Abstract
Purpose
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a predominantly type 2 inflammatory disease frequently coexisting with other type 2 conditions including asthma and non-steroidal anti-inflammatory drug-exacerbated respiratory disease (NSAID-ERD). Coexisting asthma leads to increased CRSwNP symptom burden. Dupilumab, a monoclonal antibody that blocks the shared receptor component for interleukin-4 and -13, demonstrated efficacy in adults with severe CRSwNP in the Phase 3 SINUS-24 (NCT02912468) and SINUS-52 (NCT02898454) studies, including in patients with coexisting asthma/NSAID-ERD. However, the impact of different asthma characteristics on dupilumab treatment in this population is unknown. We report CRSwNP and asthma outcomes with dupilumab in patients with CRSwNP and coexisting asthma according to baseline asthma characteristics.
Methods
Change from baseline at Week 24 (pooled studies) and Week 52 (SINUS-52) in CRSwNP outcomes (nasal polyp score, nasal congestion, 22-item Sino-Nasal Outcome Test [SNOT-22], loss of smell score, University of Pennsylvania Smell Identification Test) and asthma outcomes (5-item Asthma Control Questionnaire [ACQ-5], pre-bronchodilator forced expiratory volume in 1 second [FEV1]) were analyzed post hoc for placebo and dupilumab 300 mg every 2 weeks according to baseline blood eosinophils ≥150/≥300 cells/µL, ACQ-5 scores <1.5/≥1.5, and FEV1 <80%.
Results
In the pooled studies, 428/724 patients (59.1%) had coexisting asthma, of which 181/428 (42.3%) had coexisting NSAID-ERD. Dupilumab significantly improved all CRSwNP and asthma outcomes vs placebo at Week 24 (P < 0.001) regardless of baseline eosinophil or ACQ-5 category, or FEV1 <80%. Similar magnitude of improvement was seen at Week 52 (SINUS-52) and in patients with NSAID-ERD (pooled studies, Week 24). By Week 24, improvements with dupilumab exceeded the minimum clinically important differences for ACQ-5 and SNOT-22 in 35.2% to 74.2% and 72.0% to 78.7% of patients, respectively.
Conclusion
Dupilumab improved CRSwNP outcomes in patients with CRSwNP and coexisting asthma, and improved asthma outcomes, regardless of differences in baseline asthma characteristics.
Introduction
Type 2 inflammation plays a central role in the pathophysiology of multiple inflammatory diseases, including chronic rhinosinusitis with nasal polyps (CRSwNP) and asthma.Citation1 Approximately 85% of patients with CRSwNPCitation2 and 71% to 84% of adults with asthmaCitation3 have type 2 inflammation. CRSwNP and asthma often coexist, with studies indicating that between 40% and 67% of patients with CRSwNP have coexisting asthma.Citation4–7
CRSwNP is associated with a significant clinical, health-related quality of life (HRQoL) and economic burden.Citation8–10 Those with coexisting asthma have a more severe CRSwNP disease burden and tend to have poorer outcomes for both CRSwNP and asthma than patients without the coexisting condition, adding to the treatment challenge.Citation7,Citation8,Citation10 Asthma characteristics such as elevated type 2 inflammatory markers, poor asthma control, and reduced lung function have been shown to impact burden of asthma, risk of exacerbation, and treatment outcomes.Citation6,Citation11–14 The presence of coexisting non-steroidal anti-inflammatory drug-exacerbated respiratory disease (NSAID-ERD) has also been shown to increase disease burden in both CRSwNP and asthma.Citation15
Dupilumab is a fully human monoclonal antibody that blocks the shared receptor component for interleukin-4 and -13, which are key and central drivers of type 2 inflammation.Citation16,Citation17 In the Phase 3 SINUS-24 (NCT02912468) and SINUS-52 (NCT02898454) studies in patients with severe CRSwNP, dupilumab significantly improved objective and patient-reported CRSwNP outcomes.Citation18 Additional post hoc analyses of the SINUS trials also demonstrated that dupilumab was equally effective in improving CRSwNP, asthma, and quality-of-life outcomes in patients with CRSwNP with or without self-reported asthma.Citation19,Citation20 However, the efficacy of different treatments can be influenced by the type of asthma, or by variation in other physiologic or immunologic characteristics.Citation21 The efficacy of dupilumab in patients with CRSwNP and varying asthma characteristics is currently unknown. Therefore, the aim of this post hoc analysis of the SINUS studies was to assess the effect of dupilumab on CRSwNP and asthma outcomes in patients with severe CRSwNP and coexisting asthma stratified by baseline asthma characteristics.
Methods
Study Design and Patients
Data were included for patients with severe CRSwNP and coexisting asthma who received dupilumab 300 mg or placebo once every 2 weeks in SINUS-24 and SINUS-52; full details of these studies are published elsewhere.Citation18 Both study protocols were approved by 200 Copernicus Group IRB (Protocol # EFC14146, Tracking # SAN4-16-406; Protocol # EFC14280, Tracking # SAN4-16-417). Severe CRSwNP was defined in line with criteria from the European Forum for Research and Education in Allergy and Airway Diseases (EUFOREA), and in the eligibility criteria for the SINUS-24 and SINUS-52 studies.Citation18,Citation22 For this post hoc analysis, patients were divided into groups based on evidence of type 2 inflammation (blood eosinophil levels), asthma control, and reduced lung function, which are known to impact disease burden, exacerbation, or risk of poor treatment outcomes.Citation6,Citation11–14 Using baseline measures, patients were stratified into the following 5 groups, which were selected to represent relevant categories associated with the above asthma characteristics: 1) blood eosinophil levels ≥150 cells/µL or 2) ≥300 cells/µL (representing evidence of type 2 inflammation); 3) 5-item Asthma Control Questionnaire (ACQ-5) score <1.5 (controlled asthma) or 4) ≥1.5 (uncontrolled asthma); and 5) forced expiratory volume in 1 second (FEV1) <80% predicted (reduced lung function).
CRSwNP outcomes assessed were nasal polyp score (NPS; range 0 to 8; the sum of the right and left nostril scores, assessed by at least two physicians based on centrally read video recordings of nasal endoscopy), nasal congestion (NC; 0 to 3), loss of smell (LoS) score (0 to 3), 22-item Sino-Nasal Outcome Test (SNOT-22) total (0 to 110) and domain (ear/facial, emotion, function, nasal, and sleep, each computed as the average score of the corresponding items [0 to 5]) scores,Citation23 and the University of Pennsylvania Smell Identification Test (UPSIT) score (0 to 40). ACQ-5 score (range 0 to 6) and pre-bronchodilator predicted FEV1 were the asthma outcomes assessed.
CRSwNP and asthma outcomes were also assessed within each asthma characteristic group for patients with or without coexisting NSAID-ERD.
Statistical Analyses
Pooled data from SINUS-24 and SINUS-52 were used for the Week 24 analysis, and data from SINUS-52 were used for the Week 52 analysis. Least squares (LS) mean changes from baseline at 24 and 52 weeks for each outcome were analyzed in each asthma characteristic group using an analysis of covariance model with change from baseline in the outcome as the response, and the corresponding baseline value, treatment group, prior surgery history, regions, and study indicator as covariates. As this was a post hoc analysis, all P values are nominal.
Results
Baseline Characteristics
In the pooled intention-to-treat population, 59.1% (428/724) of patients had self-reported coexisting asthma. Of these patients, 50.9% (218/428) had uncontrolled asthma (ACQ-5 score ≥1.5) and 40.7% (174/428) had predicted FEV1 <80%. Baseline CRSwNP and asthma disease characteristics were generally balanced across the asthma characteristics groups (), except for SNOT-22, which was higher in the uncontrolled vs the controlled asthma subgroup; the difference exceeded the minimum clinically important difference (MCID) of 8.9 points.Citation24
Table 1 Baseline CRSwNP and Asthma Characteristics for Dupilumab and Placebo Treatment Groups in Patients with CRSwNP and Coexisting Asthma from the SINUS-24 and SINUS-52 Studies (Pooled), Stratified by Asthma Subgroups
CRSwNP Outcomes
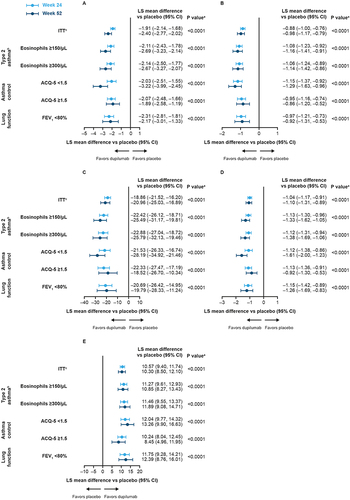
Dupilumab significantly improved NPS, NC, LoS, and HRQoL vs placebo at Week 24 across all asthma characteristics groups, with improvements maintained at Week 52 (all P < 0.0001) (). At Week 24 in the pooled studies, improvements in SNOT-22 total score with dupilumab exceeded the MCID of 8.9 points in 76.1% of patients with baseline eosinophils ≥150/μL, 77.3% of patients with baseline eosinophils ≥300/μL, 78.7% of patients with controlled asthma, 77.4% of patients with uncontrolled asthma, and 72.0% of patients with reduced lung function (all P < 0.0001 vs placebo). The corresponding proportions in SINUS-52 at Week 52 were 73.0%, 74.6%, 78.6%, 67.5%, and 67.5%, respectively (all P < 0.001 vs placebo).
Figure 1 Forest plots of least squares mean difference vs placebo in CRSwNP outcomes at Week 24 (Pooled SINUS-24 and SINUS-52) and Week 52 (SINUS-52) in patients with CRSwNP and coexisting asthma, stratified by baseline asthma characteristics (A) NPS, (B) NC, (C) SNOT-22 total, (D) LoS score, (E) UPSIT.
In addition, there were significant improvements in each SNOT-22 domain score vs placebo (Table S1).
Asthma Outcomes
Improvements in FEV1 were observed with dupilumab across all asthma characteristics groups at Week 24 and Week 52, with all changes reaching significance (Week 24, all P < 0.001; Week 52, all P < 0.05), except at Week 52 in patients with uncontrolled asthma at baseline (P = 0.0511) ().
Figure 2 Forest plots of least squares mean difference vs placebo in asthma outcomes at Week 24 (Pooled SINUS-24 and SINUS-52) and Week 52 (SINUS-52) in patients with CRSwNP and coexisting asthma, stratified by baseline asthma characteristics (A) ACQ-5, (B) FEV1.
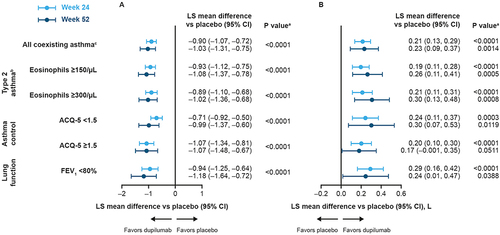
Dupilumab significantly improved ACQ-5 at Week 24 across all asthma characteristics groups, with improvements maintained at Week 52 (all P < 0.0001) (). At Week 24 in the pooled studies, improvements exceeded the MCID of 0.5 points for ACQ-5Citation25 in 53.0% of patients with baseline eosinophils ≥150/μL, 53.4% of patients with baseline eosinophils ≥300/μL, 35.2% of patients with controlled asthma, 74.2% of patients with uncontrolled asthma, and 61.7% of patients with reduced lung function (all P < 0.0001). The corresponding proportions in SINUS-52 at Week 52 were 45.9%, 45.8%, 28.6%, 62.5%, and 47.5%, respectively (all P < 0.01).
CRSwNP and Asthma Outcomes in Patients with Coexisting NSAID-ERD
In the SINUS studies, 28.2% of patients had CRSwNP with coexisting NSAID-ERD. Among patients with CRSwNP and asthma, the frequency of coexisting NSAID-ERD was similar across the asthma characteristic groups (37.9% to 42.2%) (Table S2). Improvements with dupilumab were seen for all CRSwNP and asthma outcomes across all asthma characteristics groups at Week 24 regardless of the presence of NSAID-ERD at baseline (Table S2). All changes were significant (P < 0.05), except for change in FEV1 in patients with an ACQ-5 score <1.5 and without NSAID-ERD at baseline (P = 0.0743).
Discussion
The results of this analysis demonstrate that dupilumab significantly improved both CRSwNP and asthma outcomes vs placebo, with improvements seen irrespective of relevant baseline asthma characteristics. Patients with type 2 asthma—an inflammatory characteristic that typically presents with more severe and difficult-to-treat disease, and is associated with coexisting conditions and increased risk of poor asthma outcomesCitation11—demonstrated improvement in CRSwNP and asthma outcomes. As dupilumab targets the shared receptor component for interleukin-4 and -13, which are key and central drivers of type 2 inflammation, this provides a likely mechanism to explain the clinical effects of dupilumab in patients with type 2 asthma and CRSwNP.
Patients with uncontrolled asthma also demonstrated improvement, which is significant as this characteristic is associated with increased risk of asthma exacerbation and increased burden on healthcare resources.Citation12,Citation13 Interestingly, approximately one-third of patients with controlled asthma achieved the ACQ-5 MCID, which indicates that further improvement may be gained with dupilumab in those with already well-controlled asthma. Dupilumab also demonstrated improvement in CRSwNP and asthma outcomes irrespective of the presence of coexisting NSAID-ERD.
Most (90%) of the patients with self-reported asthma in our cohort showed characteristics of type 2 asthma based on elevated blood eosinophils ≥150/μL, with approximately 70% with blood eosinophils ≥300/μL. The type 2 asthma group definition was based solely on eosinophil count, which is a narrow definition and highlights a potential limitation of the study, as other type 2 biomarkers need to be considered to fully determine the effectiveness of dupilumab treatment in patients with coexisting type 2 asthma, as well as determining that the outcomes of the current analysis are independent of other type 2 biomarkers. Other type 2 biomarkers that are common in asthma are missing from our definition, including high total immunoglobulin E and high fractional exhaled nitric oxide (FeNO).Citation26 The latter omission is particularly important as high FeNO has been shown to provide prognostic value of exacerbation risk and predict patient response to dupilumab independently of blood eosinophil count.Citation27–30 Furthermore, this study only assesses the impact of asthma severity based on airway obstruction, and patients with severe airway obstruction (FEV1 <50%) were excluded from the SINUS-24 and SINUS-52 trials, meaning that dupilumab efficacy in patients with severe airway obstruction is uncertain. Additional analyses could consider other markers for asthma disease severity, including asthma medication use, exacerbation history, and asthma severity per Global Initiative for Asthma guidelines.
More generally, in the context of other biologics and their efficacy in patients with coexisting disease, post hoc analyses of the PROXIMA (omalizumab) and ANANKE studies (benralizumab) demonstrated improvements in asthma outcomes in patients with severe eosinophilic/allergic asthma regardless of coexisting CRSwNP, though neither of these studies was focused on CRSwNP and did not report CRSwNP outcomes in these populations.Citation31,Citation32 Biologics have also demonstrated efficacy in a real-world setting, with improvements in asthma outcomes and some CRSwNP outcomes observed with dupilumab, benralizumab, mepolizumab, and omalizumab in patients with asthma, with or without coexisting CRSwNP.Citation33–40 However, the impact of baseline disease characteristics on treatment outcomes evaluated in this analysis was not assessed in these studies.
In conclusion, dupilumab significantly improved upper and lower airway outcomes in patients with severe CRSwNP and coexisting asthma, in line with other studies of biologics in the treatment of coexisting disease. Improvements were seen in patients with type 2 asthma, irrespective of relevant baseline measures of asthma control or lung function, and irrespective of coexisting NSAID-ERD. These data add to our understanding of the effects of dupilumab in patients with coexisting upper and lower airway disease.
Abbreviations
ACQ-5, 5-item Asthma Control Questionnaire; ANCOVA, analysis of covariance; CI, confidence interval; CRSwNP, chronic rhinosinusitis with nasal polyps; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; HRQoL, health-related quality of life; ITT, intention-to-treat; LoS, loss of smell; LS, least squares; MCID, minimum clinically important difference; NC, nasal congestion; NPS, nasal polyp score; NSAID-ERD, non-steroidal anti-inflammatory drug-exacerbated respiratory disease; SNOT-22, 22-item Sino-Nasal Outcome Test; UPSIT, University of Pennsylvania Smell Identification Test.
Data Sharing Statement
Qualified researchers may request access to patient-level data and related study documents including clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/.
Ethics Approval and Informed Consent
The studies were conducted in accordance with the Declaration of Helsinki, approved by the local institutional review board or ethics committee at each study site (Table S3), and all patients provided written informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all of these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
WWB reports speaker fees from GlaxoSmithKline and Regeneron Pharmaceuticals Inc., consultancy fees from Arrowhead, AstraZeneca, Genentech, GlaxoSmithKline, Knopp Biosciences, Novartis, Regeneron Pharmaceuticals Inc., and Sanofi, grants or contracts from National Institutes of Health – National Heart, Lung, and Blood Institute and National Institutes of Health – National Institute of Allergy and Infectious Diseases, book royalties from Elsevier, and support for attending meetings and/or travel from GlaxoSmithKline, Regeneron Pharmaceuticals Inc., and Sanofi. IDP reports speaker fees from Aerocrine, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Novartis, Regeneron Pharmaceuticals Inc., Sanofi, and Teva, payments for organizing educational events from AstraZeneca, GlaxoSmithKline, Regeneron Pharmaceuticals Inc., Sanofi, and Teva, consultancy fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Genentech, GlaxoSmithKline, Knopp Biosciences, Merck, Novartis, Regeneron Pharmaceuticals Inc., Sanofi, and Teva, international scientific meeting sponsorship from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Teva, research grants from Chiesi, payments to support FDA approval meetings from GlaxoSmithKline, and payments for use of the Leicester Cough Questionnaire (of which he is a co-patent holder of the rights) in clinical trials from Bayer, Insmed, and Merck, and served as an expert witness for a patent dispute involving AstraZeneca and Teva. He also reports a grant from Chiesi to support a Phase 2 clinical trial in Oxford. SS is a former employee of Regeneron Pharmaceuticals Inc. and may hold stock and/or stock options. AHK, AP, JAJ-N, and PJR are employees of Sanofi and may hold stock and/or stock options. SN and YD are employees of Regeneron Pharmaceuticals Inc. and may hold stock and/or stock options. The authors report no other conflicts of interest in this work.
Acknowledgments
This research was sponsored by Sanofi and Regeneron Pharmaceuticals Inc. ClinicalTrials.gov identifiers: NCT02912468 and NCT02898454. Medical writing support was provided by Olympia Gianfrancesco, PhD, of Adelphi Group, Macclesfield, UK, funded by Sanofi and Regeneron Pharmaceuticals Inc. in accordance with Good Publications Practice.
Additional information
Funding
References
- Gandhi NA, Bennett BL, Graham NM, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35–50. doi:10.1038/nrd4624
- Bachert C, Zhang N, Hellings PW, Bousquet J. Endotype-driven care pathways in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2018;141(5):1543–1551. doi:10.1016/j.jaci.2018.03.004
- Jackson DJ, Aljamil N, Roxas C, et al. P48 The ‘T2-low’ asthma phenotype: could it just be T2-high asthma treated with corticosteroids? Thorax. 2018;73:A124–A125.
- Khan A, Vandeplas G, Huynh TMT, et al. The Global Allergy and Asthma European Network (GALEN) rhinosinusitis cohort: a large European cross-sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology. 2019;57(1):32–42. doi:10.4193/Rhin17.255
- Philpott C, Ta NH, Hopkins C, et al. Socioeconomic, comorbidity, lifestyle, and quality of life comparisons between chronic rhinosinusitis phenotypes. Laryngoscope. 2021;131(10):2179–2186. doi:10.1002/lary.29527
- Busse WW, Kraft M, Rabe KF, et al. Understanding the key issues in the treatment of uncontrolled persistent asthma with type 2 inflammation. Eur Respir J. 2021;58(2):2003393. doi:10.1183/13993003.03393-2020
- Laidlaw TM, Mullol J, Woessner KM, Amin N, Mannent LP. Chronic rhinosinusitis with nasal polyps and asthma. J Allergy Clin Immunol Pract. 2021;9(3):1133–1141. doi:10.1016/j.jaip.2020.09.063
- Bachert C, Bhattacharyya N, Desrosiers M, Khan AH. Burden of disease in chronic rhinosinusitis with nasal polyps. J Asthma Allergy. 2021;14:127–134. doi:10.2147/JAA.S290424
- Bhattacharyya N, Villeneuve S, Joish VN, et al. Cost burden and resource utilization in patients with chronic rhinosinusitis and nasal polyps. Laryngoscope. 2019;129(9):1969–1975. doi:10.1002/lary.27852
- Khan A, Huynh TMT, Vandeplas G, et al. The GALEN rhinosinusitis cohort: chronic rhinosinusitis with nasal polyps affects health-related quality of life. Rhinology. 2019;57(5):343–351. doi:10.4193/Rhin19.158
- de Groot JC, Ten Brinke A, Bel EH. Management of the patient with eosinophilic asthma: a new era begins. ERJ Open Res. 2015;1(1):00024–2015. doi:10.1183/23120541.00024-2015
- Meltzer EO, Busse WW, Wenzel SE, et al. Use of the asthma control questionnaire to predict future risk of asthma exacerbation. J Allergy Clin Immunol. 2011;127(1):167–172. doi:10.1016/j.jaci.2010.08.042
- Sullivan PW, Globe G, Ghushchyan VH, Campbell JD, Bender B, Magid DJ. Exploring asthma control cutoffs and economic outcomes using the Asthma Control Questionnaire. Ann Allergy Asthma Immunol. 2016;117(3):251–257.e252. doi:10.1016/j.anai.2016.07.020
- Tupper OD, Ulrik CS. Long-term predictors of severe exacerbations and mortality in a cohort of well-characterised adults with asthma. Respir Res. 2021;22(1):269. doi:10.1186/s12931-021-01864-z
- Kowalski ML, Agache I, Bavbek S, et al. Diagnosis and management of NSAID-Exacerbated Respiratory Disease (N-ERD)-a EAACI position paper. Allergy. 2019;74(1):28–39. doi:10.1111/all.13599
- Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425–437. doi:10.1080/1744666X.2017.1298443
- Le Floc’h A, Allinne J, Nagashima K, et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75(5):1188–1204. doi:10.1111/all.14151
- Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650. doi:10.1016/S0140-6736(19)31881-1
- Laidlaw TM, Bachert C, Amin N, et al. Dupilumab improves upper and lower airway disease control in chronic rhinosinusitis with nasal polyps and asthma. Ann Allergy Asthma Immunol. 2021;126(5):584–592.e581. doi:10.1016/j.anai.2021.01.012
- Gevaert P, Lee SE, Settipane RA, et al. Dupilumab provides early and durable improvement of symptoms in patients with chronic rhinosinusitis with nasal polyps. Clin Transl Immunology. 2023;12(1):e1433. doi:10.1002/cti2.1433
- Muraro A, Lemanske RF, Hellings PW, et al. Precision medicine in patients with allergic diseases: airway diseases and atopic dermatitis-PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2016;137(5):1347–1358. doi:10.1016/j.jaci.2016.03.010
- Bachert C, Han JK, Wagenmann M, et al. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: definitions and management. J Allergy Clin Immunol. 2021;147(1):29–36. doi:10.1016/j.jaci.2020.11.013
- Khan A, Reaney M, Guillemin I, et al. Development of Sinonasal Outcome Test (SNOT-22) domains in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2022;132(5):933–941. doi:10.1002/lary.29766
- Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447–454. doi:10.1111/j.1749-4486.2009.01995.x
- Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553–558. doi:10.1016/j.rmed.2004.10.008
- Canonica GW, Blasi F, Crimi N, et al. Defining type 2 asthma and patients eligible for dupilumab in Italy: a biomarker-based analysis. Clin Mol Allergy. 2021;19(1):5. doi:10.1186/s12948-021-00146-9
- Busse WW, Wenzel SE, Casale TB, et al. Baseline FeNO as a prognostic biomarker for subsequent severe asthma exacerbations in patients with uncontrolled, moderate-to-severe asthma receiving placebo in the LIBERTY ASTHMA QUEST study: a post-hoc analysis. Lancet Respir Med. 2021;9(10):1165–1173. doi:10.1016/S2213-2600(21)00124-7
- Couillard S, Laugerud A, Jabeen M, et al. Derivation of a prototype asthma attack risk scale centred on blood eosinophils and exhaled nitric oxide. Thorax. 2022;77(2):199–202. doi:10.1136/thoraxjnl-2021-217325
- Pavord I, Busse W, Corren J, et al. P220 baseline exhaled nitric oxide (FeNO) as predictor of response to dupilumab in uncontrolled, moderate-to-severe asthma. Ann Allergy Asthma Immunol. 2020;125(5):S34. doi:10.1016/j.anai.2020.08.117
- Pavord I, Brusselle G, Jackson D, et al. FeNO as a potential prognostic and predictive marker of lung function decline in patients with uncontrolled, moderate-to-severe asthma: LIBERTY ASTHMA QUEST. American Thoracic Society – 118th International Conference; 2022.
- Heffler E, Saccheri F, Bartezaghi M, Canonica GW. Effectiveness of omalizumab in patients with severe allergic asthma with and without chronic rhinosinusitis with nasal polyps: a PROXIMA study post hoc analysis. Clin Transl Allergy. 2020;10:25. doi:10.1186/s13601-020-00330-1
- D’Amato M, Menzella F, Altieri E, et al. Benralizumab in patients with severe eosinophilic asthma with and without chronic rhinosinusitis with nasal polyps: an ANANKE study post-hoc analysis. Front Allergy. 2022;3:881218. doi:10.3389/falgy.2022.881218
- Dupin C, Belhadi D, Guilleminault L, et al. Effectiveness and safety of dupilumab for the treatment of severe asthma in a real-life French multi-centre adult cohort. Clin Exp Allergy. 2020;50(7):789–798. doi:10.1111/cea.13614
- Campisi R, Crimi C, Nolasco S, et al. Real-world experience with dupilumab in severe asthma: one-year data from an Italian named patient program. J Asthma Allergy. 2021;14:575–583. doi:10.2147/JAA.S312123
- Minagawa S, Araya J, Watanabe N, et al. Real-life effectiveness of dupilumab in patients with mild to moderate bronchial asthma comorbid with CRSwNP. BMC Pulm Med. 2022;22(1):258. doi:10.1186/s12890-022-02046-3
- Nolasco S, Crimi C, Pelaia C, et al. Benralizumab effectiveness in severe eosinophilic asthma with and without chronic rhinosinusitis with nasal polyps: a real-world multicenter study. J Allergy Clin Immunol Pract. 2021;9(12):4371–4380 e4374. doi:10.1016/j.jaip.2021.08.004
- Lombardo N, Pelaia C, Ciriolo M, et al. Real-life effects of benralizumab on allergic chronic rhinosinusitis and nasal polyposis associated with severe asthma. Int J Immunopathol Pharmacol. 2020;34:2058738420950851. doi:10.1177/2058738420950851
- Detoraki A, Tremante E, D’Amato M, et al. Mepolizumab improves sino-nasal symptoms and asthma control in severe eosinophilic asthma patients with chronic rhinosinusitis and nasal polyps: a 12-month real-life study. Ther Adv Respir Dis. 2021;15:17534666211009398. doi:10.1177/17534666211009398
- Gallo S, Castelnuovo P, Spirito L, et al. Mepolizumab improves outcomes of chronic rhinosinusitis with nasal polyps in severe asthmatic patients: a multicentric real-life study. J Pers Med. 2022;12(8):1304. doi:10.3390/jpm12081304
- Bidder T, Sahota J, Rennie C, Lund VJ, Robinson DS, Kariyawasam HH. Omalizumab treats chronic rhinosinusitis with nasal polyps and asthma together-a real life study. Rhinology. 2018;56(1):42–45. doi:10.4193/Rhin17.139
