Abstract
Purpose
Our study compared knowledge of, and attitudes towards, allergic rhinitis (AR) among patients and physicians in: Brazil, Japan, Korea, Mexico, Saudi Arabia, Spain, United Arab Emirates, and the United Kingdom.
Patients and Methods
Patients with AR were recruited via probability-based sampling. Data were captured via telephone interview, personal interview, or self-administered online survey. Physicians were recruited from an online physician panel and interviewed by self-administered online survey.
Results
In total, 1436 patients and 1637 physicians were surveyed. Most patients (76.9%) reported moderate-to-severe AR, whereas physicians reported more mild (mean cases ranging from 57.0–68.2) than moderate-to-severe AR (mean cases ranging from 31.8–43.0). Overall, most physicians (85.4%) and patients (77.5%) agreed AR could be controlled with treatment. Physicians preferred prescribing oral antihistamines (OAHs) for mild AR (from 45.3% of physicians in Brazil to 73.6% of physicians in Mexico). For moderate and severe AR, more physicians preferred prescribing intranasal corticosteroids (INCSs) and OAHs for moderate and severe AR than other available treatments (from 48.5% of physicians in the UK to 69.5% of physicians in Spain). Patients preferred OAHs to INCSs for treating AR (62.0%). Patients also reported a range of comorbidities: overall, sinus infections were the most common (24.7%), and comorbid asthma was present in 12.9% of patients. Per country, Saudi Arabia had the highest proportion (53.5%) and Mexico had the lowest proportion (8.0%) of patients with comorbid asthma.
Conclusion
Patient and physician perceptions of AR mostly differed between and within countries, although there was generally agreement that AR could be controlled with treatment. Differing attitudes towards AR among patients and physicians suggests a need for improved education in and communication between these groups, with subsequent implications for optimizing disease management.
Plain Language Summary
Allergic rhinitis (AR), often referred to as “hayfever” in the case of grass pollen allergy, can cause sneezing, a runny and stuffy nose, and itchy or watery eyes. It is caused by an allergy to airborne particles such as pollen, house dust mites, or molds. AR can impact sleep, work or school performance, and the ability to participate in or enjoy social activities. Other health conditions, including asthma and allergic conjunctivitis, are often associated with AR.
There are a wide range of available treatment options for AR. Treatment choice may be influenced by the severity of AR symptoms and patient preference. Differences in attitudes towards treatment choice between patients and doctors can hamper AR management. We compared the knowledge of, attitudes towards, and treatment of AR among patients and doctors in eight different countries.
Our results showed that generally, across all countries: patients reported more severe AR symptoms than doctors; doctors preferred prescribing oral antihistamines for mild AR, and intranasal corticosteroids and oral antihistamines for moderate-to-severe AR; patients preferred oral antihistamines to intranasal corticosteroids; and comorbidities such as sinus infections and asthma were common among AR sufferers.
Results from this multinational study suggest that although patients and doctors agree that AR symptoms can be controlled with treatment, their views about the severity of the symptoms and the optimal choice of treatments differ. This suggests a need for improved education and communication within and between these groups.
Introduction
Allergic rhinitis (AR) is an inflammatory disease of the nasal mucosa that is triggered by an immune response to airborne environmental allergens (including pollen, house dust mites, and molds),Citation1–4 which affected an estimated 400 million people worldwide in 2013.Citation5 AR leads to symptoms such as sneezing, a runny and stuffy nose, and itchy or watery eyes,Citation1 and can negatively affect patient quality of life by impacting upon sleep, performance at work or school, and social functioning.Citation5–9 AR also has significant associated healthcare costs and economic burden,Citation5,Citation10 and is associated with other inflammatory conditions, including asthma and relevant comorbidities, rhinosinusitis, and allergic conjunctivitis.Citation5,Citation11 Intranasal corticosteroid (INCS) treatments are widely recommended by expert panels for use as first-line choices for moderate-to-severe AR.Citation12 Although there are various local and global treatment guidelines available for INCSs,Citation13–17 treatment choice may be influenced by physician perception of disease severity and patient preference around medication use. Despite guidelines, many clinicians remain uncertain about the benefits and disadvantages of the many AR treatment options available.Citation18 Additionally, real-world evidence shows that most patients do not follow guidelines and have very poor adherence to treatment.Citation19,Citation20 Differences between patient and physician attitudes towards treatment choice may negatively affect patient experiences of managing AR. It is therefore important to understand both patient perceptions of AR and physician practices for AR treatment in more detail to ultimately improve patient experiences and optimize disease management.Citation2,Citation12 Knowledge and Attitude among Patients and Physicians on AR (KAPPA) was an international survey of patients with AR and AR physicians which had three main aims: to understand patient and physician attitudes, beliefs, and treatment practices of moderate-to-severe AR; to describe gaps between patient and physician perceptions of INCS use; and to identify key drivers and barriers to treatment adherence and treatment patterns.
Materials and Methods
Study Design
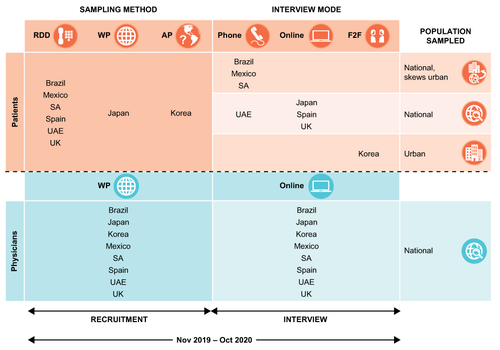
KAPPA was an international survey of patients with AR and AR physicians conducted across eight countries (Brazil, Japan, Korea, Mexico, Saudi Arabia [SA], Spain, United Arab Emirates [UAE], and the United Kingdom [UK]) between November 2019 and October 2020. Patients were recruited using a probability-based sampling methodology appropriate for each country. This included: random digit dialing (to give a nationally representative sample of households) in Brazil, Mexico, SA, UAE, Spain, and the UK; face-to-face contact (area probability sampling of households) in Korea; and sampling a large, fully certified, and data-compliant patient panel provider in Japan. Physicians were recruited from a fully certified, data-compliant web panel. Patient data were collected per local preferences and feasibility: via telephone interview (Brazil, Mexico, SA, and UAE), face-to-face interview (Korea), or self-administered online survey (Japan, Spain, and UK). Interviews were conducted in the respondents’ local language, and all physician data were collected via online survey (). Approximately 200 patients/country were targeted for inclusion from all countries except Spain and the UK, which instead targeted 100 patients/country. Approximately 200 physicians were targeted from all countries. Samples for recruiting were stratified by city size, and patients were recruited using appropriate probability-based sampling methodology where possible: random digit dialing, web panel, or area probability.
Eligibility Criteria
Eligible patients were male and female adults (≥18 years) or children with a parent or caregiver available to be interviewed on their behalf. Children were aged 2 to 17 years in Japan, Korea, UAE, SA, Mexico, and Brazil; or aged 6 to 17 years in Spain and the UK. Patients had a physician-confirmed diagnosis of AR and an INCS prescription in the past 12 months. Patients with non-allergic rhinitis were not included.
Eligible physicians were general practitioners (GPs), internal medicine physicians, pediatricians, and specialists (ear, nose, and throat physicians, and otolaryngologists) who: were responsible for the treatment and management of patients with AR; had been practicing for ≥3 years; spent >60% of their time in direct patient care in the past 12 months; had treated ≥10 patients with AR per month. Physicians were excluded if responsible for drug formulary decisions or employed by a pharmaceutical company.
Patient and Physician Survey Development
An English-language questionnaire was developed for both patients and physicians (by GSK and Abt Associates) and was translated into local languages by accredited translators. Screening questions were included to identify eligible patients and physicians. Two validated question modules (Work Productivity and Activity Impairment Questionnaire [Specific Health Problem V2.0]Citation21 and the Rhinitis Control Assessment Test)Citation22 were used for the patient questionnaire.
Survey questions collected data on patient and physician perceptions of AR severity, quality of life, disease burden, healthcare resource utilization, comorbidities, and AR indicators. Questions from past surveys (eg, the Global Asthma Physician SurveyCitation23 and the Asia Pacific Survey of Physicians on Asthma and AR)Citation24 were used as references where possible, and new questions were subject to multi-step review and revision by the study team before finalization.
The study sponsor (GSK) was not revealed until study end in non-European Union (EU) countries; collection of data in EU countries complied with General Data Protection Regulation guidelines, and so the study sponsor was revealed as part of the informed consent form. Adults provided their consent to participate, and parents or caregivers completed the survey on behalf of their children. The study complied with all applicable laws regarding subject privacy. No direct subject contact or primary collection of individual human subject data occurred. All survey responses were protected by confidentiality, and no identifying information could be linked to the survey data that were presented as aggregate analyses in tabular form. Institutional Review Board approval for the study was provided by Abt Associates (Abt IRB# 2014).
Data Analyses
For continuous variables, means, medians, and standard deviations (SDs) were calculated. For categorical, ordinal variables, and interval variables, proportions were tested for statistical significance (p-level significance was 0.05). Chi-squared tests of statistical significance were used, and one-way analysis-of-variance tests compared means between categorical groups (countries). Statistical tests were used to determine whether or not broad differences existed across comparison groups (countries) for a particular AR characteristic or indicator, rather than to determine specific group-to-group differences.
Results
Interview Summary and Demographics
Patients
Overall, 1436 patients were interviewed (mean interview length=25 minutes). Mean age ranged from 24.0–42.4 years across countries (p<0.001): SA and Korea had the most patients <18 years (30.0% and 36.0%, respectively), while the UK and Japan had the most patients >50 years (29.0% and 40.0%, respectively). Similar proportions of male (48.8%) and female (51.2%) patients were interviewed, and differences in sex across countries were significant (p<0.001) ().
Table 1 Summary of Patient Interviews and Patient Characteristics, Overall and by Country
Physicians
A total of 1637 physicians were interviewed (mean interview length=31 minutes): GPs (n=502), internal medicine physicians (n=104), pediatricians (n=613), and specialists (n=403). Japan (n=91) and Korea (n=80) interviewed the most GPs; Brazil (n=87) and Korea (n=76) interviewed the most pediatricians. Most countries interviewed 50 specialists (). There were significant differences across countries for all physician characteristics recorded (all p<0.001). For AR treatment at first mention, use of country-specific guidelines was notable in physicians in Brazil (40.6%), Spain (19.0%), and Japan (25.2%), and AR and its Impact on Asthma guidelines (year/version not specified) were used in all countries and were the most widely used guidelines overall (21.0%). One-fifth treated AR at first mention based on their experience as a physician, and 4.8% did not use any guidelines for AR treatment at first mention; however, at second and third mention, all physicians reported using some form of guidelines for AR treatment (data not shown).
Table 2 Physician Characteristics, Overall and by Country
AR Severity
The mean age of AR symptom onset varied significantly (p<0.001) between countries, ranging from mean (SD) 11.1 (8.1) years in SA to 22.9 (9.7) years in Mexico (Brazil, 16.4 [10.9] years; UAE, 14.9 [13.9] years; Japan, 18.1 [14.1] years; Korea, 20.8 [17.8] years; Spain, 18.2 [12.6] years; UK, 16.7 [12.0] years). Similarly, the mean age that patients first sought treatment for AR was significantly different (p<0.001) between countries, with youngest patients in SA (mean [SD] 11.8 [11.1] years) and eldest in Mexico (26.3 [9.6] years) (Brazil, 17.7 [11.1] years; UAE, 16.2 [14.2] years; Japan, 20.6 [15.2] years; Korea, 22.4 [18.2] years; Spain, 20.0 [12.6] years; UK, 19.2 [13.0] years).
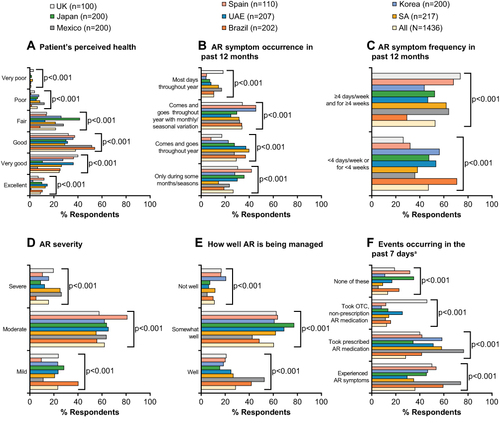
Across all countries, symptoms were subject to significant temporal variation (p<0.001), with most (33.9%) patients reporting that symptoms fluctuated throughout the year and were worse in some seasons than in others. Most patients felt that their AR was well/somewhat well managed (88.9%), and considered their AR severity to be moderate or severe (76.9%). Around a third of patients had experienced AR symptoms in the past 7 days; 38.2% had taken prescription AR medication in the past 7 days, 12.4% had taken over-the-counter oral medications in the past 7 days, and 13.3% had no symptoms or had not taken any medications in the past 7 days. Patient perceptions of AR management and severity, and approaches to symptom control, varied significantly across countries (p<0.001) ().
Figure 2 Summary of patient’s perceptions of AR severity and approaches to AR symptom control, overall and by country. (A) Patient’s perceived health; (B) AR symptom occurrence in past 12 months; (C) AR symptom frequency in past 12 months; (D) AR severity; (E) How well AR is being managed; (F) Events occurring in past 7 days.
Physician classifications of AR cases by severity (mild or moderate-to-severe), seasonality (seasonal, perennial, or perennial with seasonal exacerbations), and frequency (intermittent or persistent) all varied significantly across countries (all p<0.001). In all countries, physicians most often classified AR as mild, seasonal, and intermittent (data not shown).
Quality of Life and Disease Burden
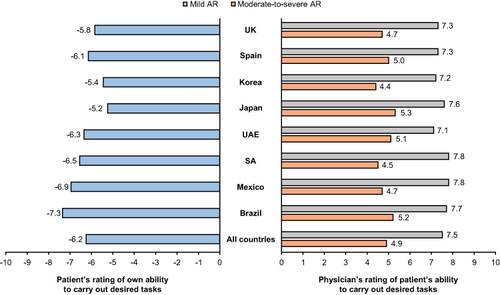
When AR symptoms were worst, patient ratings of their abilities to carry out tasks and physician ratings of patient’s abilities varied significantly between countries (p<0.001). For patients, the overall rating was 6.2/10, ranging from 5.2/10 (Japan) to 7.3/10 (Brazil). Physicians felt that patients with mild AR (7.5/10) were better able to carry out tasks when compared to patients with moderate-to-severe AR (4.9/10), a trend that was also reflected across countries ().
Figure 3 Patient’s rating of their own ability to carry out desired tasks and physician’s rating of patient’s ability to carry out desired tasks (by AR severity) when AR symptoms are at their worst, overall and by country.
Patients missed on average around half a day from work due to AR in the past 7 days, although this varied considerably between countries (from 0.3 hours/7 days in Spain to 11.7 hours/7 days in Korea; data not shown). Physician rankings of the impact of allergies on patient daily life and on patient ability to tolerate discomfort from AR symptoms varied significantly (p<0.001) across countries, and physicians mostly perceived discomfort from AR symptoms as something patients could “tolerate only with treatment”, ranging from 71.5% in UAE to 89.0% in Spain (data not shown).
Comorbidities and Healthcare Resource Utilization
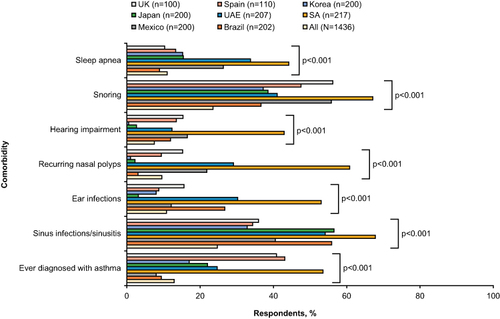
Overall, the most common comorbidities/health conditions were sinus infections/sinusitis (24.7%), snoring (23.5%), and a history of asthma (12.9%). Proportions of comorbidities/health conditions varied significantly (p<0.001) by country: SA had the highest (53.5%) proportion of patients who had ever been diagnosed with asthma, and the highest proportions of all comorbid health conditions compared to other countries. Rates of asthma diagnosis were highest in SA (53.5%), Spain (43.1%), and the UK (40.8%) compared to other countries (ranging from 8.0–24.6%) (). All recorded healthcare resource utilization incidents in the past 12 months varied significantly across countries (p<0.001); the proportions of patients requiring ≥1 emergency room (ER) visit (71.3%), ≥1 hospital stay (56.9%), and a pharmacy visit (62.5%) in the past 12 months were highest in SA compared to all other countries. SA also had a mean number of 31.7 unplanned clinic/office/ER visits in the past 12 months, far higher than any other country (unplanned clinic/office/ER visits in the past 12 months ranged from 2.0 to 9.8 in other countries) (data not shown).
Figure 4 Patient comorbidities, overall and by country.
Treatment Indicators
Patient Indicators
There was significant (p<0.001) variation across countries in the frequency of taking prescribed INCSs. In Mexico, similar proportions of patients took INCSs all, most, or some of the time throughout the year, compared to only during the allergy season (ranging from 20.0–28.5%). Almost half of patients in Brazil, Japan, and Spain only took INCSs during the allergy season, and almost half of patients in SA took INCSs most of the time (data not shown).
Around 70% of patients felt that quick and long-lasting symptom relief were very important attributes of INCS adherence, and approximately 50% considered ease of INCS use very important. Variation in the importance ranking of attributes influencing INCS adherence varied significantly (p<0.001) across countries (). There was an overall agreement that patients preferred oral antihistamine (OAH) medication to INCS medication (62.0%), and variation between countries was significant (p<0.001) (data not shown).
Table 3 Attributes and Challenges Relevant to Patient INCS Adherence, Overall and by Country
Overall, 82.0% of patients agreed that AR symptoms could be controlled with INCSs; 78.4% agreed that INCS treatment was considered safe to use, and 64.6% agreed that INCS initiation should be delayed for children until they were adults. Variation between countries was significant (p<0.001; data not shown).
Physician Indicators
The patient symptoms that most strongly influenced physicians to prescribe INCSs were nasal congestion and/or obstruction (12.6%), watery/runny nose (9.9%), and frequent itchy nose/nasal itching (9.5%) (). These findings varied significantly (p<0.001) across countries.
Table 4 Physician Treatment Decision Factors and Attitudes Towards INCSs, Overall and by Country
Physician prescribing preference and attitudes towards INCS treatment also varied significantly (both p<0.001) across countries. Physicians preferred to prescribe OAH treatments for patients with mild AR (61.4% overall), and both INCS and OAH treatments for patients with moderate AR (60.2% overall) and severe AR (57.0% overall). Overall, most physicians strongly agreed/agreed that INCSs had a good safety profile (92.1%), and most strongly disagreed/disagreed INCS initiation should be delayed for children until they were adults (65.7%). Overall, most (91.6%) physicians strongly agreed/agreed that the route of administration influenced patient adherence, and variation between countries was significant (p<0.001) ().
AR Indicators
Patient Indicators
Overall, most patients (69.1%) strongly agreed/agreed that AR was a serious disease; nonetheless, patient attitudes towards the seriousness of AR varied significantly (p<0.001) across countries. Brazil and Mexico had the highest proportions of patients who strongly agreed that AR was a serious disease (79.7% and 54.5%, respectively), and although fewer patients (10.5–28.4%) strongly agreed with this in other countries, overall agreement that AR was a serious disease remained higher than overall disagreement. Most patients agreed AR could be controlled but not cured (77.5%), and that uncontrolled AR could lead to asthma symptoms (76.5%) and complications such as ear and sinus infections (83.6%). These responses were broadly similar across countries, with significant (p<0.001) variation between countries (data not shown).
Overall, most patients considered the cost of INCSs, and doctor/ER visits and immunotherapy costs, to be minor burdens (47.5% and 43.8%, respectively).
Physicians generally agreed that AR is a chronic disease that should be treated for a long period of time (91.3%), and that AR could be controlled but not cured (85.4%). They also felt that uncontrolled AR could lead to asthma symptoms (80.8%), complications like ear and sinus infections (89.8%), and an increased economic and social burden (88.1%). Variations across countries were significant (p<0.001).
Physicians also felt that patients prescribed AR medication mostly (51.5%) followed their advice for a follow-up appointment, with lower proportions of patients deciding when to have their own follow-up (26.2%) or only having follow-up visits with worsening/uncontrolled symptoms. Very few (0.5%) patients across countries were unable to afford follow-up visits (data not shown).
Discussion
In this international survey of patients with AR and AR physicians, there was overall wide variation between patient and physician perceptions of AR severity. Perhaps most notable was that patients tended to estimate their AR as being more severe than physicians perceived it to be, which suggests a need for improved patient and physician education. This difference in physician versus patient perception of AR severity may also be the result of patients with more severe AR filling out the survey. In the UK, for example, patients are advised by government policy to seek AR treatment from a pharmacist, and are discouraged from seeing GPs unless their symptoms are severe or they experience side effects from over-the-counter medications.Citation25,Citation26 Therefore, despite patients’ high dependence on medications, AR sufferers often remain undertreated.Citation27 Physicians reported that discomfort from AR should have little overall impact on patients’ daily activities, and that most patients could tolerate discomfort from AR but only with treatment. However, both patients and physicians felt AR had a negative impact on quality of life and productivity. We found that patients missed on average around half a day from work due to AR in the past 7 days (equal to almost a month of missed work per year), which has obvious implications for a loss of productivity and income, although it is important to consider seasonal variations between countries and the limited time frame captured. Future research into the effects of AR and its symptoms on missed work hours and productivity, conducted over a longer time period, would be beneficial in exploring this further.
Although patients were in agreement that AR is a serious disease, patient attitudes towards the seriousness of AR varied significantly across countries, with Brazil and Mexico having the highest proportions of patients who strongly agreed that AR was a serious disease. The reason for this could be that affordable healthcare for patients with AR differs across countries and so a lack of access to specialist physicians and treatment could lead to more uncontrolled disease in some countries. The number of patients reporting comorbid asthma also varied across countries, with SA, Spain, and the UK reporting the highest rates. Additionally, the proportion of unplanned medical facility visits in the past 12 months was variable across countries and notably higher in SA than any other country, although the cause for these visits was not specified. Despite this, half of patients considered the cost of INCSs and medical visits only a minor burden and physicians felt that most patients could attend follow-up visits regardless of cost, suggesting overall affordable healthcare for patients with AR across countries.
Physicians prescribed INCSs (alone or in combination) for an estimated 80–90% of patients with AR across the countries surveyed, as would be expected given that INCS treatments are widely recommended for the treatment of AR.Citation12 Both patients and physicians largely agreed that INCSs were considered safe to use; however, around two-thirds of physicians strongly disagreed that INCS treatment in children should be delayed until adulthood, which contrasted with over half of patients who agreed that INCS treatment should be delayed until adulthood. As with perception of AR severity, this difference in perception of safety could indicate a need for improved education around the use of INCSs in children, as well as the undertaking of studies in pediatric patients to demonstrate safety. Patients and physicians across all countries agreed that AR symptoms could be well controlled with the use of appropriate medication. Patient preference for OAH over INCS treatment varied per country. Patient preferences could possibly be influenced by different treatment availabilities and/or reimbursement costs across countries, and could also be influenced by the characteristics of a given treatment (eg, whether it is easy to use, and whether patients have been shown how to use the device correctly),Citation28,Citation29 which could subsequently affect adherence and overall effectiveness and drive patient preference for a particular treatment.Citation29 Certainly, in our study, “easy to use” was considered an important factor that affected INCS adherence among patients surveyed. The importance of physician engagement with the patient to demonstrate appropriate nasal inhalation technique should not be underestimated, especially for INCS treatment needed only once daily, which may have the ability to alter patient perception of INCSs.
A recent report from Bousquet et al,Citation19 which integrated guidelines with real-world evidence and supportive studies, showed poor adherence to AR treatment and demonstrated that many patients with AR used on-demand treatment based on their own judgement rather than seeking advice from their physician. Our study found that most physicians used some form of local or global treatment guidelines for AR treatment, although it likely reflects differences in availabilities of medications and could also reflect lack of education about treatment guidelines within countries. Thus, it is important for physicians and patients to communicate and collaborate effectively so that AR treatment and management can be improved, and patient care can subsequently be optimized.
The strengths of the study are that it had a large sample size, included a range of countries, and covered a wide age range of patients and experience range of physicians, subsequently providing a useful general overview of patient and physicians perceptions of and attitudes towards AR across geographical boundaries. Our study also had several limitations, including country-by-country differences and variations in healthcare systems or physician standards. The survey language, mode of administration, and data collection method may have affected patient/physician responses, and it was observed that the patient sample was skewed towards being urban, employed, and beyond high school educated. Furthermore, since patients were screened for prescribed INCSs in the 12 months before the study, the sample may not have represented all patients with AR. Finally, since the original power/sample size calculations were geared toward sufficient statistical power for within-country analyses, the differences shown between countries in our study may show statistical significance that is not necessarily applicable outside of the populations studied. Nevertheless, we do still show broad trends and differences/similarities between countries that could be of interest to patients and physicians. We acknowledge also that all physician data for these analyses were pooled; therefore, we could not ascertain whether approaches to patient care varied between ear, nose, and throat physicians or surgeons. This could be explored in future studies.
Conclusion
To conclude, patient and physician attitudes within individual countries towards the management of AR were similar and they were generally in agreement that AR could be controlled with treatment. However, between-country variation in patient and physician perceptions of AR severity and optimal treatment choice was observed throughout the study. Our findings highlight an unmet need for improved patient and physician education, and improved communication between patients and physicians across the countries surveyed, and could serve as a useful starting point for optimizing AR management. Further research into the long-term impact of AR and its symptoms on missed work, productivity, and presenteeism, as well as long-term studies on the use of INCSs in children, would be beneficial.
Abbreviations
ANOVA, Analysis of variance; AP, Area probability; AR, Allergic rhinitis; ENT, Ear, nose, and throat; ER, Emergency room; EU, European Union; F2F, Face-to-face; GP, General practitioner; INCS, Intranasal corticosteroid; KAPPA, Knowledge and Attitude among Patients and Physicians on Allergic rhinitis; NE, Not evaluated; OAH, Oral antihistamine; OTC, Over the counter; RDD, Random digit dialing; SA, Saudi Arabia; SD, Standard deviation; UAE, United Arab Emirates; UK, United Kingdom; WP, Web panel.
Data Sharing Statement
Information on GSK’s data sharing commitments and requesting access to anonymized individual participant data and associated documents can be found at www.clinicalstudydatarequest.com.
Ethics Approval and Informed Consent
The study sponsor was not revealed until study end in non-EU countries; collection of data in EU countries complied with General Data Protection Regulation guidelines and so the study sponsor was revealed as part of the informed consent form. Adults provided their consent to participate, and parents or caregivers completed the survey on behalf of their children. All survey responses were protected by confidentiality and no identifying information could be linked to the survey data.
Consent for Publication
All authors critically reviewed the manuscript for important intellectual content at each draft and approved the final version for submission.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
C. Bhargave, M. Verma, and R. W. Jakes are employees of, and shareholders in, GSK. Y. Okamoto has received speaker fees from Torii Co. Ltd., Novartis, Tanabe Mitsubishi Co. Ltd., Kirin Co. Ltd., ALK Co. Ltd., Shionogi Co. Ltd., and Meiji Pharma Co. Ltd. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors would like to thank Sheila Chua (formerly of GSK) and Anup Pingle (GSK) for their contributions to study design and data acquisition, interpretation, and analysis; Priya Jain (GSK) for her contributions to data acquisition, interpretation, and analysis; and Jeanne Pimenta (formerly of GSK) for her contributions to developing the KAPPA study protocol. Trademarks are the property of their respective owners.
Additional information
Funding
References
- National Institute of Health: National Institute of Allergy and Infectious Diseases. Immunotherapy for environmental allergies; 2015. Available from: https://web.archive.org/web/20150617190743/http:/www.niaid.nih.gov/topics/environmental-allergies/Pages/immunotherapy.aspx. Accessed July 23, 2021.
- Church DS, Church MK, Scadding GK. Allergic rhinitis: impact, diagnosis, treatment and management. Pharm J. 2016;8:249–255. doi:10.1211/CP.2016.20201509
- Rosario Filho NA, Satoris RA, Scala WR. Allergic rhinitis aggravated by air pollutants in Latin America: a systematic review. World Allergy Organ J. 2021;14(8):100574. doi:10.1016/j.waojou.2021.100574
- Liva GA, Karatzanis AD, Prokopakis EP. Review of rhinitis: classification, types, pathophysiology. J Clin Med. 2021;10(14):14. doi:10.3390/jcm10143183
- Pawankar R, Canonica GW, Holgate ST, et al. World Allergy Organization (WAO) white book on allergy: update 2013. Milwaukee (WI): World Allergy Organization; 2013. Available from: https://www.worldallergy.org/wao-white-book-on-allergy. Accessed July 28, 2021.
- Meltzer EO. Quality of life in adults and children with allergic rhinitis. J Allergy Clin Immunol. 2001;108(1 Suppl):S45–53. doi:10.1067/mai.2001.115566
- Vandenplas O, Suarthana E, Rifflart C, et al. The impact of work-related rhinitis on quality of life and work productivity: a general workforce-based survey. J Allergy Clin Immunol Pract. 2020;8(5):1583–1591. doi:10.1016/j.jaip.2019.12.033
- Dykewicz MS, Wallace DV, Amrol DJ, et al. Rhinitis 2020: a practice parameter update. J Allergy Clin Immunol. 2020;146(4):721–767. doi:10.1016/j.jaci.2020.07.007
- Bedard A, Anto JM, Fonseca JA, et al. Correlation between work impairment, scores of rhinitis severity and asthma using the MASK-air® App. Allergy. 2020;75(7):1672–1688. doi:10.1111/all.14204
- Canonica GW, Mullol J, Pradalier A, et al. Patient perceptions of allergic rhinitis and quality of life: findings from a survey conducted in Europe and the United States. World Allergy Organ J. 2008;1(9):138–144. doi:10.1097/WOX.0b013e3181865faf
- Linton S, Burrows AG, Hossenbaccus L, et al. Future of allergic rhinitis management. Ann Allergy Asthma Immunol. 2021;127(2):183–190. doi:10.1016/j.anai.2021.04.029
- Brozek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi:10.1016/j.jaci.2017.03.050
- Okubo K, Kurono Y, Ichimura K, et al. Japanese guidelines for allergic rhinitis 2020. Allergol Int. 2020;69(3):331–345. doi:10.1016/j.alit.2020.04.001
- Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152(1 Suppl):S1–43. doi:10.1177/0194599814561600
- Sakano E, Sarinho ESC, Cruz AA, et al. IV Brazilian consensus on rhinitis - an update on allergic rhinitis. Braz J Otorhinolaryngol. 2018;84(1):11. doi:10.1016/j.bjorl.2017.10.006
- National Institute for Health and Care Excellence. Allergic rhinitis; 2018. Available from: https://cks.nice.org.uk/topics/allergic-rhinitis/. Accessed July 28, 2021.
- Savoure M, Bousquet J, Burte E, et al. Questionnaire as an alternative of skin prick tests to differentiate allergic from non-allergic rhinitis in epidemiological studies. Allergy. 2021;76(7):2291–2294. doi:10.1111/all.14812
- Hellings PW, Seys SF, Marien G, et al. ARIA masterclass 2018: from guidelines to real-life implementation. Rhinology. 2019;57(5):392–399. doi:10.4193/Rhin19.011
- Bousquet J, Schunemann HJ, Togias A, et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol. 2020;145(1):70–80. doi:10.1016/j.jaci.2019.06.049
- Menditto E, Costa E, Midao L, et al. Adherence to treatment in allergic rhinitis using mobile technology. The MASK study. Clin Exp Allergy. 2019;49(4):442–460. doi:10.1111/cea.13333
- Reilly MC, Tanner A, Meltzer EO. Work, classroom and activity impairment instruments. Validation studies in allergic rhinitis. Clin Drug Invest. 1996;11(5):278–288. doi:10.2165/00044011-199611050-00004
- Nathan RA, Dalal AA, Stanford RH, et al. Qualitative development of the rhinitis control assessment test (RCAT), an instrument for evaluating rhinitis symptom control. Patient. 2010;3(2):91–99. doi:10.2165/11318410-000000000-00000
- Chapman KR, Hinds D, Piazza P, et al. Physician perspectives on the burden and management of asthma in six countries: the Global Asthma Physician Survey (GAPS). BMC Pulm Med. 2017;17(1):153. doi:10.1186/s12890-017-0492-5
- Hinds D, Aggarwal B, Du X, et al. Asia Pacific survey of physicians on asthma and allergic rhinitis (ASPAIR): data from China. Chin Med J. 2019;132(11):1264–1271. doi:10.1097/CM9.0000000000000229
- National Health Service (Scotland). NHS inform: hay fever; 2021. Available from: https://www.nhsinform.scot/illnesses-and-conditions/immune-system/hay-fever. Accessed March 02, 2022.
- National Health Service. Hay fever; 2021. Available from: https://www.nhs.uk/conditions/hay-fever/. Accessed March 02, 2022.
- Tan R, Cvetkovski B, Kritikos V, et al. Management of allergic rhinitis in the community pharmacy: identifying the reasons behind medication self-selection. Pharm Pract. 2018;16(3):1332. doi:10.18549/PharmPract.2018.03.1332
- Turner RR, Testa MA, Hayes JF, et al. Validation of the allergic rhinitis treatment satisfaction and preference scale. Allergy Asthma Proc. 2013;34(6):551–557. doi:10.2500/aap.2013.34.3715
- Sher ER, Ross JA. Intranasal corticosteroids: the role of patient preference and satisfaction. Allergy Asthma Proc. 2014;35(1):24–33. doi:10.2500/aap.2014.35.3725