Abstract
Background
The purpose of this study was to evaluate the efficacy and duration of action of once-daily dosing with alcaftadine 0.25% ophthalmic solution and olopatadine 0.2% ophthalmic solution as compared with placebo in the prevention of ocular itching, and to directly compare the efficacy of alcaftadine 0.25% with olopatadine 0.2% in the prevention of ocular itching associated with allergic conjunctivitis using the conjunctival allergen challenge model.
Methods
Subjects with allergic conjunctivitis (n = 127) were enrolled in a multicenter, double-masked, randomized, active-controlled and placebo-controlled clinical trial. Using the conjunctival allergen challenge model, this study was conducted over the course of approximately 5 weeks. Subjects were randomized into one of three treatment arms: alcaftadine 0.25% ophthalmic solution, olopatadine 0.2% ophthalmic solution, or placebo. Study medications were administered twice over the course of the trial. The primary efficacy measure for the study was ocular itching evaluated by the subject at 3, 5, and 7 minutes post challenge. Secondary endpoints, measured at 7, 15, and 20 minutes post challenge, included conjunctival, ciliary, and episcleral redness, lid swelling, chemosis, and tearing. Duration of action was measured at 16 and 24 hours post-instillation of the study medication at visits 3 and 4, respectively.
Results
For the primary measure of ocular itching, both actives, alcaftadine 0.25% and olopatadine 0.2%, were statistically superior to placebo at all three measured time points for both the 16-hour and 24-hour measures (P < 0.0001). Eyes treated with alcaftadine 0.25% had numerically lower mean ocular itching scores than eyes treated with olopatadine 0.2% at every time point, and this difference was statistically significant at the 3-minute time point 16 hours post instillation (P = 0.026). Eyes treated with alcaftadine 0.25% and with olopatadine 0.2% displayed significantly less lid swelling relative to placebo at every time point for the 16-hour and 24-hour post-instillation visits (P < 0.005). Alcaftadine 0.25% was the only active treatment that provided statistically significant relief of chemosis at every time point of the 24-hour post-instillation visit.
Conclusion
Both the alcaftadine 0.25% and olopatadine 0.2% ophthalmic solutions provided highly effective relief of ocular itching at both 16 and 24 hours post-instillation. Treatment differences between the actives were most pronounced at the earliest time point (3 minutes post-challenge) following conjunctival allergen challenge (16 hours), when alcaftadine 0.25% ophthalmic solution was statistically superior to olopatadine 0.2% ophthalmic solution. Alcaftadine 0.25% was the only treatment to provide significant relief from chemosis at both 16 and 24 hours post-instillation. Both active treatments and placebo were generally safe and well tolerated.
Introduction
Allergic conjunctivitis is the most common allergic disorder of the eye, with an estimated 60 million affected individuals in the US.Citation1 The hallmark symptom of the disease is ocular itching, but patients typically experience a combination of conditions including conjunctival hyperemia, lid swelling, excessive tearing, chemosis, rhinorrhea, and nasal congestion.Citation2 Affected individuals are those with either seasonal or perennial allergies. Exposure to allergens, such as pollens, dust mites, or animal dander elicits an IgE-based hypersensitivity response. This triggers the release of histamine and other pro-allergic and pro-inflammatory mediators.Citation2
Mast cells of the immune system are found throughout the body, including the substantia propria of the conjunctiva.Citation3 Allergen binding to specific IgE molecules triggers mast cell degranulation and the subsequent increase in histamine leads to activation of both H1-type and H2-type histamine receptors.Citation2–Citation4 These stimulate itching and vasodilation, respectively, thereby initiating a response that may last minutes or hours. With prolonged or repeated allergen exposure, the conjunctiva may become infiltrated by inflammatory cells that promote continued symptoms and eventual disruption of epithelial integrity.Citation5
Antihistamines remain at the forefront of the therapeutic options for treating allergic conjunctivitis because of the central role of histamine in the pathogenesis of both itching and vasodilation. Currently available antagonists of histamine receptor activation have significant benefit in relieving the signs and symptoms of allergic conjunctivitis, and are particularly effective in relieving ocular itching.Citation4 Another advantage of these compounds is that many can be formulated for topical delivery, reducing the adverse effects associated with systemic formulations.Citation6,Citation7 Two topical antihistamines currently approved for once-daily use in the US, olopatadine hydrochloride 0.2% and alcaftadine 0.25%, are the focus of the study reported herein.
Both olopatadine 0.2% and alcaftadine 0.25% are classified as dual-action antiallergic medications, meaning they act both directly to inhibit histamine receptor activation and indirectly to reduce allergic responses by attenuating mast cell degranulation.Citation4,Citation7 Olopatadine was first used in a formulation strength of 0.1% and indicated for twice-daily use. A more concentrated formulation of 0.2% was later approved for once-daily treatment of ocular itching associated with allergic conjunctivitis.Citation8 Alcaftadine 0.25% ophthalmic solution was approved by the FDA in 2011 as a once-daily therapeutic treatment for the prevention of itching associated with allergic conjunctivitis.Citation9 These are the only topical antihistamines with this long duration of action currently available.
Olopatadine 0.2% and alcaftadine 0.25% approvals for once-daily use in the US were based upon clinical trials in which efficacy was demonstrated 16 hours post-instillation utilizing the conjunctival allergen challenge (CAC) model of allergic conjunctivitis.Citation10,Citation11 The CAC model provides a controlled alternative to environmental allergy trials that depend on variable allergen exposures and subjective patient-reported efficacy measures. It has been utilized in over 100 clinical trials, and has become a standard for the US Food and Drug Administration approval of ophthalmic antiallergics.Citation12 The goal of this study was to evaluate the efficacy of once-daily antihistamines beyond the established 16-hour post-instillation time point. This study was designed to evaluate the efficacy and duration of action of alcaftadine 0.25% ophthalmic solution and olopatadine 0.2% ophthalmic solution as compared with placebo in the prevention of ocular itching at both 16 and 24 hours post instillation, and to directly compare the efficacy of alcaftadine 0.25% with olopatadine 0.2% in the prevention of ocular itching associated with allergic conjunctivitis using CAC.
Materials and methods
Study design
This study (ClinicalTrials.gov identifier NCT01470118) protocol employed a multicenter, double-masked, randomized, active-controlled and placebo-controlled design. Key features of the protocol design are similar to those in previous studies of alcaftadine,Citation11 with key differences based on the timing of allergen challenge relative to instillation of study medication; allergen challenges were conducted 16 and 24 hours after treatment instillation to assess treatment efficacy.
Three treatments were used in this comparative study. The two actives were alcaftadine 0.25% ophthalmic solution (Lastacaft®, Allergan Inc, Irvine, CA, USA) and olopatadine 0.2% ophthalmic solution (Pataday™, Alcon Laboratories Inc, Fort Worth, TX, USA), and the placebo was 0.3% hydroxypropyl methylcellulose (Tears Naturale® II, Alcon Laboratories Inc). The protocols and informed consent were both investigational review board-approved (Alpha IRB, San Clemente, CA, USA). Three sites were used for the trials and they were conducted in accordance with International Conference on Harmonisation, Good Clinical Practice guidelines and the Declaration of Helsinki. All subjects had to be at least 10 years of age, and had to provide written, informed consent (and assent if applicable).
Eligibility criteria
Key subject inclusion was based upon entry criteria outlined as follows: subjects were required to have a history of ocular allergies and at least one positive skin test to at least one of the allergens which included cat hair, cat dander, grasses, ragweed, dog dander, cockroach, dust mites, and trees within 24 months of the trial start date. All subjects were also required to have best-corrected visual acuity ≥ 0.6 on the logMAR (Early Treatment Diabetic Retinopathy Study chart, ETDRS) scale, and all agreed to avoid disallowed medication and contact lens wear for the designated period. Subjects underwent two allergen challenge visits during screening. In order to be included in the trial, subjects were required to exhibit a positive, bilateral reaction (itching and conjunctival redness scores ≥ 2, see below) to the CAC within 10 minutes of allergen instillation at both visit 1 and visit 2.
Key exclusion criteria included any baseline itching or redness > 1 in any vessel at each visit, any known allergy, contraindications or sensitivity to any of the study medications, or any systemic or ocular condition in the opinion of the investigator that could have affected safety or trial parameters. Exclusion criteria also included ocular surgery within 3 months or refractive surgery within 6 months, or if subjects exhibited any signs of active allergic conjunctivitis at the start of any visit, had an active ocular infection (bacterial or viral), or history of herpetic ocular disease. The use of any prohibited topical or systemic medications during the designated period or concurrent enrollment in any other clinical trial excluded the subject from the study. Due to the potential for randomization into a treatment arm with a pregnancy category C therapeutic,Citation8 women who were pregnant, planning a pregnancy, lactating, or not using a medically acceptable form of birth control for the entire period of the trial were excluded from the study. Subjects planning surgical procedures during the trial period or with a history of retinal detachment, diabetic retinopathy, or progressive retinal disease were also ineligible. Based upon these inclusion and exclusion criteria, three study sites screened a total of 245 subjects. A total of 127 subjects were eligible for randomization in a 1:1:1 ratio corresponding to the three treatments.
Allergen challenge and assessment of treatment efficacy
Allergen challenge employed a five-point scale for severity of ocular itch, where 0 indicated no itch and 4 indicated an incapacitating itch with an insatiable desire to rub. This scale, which allows for half-unit measures, has been used in CAC studies previously.Citation10–Citation18 Ocular redness was scored by an investigator using a five-point ocular redness scale (0–4) where 0 = none and 4 = extremely severe with large, numerous, dilated blood vessels characterized by an unusually severe deep red color. This scale has been used to grade ocular redness in previous studies.Citation10–Citation18
For the first study visit (visit 1), subjects received bilateral ocular instillation of antigen, followed by subject-reported assessment of itching 10 minutes after instillation. Subjects were titrated with increasing allergen concentrations until the scores for both itching and redness reached ≥ 2.0. The maximal allergen concentration used in the visit 1 titration was used for all subsequent visits. All study visits included medical history updates and ocular health assessment (visual acuity measurement and slit-lamp examination).
At visit 2, a second allergen challenge provided baseline data for those subjects who continued to satisfy eligibility criteria. Following the challenge, subjects graded their ocular itching at 3, 5, and 7 minutes after allergen instillation, while the secondary endpoint measures of investigator-assessed conjunctival redness and chemosis and subject-assessed lid swelling and tearing were at 7, 15, and 20 minutes. Subjects who met the screening criteria (scores ≥ 2 for both itching and conjunctival redness within 10 minutes of CAC) at visit 2 continued to visit 3A, approximately 2 weeks after visit 2.
At visit 3A, subjects who qualified were randomized and received one drop of masked treatment (either alcaftadine 0.25%, olopatadine 0.2%, or placebo) in each eye. Subjects returned approximately 15.5 hours after treatment instillation for the visit 3B CAC. Sixteen hours after instillation of treatment, subjects’ eyes were challenged by instilling one drop of the allergen solution bilaterally. Ocular itching was graded by subjects at 3, 5, and 7 minutes after instillation. Secondary efficacy variables, ie, redness, chemosis, tearing, and lid swelling, were evaluated at 7, 15, and 20 minutes. Conjunctival, ciliary, and episcleral redness as well as chemosis were graded by the investigator, and lid swelling and tearing were graded by the subject.
Fourteen days later (± 3 days), subjects returned for visit 4A. Following ocular health assessments, study staff instilled one drop of masked treatment into each eye and the subjects returned in 23.5 hours for visit 4B. Twenty-four hours after treatment instillation, subjects were again challenged with one drop of allergen solution bilaterally and then assessed for primary and secondary variables as in previous visits.
Efficacy and safety measures
The primary efficacy variable was ocular itching, which was quantified by the subjects using a 5-unit (0–4 units) standardized scale, with half-unit increments allowed. Secondary endpoints of conjunctival redness, ciliary redness, episcleral redness, and chemosis, were graded by the investigator using a 0–4 scale in which half unit values were allowed. Lid swelling employed a 0–3 scale without half-unit increments, and ocular tearing was scored using a 0–4 scale without half-unit increments. Safety assessments included the visual acuity measure using an ETDRS chart, slit-lamp biomicroscopy examination, and adverse event assessments.
Data analysis and statistical methods
Study population information and medical histories included both quantitative data such as age and qualitative demographics. Quantitative data were analyzed using a two-sided t-test, while qualitative demographic variables were analyzed using Fisher’s Exact test or Chi-squared test. The intent-to-treat population () was defined as those who were randomized at the beginning of visit 3. Per protocol subjects were those who completed the study with no major protocol violations.
Table 1 Demographics (intent-to-treat population)
The primary efficacy measure was ocular itching and the primary endpoint was mean ocular itching at 16 and 24 hours post instillation of the study medication, and 3, 5, and 7 minutes post CAC. Data collected at visit 3B and visit 4B were used for analysis of the primary endpoint. Predefined analysis of the primary efficacy data included comparison of the number of subjects in each treatment group with minimal itch, defined as those with itch scores < 1, and the number of subjects with zero itch, defined as scores = 0. These analyses used Fisher’s Exact test for each pairwise treatment comparison (alcaftadine 0.25% versus placebo, olopatadine 0.2% versus placebo, and alcaftadine 0.25% versus olopatadine 0.2%). Descriptive statistics (number of observations, mean, standard deviation, median, minimum, and maximum values) for the primary efficacy variable were summarized by visit, time point, and treatment group. Secondary endpoints were summarized using descriptive statistics and analyzed using the same statistical methods as those used for the primary endpoints.
A sample size of 40 subjects in each treatment group was determined to provide approximately 99% power to detect a difference in means of 1.00 unit (the difference between placebo and alcaftadine 0.25%, and the difference between placebo and olopatadine 0.2%) assuming that the common standard deviation is 1.00 unit using a two-sample t-test with a 0.05 two-sided significance level. A sample size of 40 subjects in each treatment group was determined to provide approximately 87% power to detect a difference in means of 0.70 unit between alcaftadine 0.25% and olopatadine 0.2%.
Results
A total of 127 subjects were enrolled in the study following screening visits 1 and 2, and 115 completed the study. Both active treatment arms had 38 (88%) subjects complete the study, and 39 (95%) completed in the placebo group. Of the 127 subjects enrolled in the intent-to-treat population, 12 subjects did not complete the study; of these, three were because of non-treatment-related adverse events, eight because of administrative issues, and two because of other non-study-related issues. The demographic characteristics of the groups revealed no significant differences between treatment groups with respect to age, gender, race, or iris color ().
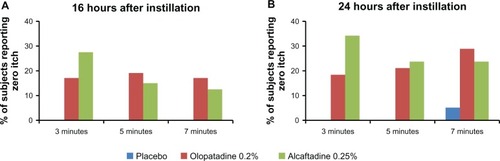
The primary endpoint for this study was mean ocular itching at 3, 5, and 7 minutes post CAC at 16 and 24 hours post instillation of treatment (; ). Sixteen hours after treatment instillation, both actives were statistically superior to placebo (P < 0.0001). The same statistical superiority compared with placebo was observed for active treatments 24 hours after instillation (P < 0.0001). Comparison of the two actives demonstrated that alcaftadine 0.25% achieved numerically lower mean itch scores than olopatadine 0.2% at all time points, both at 16 hours () and 24 hours post instillation (), with a statistically significant difference at the 3-minute time point at 16 hours (P = 0.026; ).
Figure 1 Comparison of ocular itching scores at 16 hours (A) and at 24 hours (B) after instillation of treatment. Mean itching for placebo, alcaftadine 0.25%, and olopatadine 0.2% at 3, 5, and 7 minutes after allergen challenge.
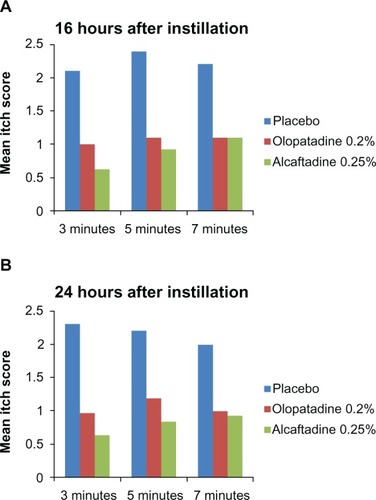
Table 2 Summary of ocular itching results (intent-to-treat population): differences versus placebo in mean ocular itching scores following CAC for alcaftadine 0.25% and olopatadine 0.2% at 16 and 24 hours
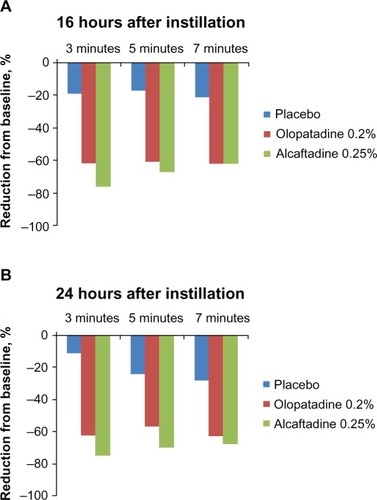
To assess the ability of the actives to reduce ocular itching within a population, comparisons between baseline itch scores for each treatment group (visit 2) and scores obtained in visits 3 and 4 were made; in this comparison, the observed treatment effects are responses in the same subjects at different visits rather than a parallel placebo population. The percent itch reduction from baseline at both 16 and 24 hours after treatment instillation revealed significant reductions from baseline ().
Figure 2 Reduction from baseline itch scores. A comparison of itch scores at visit 3 (16 hours; A) and at visit 4 (24 hours; B) with those from visit 2 (pretreatment baseline visit).
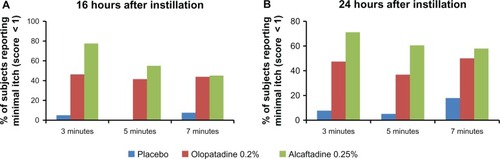
The percentage of subjects reporting minimal itch (a score of < 1.0), is plotted for all treatments at both the 16 and 24 hour assessments (). The difference between actives was greatest at 3 minutes (16 hours after instillation), when 78% of subjects treated with alcaftadine 0.25% reported itch scores < 1 as compared with 46% of those treated with olopatadine 0.2% (P = 0.006). At the same time point at 24 hours post instillation, 71% of subjects treated with alcaf-tadine 0.25% reported minimal itch, compared with 47% of subjects treated with olopatadine 0.2% (P = 0.061).
Figure 3 Comparison of minimal itch (scores < 1) data for placebo, alcaftadine 0.25%, and olopatadine 0.2% at 16 hours (A) and at 24 hours (B) after instillation of treatment.
The percentage of subjects reporting complete relief (itch score = 0) was also examined at both 16 and 24 hours after treatment instillation (). Both actives exhibited a zero itch score in 20%–30% of subjects. A greater number of subjects in the alcaftadine 0.25% arm displayed zero itch at 3 minutes (P ≥ 0.192), while more subjects in the olopatadine 0.2% arm had zero itch at the 7-minute time point (P ≥ 0.756), although differences in zero itch were not statistically significant.
Figure 4 Comparison of zero itch data for placebo, alcaftadine 0.25%, and olopatadine 0.2% at 16 hours (A) and 24 hours (B) after instillation of treatment.
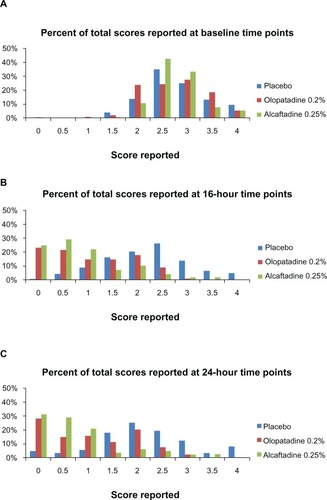
An alternative approach to the assessment of itch relief is to examine a distribution of the raw subject-reported itch scores (). For each visit, subjects reported a total of six scores for two eyes at three time points. For subject-reported itch score distributions, a leftward shift in score frequencies from baseline to drug treatment is an indicator of efficacy, both in terms of the degree of relief and the proportion of subjects whose symptoms are alleviated. In this study, the leftward shift seen in the alcaftadine group is greater than that seen in the olopatadine group, at both 16 and 24 hours after instillation.
Secondary efficacy measures for redness, chemosis, lid swelling, and tearing are summarized in . For conjunctival redness measures, both actives demonstrated statistical significance relative to placebo in each of the three vascular beds at some but not all time points evaluated, and there were no significant differences between actives (). Both treatments provided statistically significant reductions in lid swelling and ocular tearing compared with placebo, and these effects were significant at both 16 and 24 hours after treatment instillation (). For chemosis, both alcaftadine 0.25% and olopatadine 0.2% provided relief 16 hours after instillation, but only alcaftadine 0.25% demonstrated statistical significance against placebo for chemosis at every time point measured at 24 hours.
Table 3 Secondary endpoint data for both visits expressed as difference scores
There were no serious adverse events in the course of this 5-week study. There was a total of 14 adverse events experienced by nine subjects, and five of these events were reported by one subject. Three of these occurred as a result of a fall, and were not treatment-related. None of the reported adverse events were related to any study treatments.
Discussion
In this comparative study, two topical formulations of ocular antihistamines were evaluated for their ability to provide a long duration of relief from itching due to allergic conjunctivitis. Both olopatadine 0.2% and alcaftadine 0.25% are approved by the US Food and Drug Administration for once-daily dosing.Citation8,Citation9 In previous studies,Citation10,Citation11 duration of action had been measured 16 hours following instillation, and both treatments demonstrated substantial relief at that time point. The study described in this report was designed to extend that analysis to 24 hours post-instillation, while also providing a basis for direct comparison of the two antiallergic agents. Our comparison of alcaftadine 0.25%, olopatadine 0.2%, and placebo utilized the CAC model to measure duration of treatment efficacy in reducing ocular itching, ocular redness, chemosis, tearing, and lid swelling. Use of CAC is the current standard for regulatory approval of ocular antiallergic agents in the US.Citation10,Citation11,Citation13–Citation18
Both treatments provided reduction in symptoms and signs of allergic conjunctivitis in this study. The reduction in ocular itching at 16 and 24 hours after treatment instillation was statistically significant for both olopatadine 0.2% and alcaftadine 0.25% compared with placebo (P < 0.0001). Eyes treated with alcaftadine 0.25% had significantly lower scores for mean ocular itching than eyes treated with olopatadine 0.2% at 3 minutes for the 16-hour visit (P = 0.026), the earliest time point evaluated in the CAC model. In addition, eyes treated with alcaftadine 0.25% had numerically lower scores for mean itching than eyes treated with olopatadine 0.2% at all other time points for both the 16-hour and 24-hour visits. Both treatments also demonstrated reductions in all secondary endpoints. Alcaftadine 0.25% was statistically superior to olopatadine 0.2% for reducing chemosis 24 hours after instillation.
The differences in ocular itching scores between the actives and placebo, as well as the differences between the two actives, were similar both at the 16-hour and 24-hour postinstillation visits. The most significant difference between the two treatments was evidenced in the comparison of minimal itch (score < 1). At the 3-minute time point (16 hours after instillation), about 78% of subjects receiving alcaftadine reported minimal or no itch; in contrast, less than 50% of subjects receiving olopatadine demonstrated a similar level of itch relief. The difference in efficacy is perhaps most apparent in the distribution of itch scores (). These plots revealed an important difference between alcaftadine and olopatadine, where a greater proportion of scores for alcaftadine are ≤1.5, while the scores for olopatadine are evenly distributed in a range between 0 and 2.
Two previous studies have examined the therapeutic effects of both alcaftadine and olopatadine. An early CAC-based clinical study found alcaftadine 0.25% was superior to olopatadine 0.1% for both onset and duration of anti-itch effects.Citation19 The faster onset of effect is consistent with the findings of the present study in which statistically significant lower itch scores were seen at the 3-minute time point post allergen challenge (16 hours post instillation). In another preclinical study, alcaftadine was shown to have a greater effect than olopatadine on both eosinophil recruitment and epithelial junctional protein stability,Citation20 suggesting a potential for greater efficacy in late-phase allergy. This result, together with an examination of inflammation-induced disruptions in ocular epithelial stability,Citation21 suggest that efficacy differences between alcaftadine and olopatadine may be related to the greater ability of alcaftadine to prevent the disruption to the ocular surface that follows allergen exposure. Both rapid relief in ocular itching as well as long duration of action are keys to successful control of allergic conjunctivitis. Results of the current study, in combination with previous reports, establish that alcaftadine 0.25% can effectively address both of these key aspects of disease control.
Overall, this study demonstrated that both alcaftadine 0.25% and olopatadine 0.2% were effective at preventing ocular itching in a CAC model beyond 16 hours. The differences between actives and placebo and the differences between the two actives at 24 hours post instillation were similar to the findings 16 hours post instillation. Alcaftadine 0.25% was shown to achieve statistically significant lower mean ocular itch scores than olopatadine 0.2% at the earliest time point following allergen challenge. Both alcaftadine 0.25% and olopatadine 0.2% were safe and well tolerated. Further studies are warranted to delineate further the actions of these two treatments and understand better the mechanisms of actions potentially underlying any differences in efficacy.
Acknowledgment
Copy editing and styling support was provided by Diann Glickman, Evidence Scientific Solutions, and funded by Allergan Inc. The authors would like to acknowledge James McLaughlin of Ora, Inc. for writing assistance, funded by Allergan, Inc.
Disclosure
Ms Villanueva and Dr Hollander are employees of Allergan, Inc. The authors report no other conflicts of interest in this work.
References
- BlaissMSAllergic rhinoconjunctivitis: burden of diseaseAllergy Asthma Proc200728439339717883905
- CollumLMKilmartinDJAcute allergic conjunctivitisAbelsonMBAllergic Diseases of the EyePhiladelphia, PAWB Saunders2000
- AbelsonMBSchaeferKFunctional anatomy of the human conjunctiva and corneaAbelsonMBAllergic Diseases of the EyePhiladelphia, PAWB Saunders2000
- AbelsonMBMcLaughlinJTGomesPJAntihistamines in ocular allergy: are they all created equal?Curr Allergy Asthma Rep201111320521121437647
- HughesJLLackiePMWilsonSJChurchMKMcGillJIReduced structural proteins in the conjunctival epithelium in allergic eye diseaseAllergy200661111268127417002701
- [No authors]Drugs for allergic disordersTreat Guidel Med Lett201089091820094023
- ManzouriBFlynnTHLarkinFOnoSJWyseRPharmaco-therapy of allergic eye diseaseExpert Opin Pharmacother2006791191120016732705
- Pataday® (olopatadine hydrochloride ophthalmic solution) 0.2%. [package insert]Fort Worth, TXAlcon Laboratories Inc1996
- Lastacaft® (alcaftadine ophthalmic solution) 0.25%. [package insert]Irvine, CAAllergan Inc2010
- AbelsonMBGomesPJOlopatadine 0.2% ophthalmic solution: the first ophthalmic antiallergy agent with once-daily dosingExpert Opin Drug Metab Toxicol20084445346118433347
- TorkildsenGSheddenAThe safety and efficacy of alcaftadine 0.25% ophthalmic solution for the prevention of itching associated with allergic conjunctivitisCurr Med Res Opin201127362363121250860
- AbelsonMBChambersWASmithLMConjunctival allergen challenge. A clinical approach to studying allergic conjunctivitisArch Ophthalmol1990108184882297337
- AbelsonMBTorkildsenGLWilliamsJIGowJAGomesPJMcNamaraTRBepotastine Besilate Ophthalmic Solutions Clinical Study GroupTime to onset and duration of action of the antihistamine bepotastine besilate ophthalmic solutions 1.0% and 1.5% in allergic conjunctivitis: a phase III, single-center, prospective, randomized, double-masked, placebo-controlled, conjunctival allergen challenge assessment in adults and childrenClin Ther20093191908192119843481
- AbelsonMBEvaluation of olopatadine, a new ophthalmic antiallergic agent with dual activity, using the conjunctival allergen challenge modelAnn Allergy Asthma Immunol19988132112189759796
- AbelsonMBSpitalnyLCombined analysis of two studies using the conjunctival allergen challenge model to evaluate olopatadine hydrochloride, a new ophthalmic antiallergic agent with dual activityAm J Ophthalmol199812567978049645717
- AbelsonMBChapinMJKapikBMShamsNBEfficacy of ketotifen fumarate 0.025% ophthalmic solution compared with placebo in the conjunctival allergen challenge modelArch Ophthalmol2003121562663012742839
- AbelsonMBGomesPCramptonHJSchiffmanRMBradfordRRWhitcupSMEfficacy and tolerability of ophthalmic epinastine assessed using the conjunctival antigen challenge model in patients with a history of allergic conjunctivitisClin Ther2004261354714996516
- AbelsonMBSpanglerDLEpsteinABMahFSCramptonHJEfficacy of once-daily olopatadine 0.2% ophthalmic solution compared to twice-daily olopatadine 0.1% ophthalmic solution for the treatment of ocular itching induced by conjunctival allergen challengeCurr Eye Res200732121017102218085465
- GreinerJVEdwards-SwansonKIngermanAEvaluation of alcaftadine 0.25% ophthalmic solution in acute allergic conjunctivitis at 15 minutes and 16 hours after instillation versus placebo and olopatadine 0.1%Clin Ophthalmol20115879321339800
- OnoSJLaneKComparison of effects of alcaftadine and olopatadine on conjunctival epithelium and eosinophil recruitment in a murine model of allergic conjunctivitisDrug Des Devel Ther201157784
- Contreras-RuizLSchulzeUGarcía-PosadasLStructural and functional alteration of corneal epithelial barrier under inflammatory conditionsCurr Eye Res2012371197198122738643