Abstract
Purpose
The US National Asthma Education and Prevention Program updates and Global Initiative for Asthma report encourage considering the patient perspective to improve asthma control. The objective of the present study was to collect data about the perceptions, experiences, and concerns of adult patients and caregivers of children with asthma regarding rescue, maintenance, and oral corticosteroid treatments.
Patients and Methods
In-person focus groups were conducted in three cities across the US. Participants also completed patient-reported outcome measures assessing asthma control and experiences.
Results
Focus groups were conducted in demographically and clinically diverse adults with asthma (five groups, n=34), caregivers of children with asthma (five groups, n=35), and adults with a dual diagnosis of asthma and chronic obstructive pulmonary disease (one group, n=5). Only 28% of patients were well-controlled by Asthma Control Test/Asthma Control Test-Caregiver Report and 18% by Asthma Impairment and Risk Questionnaire. Forty-four percent of participants reported not following their prescribed medical plan. Four key themes emerged from the focus groups: (1) asthma symptom control and monitoring are often inadequate; (2) treatments are often used incorrectly; (3) communication between health care professionals and patients or caregivers is often ineffective; and (4) concerns related to treatment and desires to improve treatment.
Conclusion
Control of asthma symptoms is suboptimal in the vast majority of patients and both patients and caregivers do not feel sufficiently informed about asthma. Health care providers should be encouraged to engage patients and caregivers in shared decision making for managing asthma and selecting treatments that integrate patient values, preferences, and lifestyles.
Keywords:
- chronic obstructive pulmonary disease
- qualitative
- inhaler
- maintenance therapy
- rescue therapy
- corticosteroid
- asthma management
- asthma control
- shared decision-making
- Asthma Control Test
- ACT
- Asthma Impairment and Risk Questionnaire
- AIRQ
- Adult Asthma Adherence Questionnaire
- AAAQ
- Treatment Satisfaction Questionnaire for Medication
- TSQM
Introduction
Despite advances in understanding of asthma pathophysiology and the availability of novel precision therapies,Citation1–4 individuals with asthma continue to experience symptoms requiring frequent use of rescue medications and are at risk for exacerbations and death.Citation5–7 In 2022, the US Centers for Disease Control and Prevention reported that 44% of children and more than 60% of adults with asthma in the US were uncontrolled, meaning that they continue to experience morbidity from asthma symptoms.Citation3,Citation4 Poor asthma control increases the risk for exacerbations and death, reduces productivity at work and school, and results in substantial direct and indirect costs.Citation8–10 Over the next 20 years in the US, direct costs associated with uncontrolled asthma are estimated to be $300.6 billion and the total direct and indirect economic burden is estimated to be $963.5 billion.Citation8 Recently, the level of asthma control as determined by the Asthma Impairment and Risk Questionnaire (AIRQ) was found to be a predictor of patient-reported asthma exacerbations over the subsequent 12 months and the probable amount of time to first exacerbation.Citation11 Therefore, as well as reducing morbidity and death, improved asthma control would be expected to deliver considerable cost savings and reduce future asthma exacerbations.
At the patient level, lack of adherence to treatment recommendations, poor inhaler technique, and concerns about the safety and efficacy of inhaled corticosteroids (ICS) are key factors underlying poor asthma control.Citation12–14 Further, many patients rely on short-acting beta agonists (SABA) without concomitant anti-inflammatory medication for symptom relief.Citation15 However, the Global Initiative for Asthma (GINA) no longer recommends SABA-only as the preferred rescue therapy for patients aged ≥12 years.Citation2 Additionally, the US National Asthma Education and Prevention Program (NAEPP) does not include SABA in their preferred treatment regimen for patients aged ≥4 years with moderate to severe persistent asthma (steps 3 and 4).Citation1
To help address barriers to adherence, improve communication about risks, and choose treatments with the best balance of benefits and risks according to each patient’s values, preferences, and lifestyle, both the NAEPPCitation1 and GINACitation2 have long encouraged shared decision making between clinicians and patients. Shared decision making occurs when clinicians and patients make health care and treatment decisions together with the goal of moving patient preferences to more closely align with evidence-based guidelines. Prior research, however, has consistently shown that shared decision making often does not occur during asthma visits,Citation16–18 which manifests itself in poor provider-patient communication. Shared decision making depends on medical practitioners understanding what patients feel is the burden of their disease, how they regard their past and current treatments, and the level of risk they are willing to tolerate. To support and encourage shared-decision making, the objective of the present study was to use focus groups to collect data about the perceptions, experiences, and concerns of adult patients and caregivers of children with asthma regarding rescue, maintenance, and oral corticosteroid treatments.
Methods
Study Design
Between November 2019 and March 2020, 11 in-person focus groups of approximately seven participants each were conducted in New York, Dallas, and Indianapolis (Supplementary Table 1). Five of the focus groups included adults with asthma, five included caregivers of children or adolescents with asthma 4–17 years of age, and one included adults with a dual diagnosis of asthma and chronic obstructive pulmonary disease (COPD). To assure demographic and socioeconomic diversity, five of the focus groups were recruited through patient panels and were held at central sites and six through allergy/immunology or primary care practices and held at the patient’s clinical site. In this manner, patients who might not have internet access would be included. Clinical site patients were recommended by their health care providers.
Prior to the focus groups, all participants completed an online consent form and a questionnaire containing sociodemographic and clinical items and short patient-reported outcome (PRO) instruments related to their condition, treatment satisfaction, and treatment adherence. The Asthma Control Test (ACT)Citation19 is a five-item questionnaire that assesses shortness of breath, asthma symptoms, rescue medications, daily functioning, and asthma control on a 5-point scale of “not controlled at all” to “completely controlled”. The Asthma Control Test-Caregiver Report (ACT-CR)Citation20 is a version of the ACT used for caregivers. The Asthma Impairment and Risk Questionnaire (AIRQ)Citation21 is a 10-question instrument for assessing symptom impairment and exacerbation risk on a scale of from 0 (“best control”) to 10 (“worst control”). The Adult Asthma Adherence Questionnaire (AAAQ)Citation22 is a five-item questionnaire about adherence and barriers to adherence. Each item is scored on a six-point Likert scale from 1 “completely agree” to 6 “disagree completely”. Finally, the Treatment Satisfaction Questionnaire for Medication (TSQM-9)Citation23 is a 14-item questionnaire that assesses patient satisfaction with medication for effectiveness, side effects, convenience, and global satisfaction. The AIRQ, ACT, and TSQM-9 were only completed by adults with asthma, and the ACT-CR was only completed by caregivers. The AAAQ was completed by all participants. Participants could complete the electronic data entry independently or in person with assistance, during a clinical site visit, or just prior to their scheduled focus group interview. Further details of the PRO instruments are included in the Supplement.
The study was approved by the Advarra Institutional Review Board (protocol reference no. Pro00039195) and was conducted and reported in accordance with the Consolidated Criteria for Reporting Qualitative Research guidelines.Citation24 All data collected in this study were strictly confidential in accordance with local, state, and US federal law.
Participants and Eligibility
Potential participants were contacted via e-mail or telephone with information about the study and contact details. Interested individuals were screened for eligibility by telephone prior to the scheduled focus group. To be eligible, panel participants had to report a diagnosis of asthma for themselves (adults) or their child (caregivers) occurring ≥ 6 months before screening and attest to having in their possession ≥ 1 canister of SABA rescue medication in the past 6 months and to using a SABA at least once within the 3 months prior to screening. Potential participants were excluded if they could not provide proof of their asthma medications in the form of a photograph. For participants recruited through clinical sites, staff identified and verified eligibility through a review of patient records.
Participants with a dual diagnosis of asthma and COPD were adults aged ≥ 40 years who met the inclusion criteria for self-reported asthma and COPD (self-reported diagnosis of COPD with a duration of at least six months and also a current smoker or previous smoker with at least a 10 pack-year history: approximately one pack or 20 cigarettes every day for at least 10 years). Potential participants were excluded if they had a diagnosis of a chronic lower respiratory disorder other than asthma or asthma plus COPD or any condition that could result in significant dyspnea independent of asthma or COPD (eg, neuromuscular disease). All focus group participants self-reported being able to speak, read, write, and understand English.
Focus Groups
Focus groups were conducted at a clinical site (for patients recruited through an allergy/immunology or primary care provider) or a central facility (for patients recruited through a panel). Each focus group lasted for approximately 2.5 hours. The focus groups were led by a moderator (ZB or MJB) who was assisted by a co-moderator (TT, SM, or JC). Both groups consisted of individuals trained and experienced in running focus group interviews (Supplementary Table 2).
Focus groups were conducted using a semi-structured interview guide developed using standard approaches. Questions examined perceptions of, preferences for, and experiences with asthma rescue, maintenance, and oral corticosteroid (OCS) treatments, as well as the perceived impacts of these treatments on their asthma and quality of life. Other topics included the participants’ perceptions of different devices used for the asthma treatment, as well as behaviors around scheduled and unscheduled visits to different points of care (eg, emergency department, urgent care, hospitals) for asthma symptoms and exacerbations. During each focus group interview, new concepts or key topics of interest were recorded and probed in subsequent focus groups if they were not covered spontaneously. Due to this approach and because some concepts were raised spontaneously by participants, not all participants provided information relevant to all concepts. Themes were not pre-defined before the focus groups; rather, they emerged during the focus groups. Focus groups were audio recorded and professionally transcribed.
Asthma Severity and Monitoring
Asthma severity was categorized based on current treatments as defined by the GINA 2018 reportCitation25 and the 2007 NAEPP guidelines.Citation26 Briefly, severity was defined as mild for patients treated with GINA steps 1–2/NAEPP steps 1–2 and moderate-to-severe for those treated with GINA steps 3–5/NAEPP steps 3–6. Asthma monitoring by patients and caregivers included asking if and how they monitored asthma, and whether they found asthma monitoring easy.
Analysis
A data coding framework based on the structure of the interview guide was developed by investigators trained in encoding qualitative data (ZB and TT) and refined through discussion with the study team. Transcripts were coded by two investigators (JC, SM) and reviewed by a third investigator (ZB), who discussed discrepancies with the coders. For discrepancies that could not be reconciled, the final coding was determined by the principal investigator (TT). ATLAS.ti version 8 (ATLAS.ti, Berlin, Germany) was used to manage the qualitative data. For qualitative data, percentages were calculated using the number of participants responding or providing information relevant to each concept.
Quantitative Data
The personal characteristics of the participants, answers to the PRO and the PRO scores were analyzed with descriptive statistics. The frequency and percentage for categorical variables (eg, sex) were determined and the mean and standard deviation (SD) were determined for continuous variables (eg, age). PRO scores were compared between participants with mild and moderate-to-severe asthma using the Welch t-test for continuous variables and the Chi-squared test for categorical variables in R version 3.6.1 (R Foundation, Vienna, Austria).
Results
Participants
Focus groups were conducted in adults with asthma (34 participants in five focus groups), caregivers of children or adolescents with asthma 4–17 years of age (35 participants in five focus groups), and adults with a dual diagnosis of asthma and COPD (five participants in one focus group). Thirty-three patients were recruited through panels and 41 by their health care provider (Supplementary Table 1). Most of the participants were female (78%), and the mean age was 42.1 years (). Nearly half (47%) were White and 30% were Black or African American. Overall, 41% were of Hispanic/Latino ethnicity. Most participants (72%) had at least some university or college education. Nearly all adults and children with asthma had medical insurance (97% of adults, 97% of children), including 49% on Medicaid, 35% on employer-provided insurance, and 14% on Medicare. Asthma severity based on treatment was mild (intermittent or mild persistent) for 42% of patients and moderate-to-severe (moderate-to-severe persistent) for 58%.
Table 1 Sociodemographic and Clinical Characteristics of Participants
Quantitative Findings from Questionnaires: Asthma Management
Rescue inhalers were used at least three times weekly by 50% of patients and daily by 25%. Among patients with nebulizers, nebulizers were used at least three times weekly by 31% of patients and daily by 17% (, Supplementary Table 3). Over three-quarters (82%) of patients, including both adults with asthma and children with asthma, had taken OCS for their asthma on 3 or more consecutive days within the last year. Most participants (78%) who reported experience with OCS disliked taking or having their child take OCS. Dual ICS/long-acting beta agonist (LABA) therapies were the most common controller medications reported by adults with asthma (59%) and adults with a dual diagnosis of asthma and COPD (60%), whereas ICS therapies were the most common controller medications reported by caregivers of children with asthma (51%) (Supplemental Table 4).
Most adults with asthma (65%) and children with asthma (74%) saw a primary care provider for the condition, whereas most patients (80%) with a dual diagnosis of asthma and COPD saw a pulmonologist (Supplementary Table 5). Overall, most participants (69%) reported having an asthma action plan created by their doctor and of those who had an action plan, most (84%) of them reported referring to it. Approximately three-quarters (76%) of participants reported monitoring asthma, and most (87%) considered it to be easy. Most caregivers reported that their child at least sometimes monitored their own asthma (88%), although 61% reported needing to assist their child with managing their care at least once a day. Monitoring usually involved counting the number of occurrences of difficulty breathing (62% for adults with asthma, 69% for caregivers of children with asthma, 40% for adults with a dual diagnosis of asthma and COPD) or the number of occurrences of needing to use a rescue inhaler (47% for adults with asthma, 43% for caregivers of children with asthma, 40% for adults with a dual diagnosis of asthma and COPD).
Quantitative Findings from PROs: Asthma Control, Barriers to Treatment, and Satisfaction with Treatment
Asthma control was assessed by ACT/ACT-CR and AIRQ classifications. Overall, only 28% of patients were well controlled according to symptom impairment measures (ACT and ACT-CR scores) and only 18% according to a composite symptom impairment and exacerbation risk instrument (AIRQ scores) (); mean scores for all instruments used did not significantly differ by asthma severity designation.
Table 2 Patient-Reported Outcomes
As assessed using the AAAQ, 44% of all participants reported that they or their child did not follow the medication plan. Participants with both mild and moderate to severe asthma had problems with medication adherence; 50% of participants with mild asthma and 39% of participants with moderate to severe asthma reported barriers in respect to their asthma medication plan. The main contributors to lack of medication adherence varied by asthma severity. For instance, participants with mild asthma often thought they did not need treatment (73%), whereas participants with moderate to severe asthma were more concerned about treatment side effects (54%). Overall, 44% felt that their regular controller medication was not needed, 41% had concerns about side-effects of ICS, 37% said that they forgot to take or have their child take ICS, and 30% reported not being able to afford ICS. Proportions of participants not following the medication plan (p=0.497), forgetting to take ICS (p=0.450), and not being able to afford ICS (p>0.999) did not differ by asthma severity designation; however, feeling that their regular controller medication was not needed was reported more often for patients with less severe asthma (73% for mild vs 22% for moderate to severe; p<0.001), while concerns about side-effects of ICS were reported more often for patients with more severe asthma (23% for mild vs 54% for moderate to severe; p=0.020). Satisfaction with controller treatment was assessed using the TSQM on a scale of 0 to 100, with a higher score representing greater satisfaction. On average, the satisfaction scores for controller treatment were 61.5 for effectiveness, 70.7 for convenience, and 68.5 overall. TSQM scores did not differ significantly according to asthma severity designation.
Qualitative Findings from Focus Group Interviews
Four key themes emerged from the focus group interviews: (1) asthma symptom control and monitoring are often inadequate; (2) treatments are often used incorrectly; (3) communication between health care professionals and patients or caregivers is often ineffective; and (4) concerns related to treatment and desires to improve treatment. Saturation was reached by the end of the 11th focus group and no new concepts were identified at that point.
Theme 1: Asthma Symptom Control and Monitoring are Often Inadequate
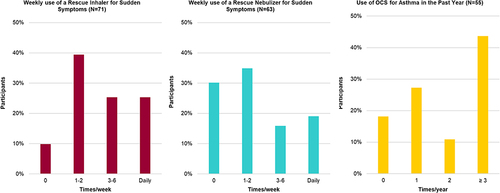
Participants reported varying frequency of rescue medication use (from every few hours to twice weekly), increasing use when symptoms worsened or flare-ups appeared, before or after exercise, or when sick. Among participants who had used OCS, the need for OCS to control symptoms varied from daily to once or twice per year (). Daily to monthly use of OCS was reported by about one-quarter (18/74) of participants. Despite being generally aware of the symptoms and triggers of asthma, only about half (35/74) of participants reported some form of monitoring.
Table 3 Sample Quotes for Theme 1: Asthma Symptom Control and Monitoring are Often Inadequate
Theme 2: Treatments are Often Used Incorrectly
Participants frequently reported not using treatments correctly (). All but two of 23 participants queried about whether they had used someone else’s rescue treatment said they had, and about half of them (n=12) said they had used a family member’s treatment. Further, not using maintenance treatments as often as prescribed was reported by over half (24/40) of participants queried, mostly (20/24) less often than prescribed. When asked why maintenance medication was taken less than prescribed, the most common response was “forgetfulness” due to “busy schedules” or being “tired”. Participants who reported using the maintenance inhaler more than prescribed felt the controller was not effective and hoped increasing the use of the inhaler would improve effectiveness. A few participants mentioned disliking the treatment they were prescribed due to lack of efficacy (n=1), side-effects (n=1), or needing to take too many medications (n=1). Two participants mentioned situations where taking the treatment was not convenient, either because of location or because they felt uncomfortable taking treatment in front of others. Finally, about half of participants queried (17/33) indicated they had used their maintenance medication as a rescue treatment.
Table 4 Sample Quotes for Theme 2: Treatments are Often Used Incorrectly
Theme 3: Communication Between Health Care Professionals and Patients or Caregivers is Often Ineffective
Participants frequently reported insufficient understanding or use of asthma action plans (). Only around half of participants queried (26/49) reported ever having an asthma action plan developed in consultation with their clinician. Not having an action plan was reported more often by participants without a college degree (16/26) and by those with or caring for a child with uncontrolled asthma (ACT-CR score <20; 19/26). Of 17 participants with an action plan who were queried, 12 indicated that they had not followed it or followed it irregularly.
Table 5 Sample Quotes for Theme 3: Communication Between Health Care Professionals and Patients or Caregivers is Often Ineffective
Most participants queried (14/19) said that health care professionals were their primary source of information about asthma, but they frequently felt insufficiently informed or involved in their own care (). Most queried (7/11) felt that they did not receive enough information from their clinicians about their asthma, and most (35/52) did not feel fully or at all involved in decision making when selecting asthma treatments. Most of these (27/35) were adult patients (ACT score <20, AIRQ ≥2) or caregivers of children with uncontrolled asthma (ACT-CR score <20).
Theme 4: Concerns Related to Treatment and Desires to Improve Treatment
The most common concerns about rescue treatments were related to device features, side-effects, and cost or insurance (, ). Similarly, the most common concerns about maintenance were related to device features, side-effects, and cost or insurance, although taste of the formulation was also a common concern (, ). For oral and injectable steroids, the most common concerns were related to side-effects (Supplementary Table 6).
Table 6 Sample Quotes for Theme 4: Treatment Concerns and Desires
Figure 2 Most concerning treatment attributes for rescue (A) and maintenance (B) medications. There are >15 different desired attributes or concerns related to device characteristics reported. The three most mentioned were related to convenience/ease of use, size/discreteness, and dosing-related features (eg, dose counter).
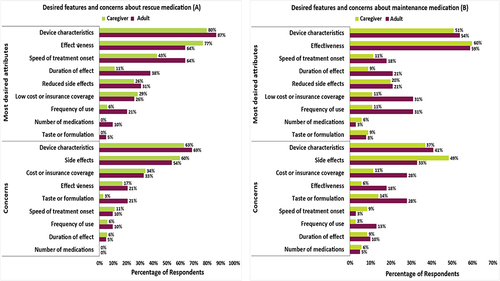
Nearly half of the participants reported concerns about the effectiveness of current asthma treatments (Supplementary Table 7). Most of these participants described concerns that their own or their child’s current asthma treatments were ineffective, half said that the rescue treatment did not work quickly enough, and about one-quarter had concerns about the duration of effectiveness.
Concerns about device features were most often related to rescue treatments (Supplementary Table 7). The most common of these was an inability to properly inhale or feel that the entire dose was inhaled. Lack of a counter, a limited number of doses for each inhaler, inaccurate or malfunctioning counters, and lack of a locking mechanism to avoid accidental pumping and leakage were also mentioned as concerns. Concerns about the pumping mechanism of the inhaler were reported by eight participants, most frequently because of the pump “getting stuck”; however, half of these participants mistakenly discussed features of maintenance inhalers. One caregiver noted that a child could easily remove the canister from the device. Of the 24 participants (11 patients, 13 caregivers) who mentioned preferences for nebulizer- vs inhaler-based rescue therapy, most (7 patients, 8 caregivers) reported preferring nebulizer-based therapy because they believed it to be more effective.
Concerns about side-effects, reported by about three-quarters of participants, were also most often related to rescue treatments (Supplementary Table 7), specifically increased heart rate, jitters or shakiness, and nervousness. For maintenance therapies, the most frequently reported side-effect of concern was oral side-effects (ie, dry mouth, bad breath, bad taste), mostly due to oral thrush. For oral or injectable steroids, the main concerns were weight gain, swelling, bloating, or puffiness; increased hunger or appetite; and bone loss or osteoporosis. Many participants mentioned that they preferred to avoid using OCS and injectable steroids due to the number and degree of side-effects. A few participants reported seeking out other options and using corticosteroids as a “last resort”.
Participants frequently discussed difficulties with affordability due to fluctuating prices of treatments, high co-pays, lack of or limited insurance coverage for different inhalers or treatments, lack of coverage when switching between health insurance policies, and delays in approval by health care providers or insurance companies. Some participants mentioned difficulty obtaining an inhaler or having to use only a treatment covered by insurance.
Desires for rescue medications included device characteristics that improve ease of use and treatments that are faster in onset, have a longer duration of effectiveness, produce fewer side-effects, and are more affordable (, ). For maintenance therapies, patients mentioned the same desires as expressed for rescue treatments, in addition to wanting treatments that could be used less frequently (, ). Participants also mentioned desiring a more convenient rescue or maintenance treatment that does not come in multiple devices. Similarly, for oral and injectable steroids, desires included a treatment that is more effective, more affordable, and has an earlier onset of action (Supplementary Table 6).
Discussion
Using a combination of questionnaires, PROs, and focus groups, this study illustrates that, despite recent advancements in treatment, patients with asthma continue to experience substantial challenges to achieving and sustaining asthma control. Many patients did not have their asthma under control, did not use treatments as prescribed, and did not have, follow, or understand their asthma action plan. Insufficient control was found across asthma severities and in a diverse population, although many participants were highly educated, employed full-time, and had access to specialists and private insurance. Many of these findings are consistent with inadequate uptake of evidence-based treatment guidelines.
A variety of interventions and approaches have been proposed and investigated to improve asthma control, including asthma action plans, patient education, and medication reminders, but results have been inconsistent.Citation27 The patient perspective is increasingly being sought in making treatment decisions, with the aim of empowering patients, improving adherence and, therefore, asthma control. In shared decision making, the patient or caregiver and the clinician discuss treatment options and their risks and benefits as well as the patient’s preferences, values, and lifestyle, with the aim of agreeing on a treatment that is best adapted to the patient while achieving their treatment goals.Citation23 Improved communication and shared decision making between health care professionals and patients with asthma or their caregivers has been shown to improve asthma control in a cross-sectional survey,Citation28 literature reviews,Citation29,Citation30 a focus group study,Citation31 a group-randomized trial of a shared decision-making intervention,Citation32 and a cross-sectional analysis of audio-recorded medical consultations.Citation33 However, further investigation is needed because the results of the few randomized controlled studies performed so far have been mixed.Citation34 Increased education and communication by health care providers to address fears of side-effects may also be warranted, particularly for ICS.Citation35,Citation36 GINA and NAEPP have long recommended shared decision making to help achieve asthma control, and their guidance documents provide specific instructions to clinicians about what information to discuss with patients and caregivers and how to conduct shared decision making.Citation1,Citation2 They also state that a partnership between patients and their health care providers can help patients manage their own asthma, and that patients should be given the opportunity to express their expectations and encouraged to participate in decisions about their treatment. Despite these recommendations, this study found that few patients or caregivers felt adequately informed about asthma medications or sufficiently involved in decisions about their treatment. It appears that efforts to improve asthma control continue to be hampered by a lack of shared decision making between patients and their clinicians.
Asthma control could be improved if the four key themes identified in this study were to be considered during shared decision making. For example, in a randomized clinical trial using BREATHE (BRief intervention to Evaluate Asthma THErapy), a shared decision making intervention, participants included in shared decision making with their physician had better asthma control and reduced symptoms at follow-up compared to participants who received a control intervention.Citation32 Additionally, asthma control could be improved if health care professionals clearly communicate to patients the benefits of pairing ICS with a fast-acting bronchodilator for rescue treatment, as recommended in the newest treatment guidelines.Citation2 These guidelines should be discussed during shared decision making, which should take into account treatment efficacy and patient preferences.
The results of this study may also help guide the development of new medications or devices. Participants mentioned concerns about device-specific features and the frequency of use for both rescue and maintenance therapies. Device characteristics appeared to be particularly important for rescue treatments. The most common concerns about devices were related to dosing, especially an inability to properly inhale or feel that the entire dose was inhaled and not being able to determine the number of remaining doses. As most rescue therapies are designed such that patients can easily determine how many doses remain, it appears that more effective instructions from health care providers may be needed for patients to fully understand the operational characteristics of their inhalation devices. Additional concerns included too few doses in each canister, a malfunctioning pump or counter, lack of a child safety feature, and difficulty removing the cap from the device. Asthma treatment guidelines recommend that providers teach patients to use their inhalers, but research has routinely shown that this practice is not common during asthma visits, possibly because providers do not know how to use these devices themselves.Citation37,Citation38
Participants would like to have treatments with minimal side-effects in an easy-to-use, portable, compact, discrete, and easily stored device. This is consistent with a recent discrete choice experiment showing that asthma and COPD patients prefer maintenance treatments with a reduced risk for side-effects and a pressurized device that needs less frequent dosing and contains a more precise dose counter.Citation39 As in other studies, participants also liked the idea of combining therapies in a single device to help reduce the number of inhalers they need.
Based on the results of the AAAQ, about a third of participants noted that they were unable to afford their ICS. It may prove beneficial for health care providers to discuss co-pay assistance programs or other cost savings programs with their patients to help alleviate cost barriers to treatment adherence. Additionally, generic versions of asthma medications may also provide cost savings to patients and improve adherence.
By collecting information through a combination of focus group interviews, customized questionnaires, and validated PRO measures, this study provided extensive information on perceptions, experiences, and concerns of both patients and caregivers about asthma treatments. A potential limitation is that, in some cases, results from the quantitative and qualitative approaches differed. For example, fewer participants reported using an asthma action plan during interviews than when answering questionnaires. This is not unexpected because, during focus groups, some participants were not asked about or did not volunteer certain information or may not have understood some terms like “asthma action plan”. Further, answers could vary, and tabulation of qualitative responses depended on interpretation and encoding. By contrast, when answering questionnaires, all participants were obliged to respond to questions with a “yes” or “no”, which allows precise tabulation. Finally, although the participants had a wide range of racial, ethnic, and social backgrounds and had varying asthma severities, generalizability may be limited because all participants were from the US and few had a dual diagnosis of asthma and COPD. Another limitation is that panel participants self-reported their diagnosis of asthma, although they were required to provide photographic evidence that they used asthma medications. Additionally, asthma severity was inferred based on patients’ current treatments and was not confirmed by spirometry or the level of therapy needed to achieve asthma control. This may have contributed to misclassification of the severity of asthma in participants, in which patients who were considered to have mild asthma may have had more severe asthma. Moreover, the percentages of women (78%) and those with college degrees (44%) in our study population were slightly higher than those in the overall asthma population (64% female)Citation40 or the general US population (38% with a college degree)Citation41 and may have affected the generalizability of the results. Also, we did not analyze how confounders affected patient use of asthma medications. However, discrete choice experiments that were informed by the results of the current study will be published that analyze the role of confounders on patient preferences of asthma treatments. Additionally, although needs for care may differ depending on the age of a child, we did not collect information based on the age group of the children represented in the caregiver group; rather, this group was included to ensure we had proper asthma patient representation. Lastly, although we highlight the importance of shared decision making between patients and clinicians, other treatment aspects such as cost, side effects, and device characteristics may not always be solved by the clinician.
Conclusions
Despite advances in treatments and long-standing recommendations to include patients with asthma and their caregivers in management decisions, the vast majority of patients in the US have suboptimal control and do not feel sufficiently informed about or involved in their care, regardless of demographic, socioeconomic, and clinical status. Additionally, participants brought up concerns about device characteristics, side effects, and cost of asthma treatments. To address these shortcomings and concerns, health care providers should be encouraged to engage in shared decision making with patients and their caregivers in managing asthma and selecting treatments that reflect their values, preferences, and lifestyles.
Abbreviations
AAAQ, Adult Asthma Adherence Questionnaire; ACT, Asthma Control Test; ACT-CR, Asthma Control Test-Caregiver Report; AIRQ, Asthma Impairment and Risk Questionnaire; COPD, chronic obstructive pulmonary disease; GINA, Global Initiative for Asthma; ICS, inhaled corticosteroids; NAEPP, National Asthma Education and Prevention Program; OCS, oral corticosteroids; PRO, patient-reported outcome; SABA, short-acting beta-agonist; TSQM-9; Treatment Satisfaction Questionnaire for Medication.
Data Sharing Statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, therefore, the data is not available.
Ethics Approval and Informed Consent
The study was approved by the Advarra Institutional Review Board (protocol reference no. Pro00039195). Prior to the focus groups, all participants completed an online consent form. This study complied with the Declaration of Helsinki.
Author Contributions
M. George, Z. Balantac, C. Gillette, N. Farooqui, T. Tervonen, C. Thomas, I. Gilbert, H. Gandhi, and E. Israel contributed to the conception and design of the study. Z. Balantac, T. Tervonen, and C. Thomas contributed to the acquisition of the data. M. George, Z. Balantac, C. Gillette, N. Farooqui, T. Tervonen, C. Thomas, I. Gilbert, H. Gandhi, and E. Israel contributed to the analysis and interpretation of the data, drafted or revised the article; agreed on the journal to which the article was submitted; gave final approval of the version to be published, and agree to be accountable for all aspects of the work and to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
Maureen George works as a consultant for AstraZeneca, Genentech, Sanofi Genzyme/Regeneron, Teva. She also serves as a speaker for AstraZeneca. Zaneta Balantac, Tommi Tervonen, and Caitlin Thomas are employees of Evidera, which was paid by AstraZeneca for work related to this study. Chris Gillette works as a consultant for AstraZeneca. Nabeel Farooqui is part of a speaker bureau for AstraZeneca, GlaxoSmithKline, Teva, Regeneron, and Genentech. Ileen Gilbert and Hitesh Gandhi are employees and shareholders of AstraZeneca. Elliot Israel reports personal fees from AB Science, Amgen, Biometry, Cowen, Equillium, Glaxo, Merck, NIH, PCORI, Allergy and Asthma Network, Genentech, NHLBI, Westchester Medical Center, Yale School of Medicine, Pneuma Respiratory, PPS Health, Regeneron, Sanofi Genzyme, grants and personal fees from AstraZeneca, Avillion, Novartis, personal fees and non-financial support from TEVA, grants from Gossamer Bio, non-financial support from Circassia, NAEPP: National Asthma Education Prevention Program, Journal of Allergy and Clinical Immunology, royalties from UpToDate, and stock option from Vorso Corp, outside the submitted work.
Acknowledgments
The authors thank Phil Leventhal, PhD, Holly Richendrfer, PhD, and Stephen Gilliver, PhD of Evidera for providing medical writing support, which was funded by AstraZeneca in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Additional information
Funding
References
- Cloutier MM, Baptist AP, Blake KV; Expert Panel Working Group of the National Heart, Lung and Blood Institute administered and coordinated National Asthma Education Prevention Program Coordinating Committee (NAEPPCC). 2020 focused updates to the asthma management guidelines: a report from the National Asthma education and prevention program coordinating committee expert panel working group. J Allergy Clin Immunol. 2020;146(6):1217–1270. doi:10.1016/j.jaci.2020.10.003
- Global Initiative for Asthma. Global strategy for asthma management and prevention; 2020. Available from: https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_04-1-wms.pdf. Accessed February 16, 2021.
- US Center for Disease Control and Prevention. Uncontrolled asthma among children with current asthma, 2018–2020; 2022. Available from: https://www.cdc.gov/asthma/asthma_stats/uncontrolled-asthma-children-2018-2020.htm. Accessed August 25, 2022.
- US Centers for Disease Control and Prevention. Uncontrolled asthma among adults, 2019; 2022. Available from: https://www.cdc.gov/asthma/asthma_stats/uncontrolled-asthma-adults-2019.htm. Accessed August 25, 2022.
- Loo SL, Wark PA. Recent advances in understanding and managing asthma. F1000Res. 2016;5. doi:10.12688/f1000research.9236.1
- Martinez FD. The state of asthma research: considerable advances, but still a long way to go. Am J Respir Crit Care Med. 2019;199(4):397–399. doi:10.1164/rccm.201901-0013ED
- Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. 2015;46(3):622–639. doi:10.1183/13993003.00853-2015
- Yaghoubi M, Adibi A, Safari A, FitzGerald JM, Sadatsafavi M. The projected economic and health burden of uncontrolled asthma in the United States. Am J Respir Crit Care Med. 2019;200(9):1102–1112. doi:10.1164/rccm.201901-0016OC
- Sullivan PW, Slejko JF, Ghushchyan VH, et al. The relationship between asthma, asthma control and economic outcomes in the United States. J Asthma. 2014;51(7):769–778. doi:10.3109/02770903.2014.906607
- Bateman ED, Reddel HK, Eriksson G, et al. Overall asthma control: the relationship between current control and future risk. J Allergy Clin Immunol. 2010;125(3):600–608. doi:10.1016/j.jaci.2009.11.033
- Beuther DA, Murphy KR, Zeiger RS, et al. Asthma Impairment and Risk Questionnaire (AIRQ) control level predicts future risk of asthma exacerbations. J Allergy Clin Immunol Pract. 2022. doi:10.1016/j.jaip.2022.08.017
- Larsson K, Kankaanranta H, Janson C, et al. Bringing asthma care into the twenty-first century. NPJ Prim Care Respir Med. 2020;30(1):25. doi:10.1038/s41533-020-0182-2
- Heiner MM. Key barriers to optimal management of adult asthma in Australia: physician and patient perspectives. Curr Med Res Opin. 2007;23(8):1799–1807. doi:10.1185/030079907X210714
- George M, Bender B. New insights to improve treatment adherence in asthma and COPD. Patient Prefer Adherence. 2019;13:1325–1334. doi:10.2147/PPA.S209532
- Kaplan A, Mitchell PD, Cave AJ, Gagnon R, Foran V, Ellis AK. Effective asthma management: is it time to let the AIR out of SABA? J Clin Med. 2020;9:4. doi:10.3390/jcm9040921
- Norful AA, Bilazarian A, Chung A, George M. Real-world drivers behind communication, medication adherence, and shared decision making in minority adults with asthma. J Prim Care Community Health. 2020;11:2150132720967806. doi:10.1177/2150132720967806
- Sleath B, Ayala GX, Washington D, et al. Caregiver rating of provider participatory decision-making style and caregiver and child satisfaction with pediatric asthma visits. Patient Educ Couns. 2011;85(2):286–289. doi:10.1016/j.pec.2010.09.016
- Sleath B, Carpenter DM, Coyne I, et al. Provider use of a participatory decision-making style with youth and caregivers and satisfaction with pediatric asthma visits. Patient Relat Outcome Meas. 2018;9:147–154. doi:10.2147/PROM.S152068
- Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi:10.1016/j.jaci.2003.09.008
- QualityMetric Incorporated. Asthma Control Test Caregiver Report (ACT-CR™); 2013. Available from: https://www.xolair.com/content/dam/gene/xolair/PDFs/asthma-control-test-caregiver-report-act-cr.pdf. Accessed July 08, 2020.
- Murphy KR, Chipps B, Beuther DA, et al. Development of the Asthma Impairment and Risk Questionnaire (AIRQ): a composite control measure. J Allergy Clin Immunol Pract. 2020;8(7):2263–2274 e2265. doi:10.1016/j.jaip.2020.02.042
- Schatz M, Zeiger RS, Yang SJ, et al. Development and preliminary validation of the adult asthma adherence questionnaire. J Allergy Clin Immunol Pract. 2013;1(3):280–288. doi:10.1016/j.jaip.2013.03.001
- Bharmal M, Payne K, Atkinson MJ, Desrosiers MP, Morisky DE, Gemmen E. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7:36. doi:10.1186/1477-7525-7-36
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi:10.1093/intqhc/mzm042
- Global Initiative for Asthma. Global strategy for asthma management and prevention; 2018. Available from: https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. Accessed March 10, 2022.
- Expert Panel Working Group of the National Asthma Education Prevention Program Coordinating Committee (NAEPPCC). Expert panel report 3: guidelines for the diagnosis and management of asthma; 2007. Available from: https://www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf. Accessed March 28, 2022.
- Pollard S, Bansback N, FitzGerld JM, Bryan S. The burden of nonadherence among adults with asthma: a role for shared decision-making. Allergy. 2017;72(5):705–712. doi:10.1111/all.13090
- Al-kalemji A, Johannesen H, Dam Petersen K, Sherson D, Baelum J. Asthma from the patient’s perspective. J Asthma. 2014;51(2):209–220. doi:10.3109/02770903.2013.860162
- Amin S, Soliman M, McIvor A, Cave A, Cabrera C. Understanding patient perspectives on medication adherence in asthma: a targeted review of qualitative studies. Patient Prefer Adherence. 2020;14:541–551. doi:10.2147/PPA.S234651
- Lycett H, Wildman E, Raebel EM, Sherlock JP, Kenny T, Chan AHY. Treatment perceptions in patients with asthma: synthesis of factors influencing adherence. Respir Med. 2018;141:180–189. doi:10.1016/j.rmed.2018.06.032
- Lingner H, Burger B, Kardos P, Criee CP, Worth H, Hummers-Pradier E. What patients really think about asthma guidelines: barriers to guideline implementation from the patients’ perspective. BMC Pulm Med. 2017;17(1):13. doi:10.1186/s12890-016-0346-6
- George M, Bruzzese JM, Lynn SSM, et al. Group-randomized trial of tailored brief shared decision-making to improve asthma control in urban black adults. J Adv Nurs. 2021;77(3):1501–1517. doi:10.1111/jan.14646
- Gillette C, Blalock SJ, Rao JK, Williams D, Loughlin CE, Sleath B. Provider-caregiver-child discussions about risks associated with asthma control medications: content and prevalence. Pediatr Pulmonol. 2014;49(8):727–733. doi:10.1002/ppul.22892
- Kew KM, Malik P, Aniruddhan K, Normansell R. Shared decision-making for people with asthma. Cochrane Database Syst Rev. 2017;10:CD012330. doi:10.1002/14651858.CD012330.pub2
- Kaplan A, Small I. Our patients’ fears may be getting the better of them: how do we deal with it? Prim Care Respir J. 2011;20(3):233–234. doi:10.4104/pcrj.2011.00068
- Menckeberg TT, Bouvy ML, Bracke M, et al. Beliefs about medicines predict refill adherence to inhaled corticosteroids. J Psychosom Res. 2008;64(1):47–54. doi:10.1016/j.jpsychores.2007.07.016
- Dominelli GS, Dominelli PB, Rathgeber SL, Webster SB. Effect of different single-session educational modalities on improving medical students’ ability to demonstrate proper pressurized metered dose inhaler technique. J Asthma. 2012;49(4):434–439. doi:10.3109/02770903.2012.672609
- Leung J, Bhutani M, Leigh R, Pelletier D, Good C, Sin DD. Empowering family physicians to impart proper inhaler teaching to patients with chronic obstructive pulmonary disease and asthma. Can Respir J. 2015;22(5):266–270. doi:10.1155/2015/731357
- Tervonen T, Hawken N, Hanania NA, Martinez FJ, Heidenreich S, Gilbert I. Maintenance inhaler therapy preferences of patients with asthma or chronic obstructive pulmonary disease: a discrete choice experiment. Thorax. 2020;75(9):735–743. doi:10.1136/thoraxjnl-2019-213974
- Center for Disease Control (CDC). Most recent national asthma data. Available from: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed August 23, 2022.
- Pew Research Center. 10 facts about today’s college graduates; 2022. Available from: https://www.pewresearch.org/fact-tank/2022/04/12/10-facts-about-todays-college-graduates/. Accessed August 25, 2022.