp Allergy. 2004;34:259–267.
- Benedek TG. History of the development of corticosteroid therapy. Clin Exp Rheumatol. 2011;29(5 Suppl 68):S-5-1.
- Siegel SC. Topical intranasal corticosteroid therapy in rhinitis. J Allergy Clin Immunol. 1988;81(5 Pt 2):984–991. doi:10.1016/0091-6749(88)90166-2
- Hox V, Lourijsen E, Jordens A, et al. Benefits and harm of systemic steroids for short- and long-term use in rhinitis and rhinosinusitis: an EAACI position paper. Clin Transl Allergy. 2020;10(1):1. doi:10.1186/s13601-019-0303-6
- Brooks CD, Karl KJ, Francom SF. Oral methylprednisolone acetate (Medrol Tablets) for seasonal rhinitis: examination of dose and symptom response. J Clin Pharmacol. 1993;33(9):816–822. doi:10.1002/j.1552-4604.1993.tb01957.x
- Laursen LC, Faurschou PH, Svendsen UG, Weeke B. Intramuscular betamethasone dipropionate vs oral prednisolone in hay fever patients. Allergy. 1987;42(3):168–172. doi:10.1111/j.1398-9995.1987.tb02194.x
- Karaki M, Akiyama K, Mori N. Efficacy of intranasal steroid spray (mometasone furoate) on treatment of patients with seasonal allergic rhinitis: comparison with oral corticosteroids. Auris Nasus Larynx. 2013;40(3):277–281. doi:10.1016/j.anl.2012.09.004
- Wise SK, Lin SY, Toskala E, et al. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. 2018;8(2):108–352. doi:10.1002/alr.22073
- Klimeka L, Sperla A, Beckerb S, Mösgesc R, Tomazicd PV. Current therapeutical strategies for allergic rhinitis. Expert Opin Pharmacother. 2019;20(1):83–89. doi:10.1080/14656566.2018.1543401
- Kaya Z, Tuncez A. Adverse cardiac effects of decongestant agents. Eur J Gen Med. 2013;10:32–35.
- Pritchard S, Glover M, Guthrie G, et al. Effectiveness of 0.05% oxymetazoline (Vicks Sinex Micromist) nasal spray in the treatment of objective nasal congestion demonstrated to 12 h post-administration by magnetic resonance imaging. Pulm Pharmacol Ther. 2014;27(1):121–126. doi:10.1016/j.pupt.2013.08.002
- Shao IH, Wu CC, Tseng HJ, et al. Voiding dysfunction in patients with nasal congestion treated with pseudoephedrine: a prospective study. Drug Des Devel Ther. 2016;10:2333–2339. doi:10.2147/DDDT.S108819
- Yuta A, Ogawa Y. Clinical review of 33 cases of rhinitis medicamentosa by decongestant nasal spray. Arerugi. 2013;62:1623–1630.
- Deckx L, de Sutter AI, Guo L, Mir NA, van Driel ML. Nasal decongestants in monotherapy for the common cold. Cochrane Database Syst Rev. 2016;10:CD009612. doi:10.1002/14651858.CD009612.pub2
- Malone M, Kennedy TM. Side effects of some commonly used allergy medications (decongestants, anti-leukotriene agents, antihistamines, steroids and zinc) and their safety in pregnancy. Int J Aller Medications. 2017;3(1):024. doi:10.23937/2572-3308.1510024
- Thongngarm T, Assanasen P, Pradubpongsa P, Tantilipikorn P. The effectiveness of oxymetazoline plus intranasal steroid in the treatment of chronic rhinitis: a randomised controlled trial. Asian Pac J Allergy Immunol. 2016;34(1):30–37. doi:10.12932/AP0649.34.1.2016
- Baroody F, Brown D, Gavanescu L, DeTineo M, Naclerio RM. Oxymetazoline adds to the effectiveness of fluticasone furoate in the treatment of perennial allergic rhinitis. J Allergy Clin Immunol. 2011;127(4):927–934. doi:10.1016/j.jaci.2011.01.037
- Khattiyawittayakun L, Seresirikachorn K, Chitsuthipakorn W, Kanjanawasee D, Snidvongs K. Effects of decongestant addition to intranasal corticosteroid for chronic rhinitis: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2018;8(12):1445–1453. doi:10.1002/alr.22193
- Scadding G. Cytokine profiles in allergic rhinitis. Curr Allergy Asthma Rep. 2014;14(5):435. doi:10.1007/s11882-014-0435-7
- Pfaar O, Bachert C, Bufe A, et al. Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases. Allergo J Int. 2014;23(8):282–319. doi:10.1007/s40629-014-0032-2
- Singulair (montelukast sodium) [prescribing information]. Whitehouse Station N.J.: Merck & Co.; 2006.
- United States Department of Health and Human Services – Food and Drug Administration. Use of real-world evidence to support regulatory decision making for medical devices. guidance for industry and food and drug administration staff; 2017. Available from: https://www.fda.gov/media/99447/download. Accessed October 12, 2021.
- Scadding GK. Optimal management of allergic rhinitis. Arch Dis Child. 2015;100(6):576–582. doi:10.1136/archdischild-2014-306300
- Roberts G, Pfaar O, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73(4):765–798. doi:10.1111/all.13317
- Zielen S, Devillier P, Heinrich J, Richter H, Wahn U. Sublingual immunotherapy provides long-term relief in allergic rhinitis and reduces the risk of asthma: a retrospective, real-world database analysis. Allergy. 2018;73(1):165–177. doi:10.1111/all.13213
- Meltzer EO. The treatment of vasomotor rhinitis with intranasal corticosteroids. World Allergy Organ J. 2020;2(8):166–179. doi:10.1097/WOX.0b013e3181af7c93
- Wilson AM, O’Byrne PM, Parameswaran K. Leukotriene receptor antagonists for allergic rhinitis: a systematic review and meta-analysis. Am J Med. 2004;116(5):338–344. doi:10.1016/j.amjmed.2003.10.030
- Krishnamoorthy M, Mohd Noor N, Mat Lazim N, Abdullah B. Efficacy of montelukast in allergic rhinitis treatment: a systematic review and meta-analysis. Drugs. 2020;80:1831–1851.
- Hirano K, Suzaki I, Uruma S, et al. Impact of omalizumab on pollen-induced seasonal allergic rhinitis: an observational study in clinical practice. Int Forum Allergy Rhinol. 2021;11(11):1588–1591. doi:10.1002/alr.22827
- Wright JD, Chu H-M, Huang C-H, Ma C, Chang TW, Lim C. Structural and physical basis for anti-IgE therapy. Sci Rep. 2015;5(1):11581. doi:10.1038/srep11581
- Vignola AM, Humbert M, Bousquet J, et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy. 2004;59(7):709–717. doi:10.1111/j.1398-9995.2004.00550.x
- Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128(5):989–995. doi:10.1016/j.jaci.2011.07.056
- Long AA. Monoclonal antibodies and other biologic agents in the treatment of asthma. MAbs. 2009;1(3):237–246. doi:10.4161/mabs.1.3.8352
- Pelaia C, Calabrese C, Vatrella A, et al. Benralizumab: from the basic mechanism of action to the potential use in the biological therapy of severe eosinophilic asthma. Biomed Res Int. 2018;2018:4839230. doi:10.1155/2018/4839230
- Ghadersohi S, Tan BK. Contemporary pharmacotherapy for allergic rhinitis and chronic rhinosinusitis. Otolaryngol Clin North Am. 2017;50(6):1135–1151. doi:10.1016/j.otc.2017.08.009
- Adelroth E, Rak S, Haahtela T, et al. Recombinant humanized mAb-E25, an anti-IgE mAb, in birch pollen-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2000;106(2):253–259. doi:10.1067/mai.2000.108310
- Chervinsky P, Casale T, Townley R, et al. Omalizumab, an anti-IgE antibody, in the treatment of adults and adolescents with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2003;91(2):160–167. doi:10.1016/S1081-1206(10)62171-0
- Kuehr J, Brauburger J, Zielen S, et al. Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;109(2):274–280. doi:10.1067/mai.2002.121949
- Rolinck-Werninghaus C, Hamelmann E, Keil T, et al. The co-seasonal application of anti-IgE after preseasonal specific immunotherapy decreases ocular and nasal symptom scores and rescue medication use in grass pollen allergic children. Allergy. 2004;59(9):973–979. doi:10.1111/j.1398-9995.2004.00552.x
- Casale TB, Busse WW, Kline JN, et al. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2006;117(1):134–140. doi:10.1016/j.jaci.2005.09.036
- Klunker S, Saggar LR, Seyfert-Margolis V, et al. Combination treatment with omalizumab and rush immunotherapy for ragweed-induced allergic rhinitis: inhibition of IgE-facilitated allergen binding. J Allergy Clin Immunol. 2007;120(3):688–695. doi:10.1016/j.jaci.2007.05.034
- Kopp MV, Brauburger J, Riedinger F, et al. The effect of anti-IgE treatment on in vitro leukotriene release in children with seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;110(5):728–735. doi:10.1067/mai.2002.128804
- Massanari M, Nelson H, Casale T, et al. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol. 2010;125(2):383–389. doi:10.1016/j.jaci.2009.11.022
- Kopp MV, Hamelmann E, Zielen S, et al. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and comorbid seasonal allergic asthma. Clin Exp Allergy. 2009;39(2):271–279. doi:10.1111/j.1365-2222.2008.03121.x
- Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126(3):466–476. doi:10.1016/j.jaci.2010.06.047
- Tsabouri S, Tseretopoulou X, Priftis K, Ntzani EE. Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. J Allergy Clin Immunol Pract. 2014;2:332–340.e1.
- Pinto JM, Mehta N, DiTineo M, Wang J, Baroody FM, Naclerio RM. A randomized, double-blind, placebo-controlled trial of anti-IgE for chronic rhinosinusitis. Rhinology. 2010;48(3):318–324. doi:10.4193/Rhino09.144
- Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341(7):468–475. doi:10.1056/NEJM199908123410702
- Didier A, Malling HJ, Worm M, et al. Post-treatment efficacy of discontinuous treatment with 300IR 5-grass pollen sublingual tablet in adults with grass pollen-induced allergic rhinoconjunctivitis. Clin Exp Allergy. 2013;43(5):568–577. doi:10.1111/cea.12100
- Durham SR, Emminger W, Kapp A, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129(3):717–725. doi:10.1016/j.jaci.2011.12.973
- Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;1:558–562. doi:10.1016/S0091-6749(98)70271-4
- Canonica GW, Cox L, Pawankar R, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7(1):6. doi:10.1186/1939-4551-7-6
- Passalacqua G, Durham SR. Allergic rhinitis and its impact on asthma update: allergen immunotherapy. J Allergy Clin Immunol. 2007;119(4):881–891. doi:10.1016/j.jaci.2007.01.045
- Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S, Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;1:CD001936. doi:10.1002/14651858.CD001936.pub2
- Eifan AO, Calderon MA, Durham SR. Allergen immunotherapy for house dust mite: clinical efficacy and immunological mechanisms in allergic rhinitis and asthma. Expert Opin Biol Ther. 2013;13(11):1543–1556. doi:10.1517/14712598.2013.844226
- Alvarez-Cuesta E, Cuesta-Herranz J, Puyana-Ruiz J, Cuesta-Herranz C, Blanco-Quiros A. Monoclonal antibody-standardized cat extract immunotherapy: risk-benefit effects from a double-blind placebo study. J Allergy Clin Immunol. 1994;93(3):556–566. doi:10.1016/S0091-6749(94)70067-2
- Varney VA, Edwards J, Tabbah K, Brewster H, Mavroleon G, Frew AJ. Clinical efficacy of specific immunotherapy to cat dander: a double-blind placebo-controlled trial. Clin Exp Allergy. 1997;27(8):860–867. doi:10.1111/j.1365-2222.1997.tb01225.x
- Epstein TG, Murphy-Berendts K, Liss GM, Bernstein DI. Risk factors for fatal and nonfatal reactions to immunotherapy (2008–2018): postinjection monitoring and severe asthma. Ann Allergy Asthma Immunol. 2021;127(1):64–69.e1. doi:10.1016/j.anai.2021.03.011
- Dhami S, Nurmatov U, Arasi S, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta-analysis. Allergy. 2017;72:1597–1631.
- Bufe A, Eberle P, Franke-Beckmann E, et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. 2009;123(1):167–173. doi:10.1016/j.jaci.2008.10.044
- Didier A, Malling H-J, Worm M, et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;120(6):1338–1345. doi:10.1016/j.jaci.2007.07.046
- Halken S, Agertoft L, Seidenberg J, et al. Five-grass pollen 300IR SLIT tablets: efficacy and safety in children and adolescents. Pediatr Allergy Immunol. 2010;21(6):970–976. doi:10.1111/j.1399-3038.2010.01050.x
- Maloney J, Bernstein DI, Nelson H, et al. Efficacy and safety of grass sublingual immunotherapy tablet, MK-7243: a large randomized controlled trial. Ann Allergy Asthma Immunol. 2014;112(2):146–153. doi:10.1016/j.anai.2013.11.018
- Calderon MA, Simons FE, Malling HJ, Lockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy. 2012;67(3):302–311. doi:10.1111/j.1398-9995.2011.02761.x
- Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62(8):943–948. doi:10.1111/j.1398-9995.2007.01451.x
- Des RA, Paradis L, Menardo JL, Bouges S, Daures JP, Bousquet J. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. VI. Specific immunotherapy prevents the onset of new sensitizations in children. J Allergy Clin Immunol. 1997;99(4):450–453. doi:10.1016/S0091-6749(97)70069-1
- Pajno GB, Barberio G, De LF, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy. 2001;31(9):1392–1397. doi:10.1046/j.1365-2222.2001.01161.x
- Incorvaia C, Masieri S, Berto P, Scurati S, Frati F. Specific immunotherapy by the sublingual route for respiratory allergy. Allergy Asthma Clin Immunol. 2010;6(1):29. doi:10.1186/1710-1492-6-29
- Scurati S, Frati F, Passalacqua G, Puccinelli P, Hilaire C, Incorvaia C. Italian Study Group on SLIT Compliance. Adherence issues related to sublingual immunotherapy as perceived by allergists. Patient Prefer Adherence. 2010;4:141–145. doi:10.2147/ppa.s10217
- Egert-Schmidt AM, Kolbe JM, Mussler S, Thum-Oltmer S. Patients’ compliance with different administration routes for allergen immunotherapy in Germany. Patient Prefer Adherence. 2014;8:1475–1481. doi:10.2147/PPA.S70326
- Kiel MA, Röder E, van Wijk RG, Al MJ, Hop WC, Rutten-van Mölken MP. Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol. 2013;132(2):353–360. doi:10.1016/j.jaci.2013.03.013
- Vaswani R, Garg A, Parikh L, Vaswani S. Non-adherence to subcutaneous allergen immunotherapy: inadequate health insurance coverage is the leading cause. Ann Allergy Asthma Immunol. 2015;115(3):241–243. doi:10.1016/j.anai.2015.06.018
- Leader BA, Rotella M, Stillman L, DelGaudio JM, Patel ZM, Wise SK. Immunotherapy compliance: comparison of subcutaneous versus sublingual immunotherapy. Int Forum Allergy Rhinol. 2016;6(5):460–464. doi:10.1002/alr.21699
- Savi E, Peveri S, Senna G, Passalacqua G. Causes of SLIT discontinuation and strategies to improve the adherence: a pragmatic approach. Allergy. 2013;68(9):1193–1195. doi:10.1111/all.12198
- Makatsori M, Scadding GW, Lombardo C, et al. Dropouts in sublingual allergen immunotherapy trials – a systematic review. Allergy. 2014;69(5):571–580. doi:10.1111/all.12385
- Berman BA, MM Fenton, Girsh LS, et al. Cromolyn sodium in the treatment of children with severe perennial asthma. Pediatrics. 1975;55(5):621–629.
- Howell JBL, Altounyan RE. A double-blind trial of disodium cromoglycate in the treatment of allergic bronchial asthma. Lancet. 1967;2(7515):539–542. doi:10.1016/S0140-6736(67)90499-0
- Storms W, Kaliner MA. Cromolyn sodium: fitting an old friend into current asthma treatment. J Asthma. 2005;42:79.
- Ratner PH, Ehrlich PM, Fineman SM, Meltzer EO, Skoner DP. Use of intranasal cromolyn sodium for allergic rhinitis. Mayo Clin Proc. 2002;77(4):350–354. doi:10.4065/77.4.350
- Long A, McFadden C, DeVine D, Chew P, Kupelnick B, Lau J. Management of Allergic and Nonallergic Rhinitis. Evid Rep Technol Assess. 2002;54:1–6.
- Lejeune M, Lefebvre PP, Delvenne P, et al. Nasal sodium cromoglycate (Lomusol) modulates the early phase reaction of mild to moderate persistent allergic rhinitis in patient. Int Immunopharmacol. 2015;26(1):272–276. doi:10.1016/j.intimp.2015.02.004
- Lange B, Lukat KF, Rettig K, Holtappels G, Bachert C. Efficacy, cost- effectiveness, and tolerability of mometasone furoate, levocabastine, and disodium cromoglycate nasal sprays in the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2005;95(3):272–282. doi:10.1016/S1081-1206(10)61225-2
- Keleş N. Treatment of allergic rhinitis during pregnancy. Am J Rhinol. 2004;18(1):23–28. doi:10.1177/194589240401800106
- NasalCrom. Cromolyn nasal. [drug monograph]. Epocrates Inc.; 2021.
- Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122(3):S1–S84. doi:10.1016/j.jaci.2008.06.003
- Tran NP, Vickery J, Blaiss MS. Management of rhinitis: allergic and non-allergic. Allergy Asthma Immunol Res. 2011;3(3):148–156. doi:10.4168/aair.2011.3.3.148
- Quraishi SA, Davies MJ, Craig TJ. Inflammatory responses in allergic rhinitis: traditional approaches and novel treatment strategies. J Am Osteopath Assoc. 2004;104:7–15.
- Meltzer EO, Orgel HA, Bronsky EA. Perennial allergic rhinitis: a study of its effect on their symptoms, quality of life, and nasal cytology. J Allergy Clin Immunol. 2000;90:242–249. doi:10.1016/0091-6749(92)90078-G
- Dockhorn R, Aaronson D, Bronsky E, et al. Ipratropium bromide nasal spray 0.03% and beclomethasone nasal spray alone and in combination for the treatment of rhinorrhea in perennial rhinitis. Ann Allergy Asthma Immunol. 1999;82(4):349–359. doi:10.1016/S1081-1206(10)63284-X
- Kim KT, Kerwin E, Landwehr L, et al.; Pediatric Atrovent Nasal Spray Study Group. Use of 0.06% ipratropium bromide nasal spray in children aged 2 to 5 years with rhinorrhea due to a common cold or allergies. Ann Allergy Asthma Immunol. 2005;94(1):73–79. doi:10.1016/S1081-1206(10)61289-6
- Ensing K, de Zeeuw RA, Nossent GD, Koeter GH, Cornelissen PJ. Pharmacokinetics of ipratropium bromide after single dose inhalation and oral and intravenous administration. Eur J Clin Pharmacol. 1989;36(2):189–194. doi:10.1007/BF00609193
- Slavin RG. Special considerations in treatment of allergic rhinitis in the elderly: role of intranasal corticosteroids. Allergy Asthma Proc. 2010;31(3):179–184. doi:10.2500/aap.2010.31.3342